Published online Aug 16, 2025. doi: 10.12998/wjcc.v13.i23.105022
Revised: April 4, 2025
Accepted: April 25, 2025
Published online: August 16, 2025
Processing time: 146 Days and 16.6 Hours
The Rome Foundation’s questionnaires, including the latest version, Rome IV diagnostic criteria since 2016, are widely used globally for diagnosing functional gastrointestinal disorders (FGIDs). However, a tailored Thai version for diagno
To develop and validate the Thai version of the Rome IV diagnostic questionnaire for FGIDs in neonates and toddlers.
This study was conducted at a tertiary hospital in Bangkok. The Rome IV diag
A total of 58 complete questionnaires were returned. The median interval between the first and second time was 7 days (range: 4 days to 15 days). The item-objective congruence index for the Thai-adapted Rome IV diagnostic questionnaire was 0.74. Internal consistency, as indicated by Cronbach’s alpha, was 0.753, 0.712, and 0.750 for the three respective sections. The intraclass correlation coefficients for test-retest reliability were 0.782, 0.782, and 0.807.
The Thai Rome IV diagnostic questionnaire for FGIDs in neonates and toddlers demonstrates acceptable validity and reliability, supporting its use in future clinical and research applications.
Core Tip: The Thai version of the Rome IV diagnostic questionnaire demonstrates acceptable validity and reliability as a diagnostic tool for functional gastrointestinal disorders in young Thai children. It achieved an item-objective congruence of 0.74, with Cronbach’s alpha and intra-class correlation coefficients ranging from 0.712 to 0.807. This validated questionnaire has the potential to significantly improve the accuracy of diagnoses and treatment, thereby enhancing the quality of care for affected children. The findings highlight its value as a crucial resource for clinical practice, future research, and advancing the study of pediatric gastrointestinal health in Thailand.
- Citation: Chatpermporn K, Chongpison Y, Ngoenmak T, Treepongkaruna S, Sintusek P. Validity and reliability of the Thai “Rome IV diagnostic questionnaires” for functional gastrointestinal disorders in neonates and toddlers. World J Clin Cases 2025; 13(23): 105022
- URL: https://www.wjgnet.com/2307-8960/full/v13/i23/105022.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i23.105022
Functional gastrointestinal disorders (FGIDs) are a complex group of clinical presentations that cannot be attributed to structural or biochemical abnormalities[1,2]. The Rome Foundation was the first to describe the symptom-based diagno
In Thailand, Siajunboriboon et al[13] undertook the translation and validation of two of the Rome IV diagnostic questionnaires for children and adolescents, specifically the self-report form and the parental-report form for children aged 4-10 years. However, no study to date has determined the prevalence of FGIDs in neonates and toddlers using the Rome IV diagnostic criteria, primarily because of the lack of a standardized diagnostic questionnaire for this age group. The present study aimed to develop and evaluate the validity and reliability of the Thai Rome IV diagnostic questionnaire for neonates and toddlers, which would meet the qualification standards set by the Rome Foundation and be authorized for use by the Rome Foundation.
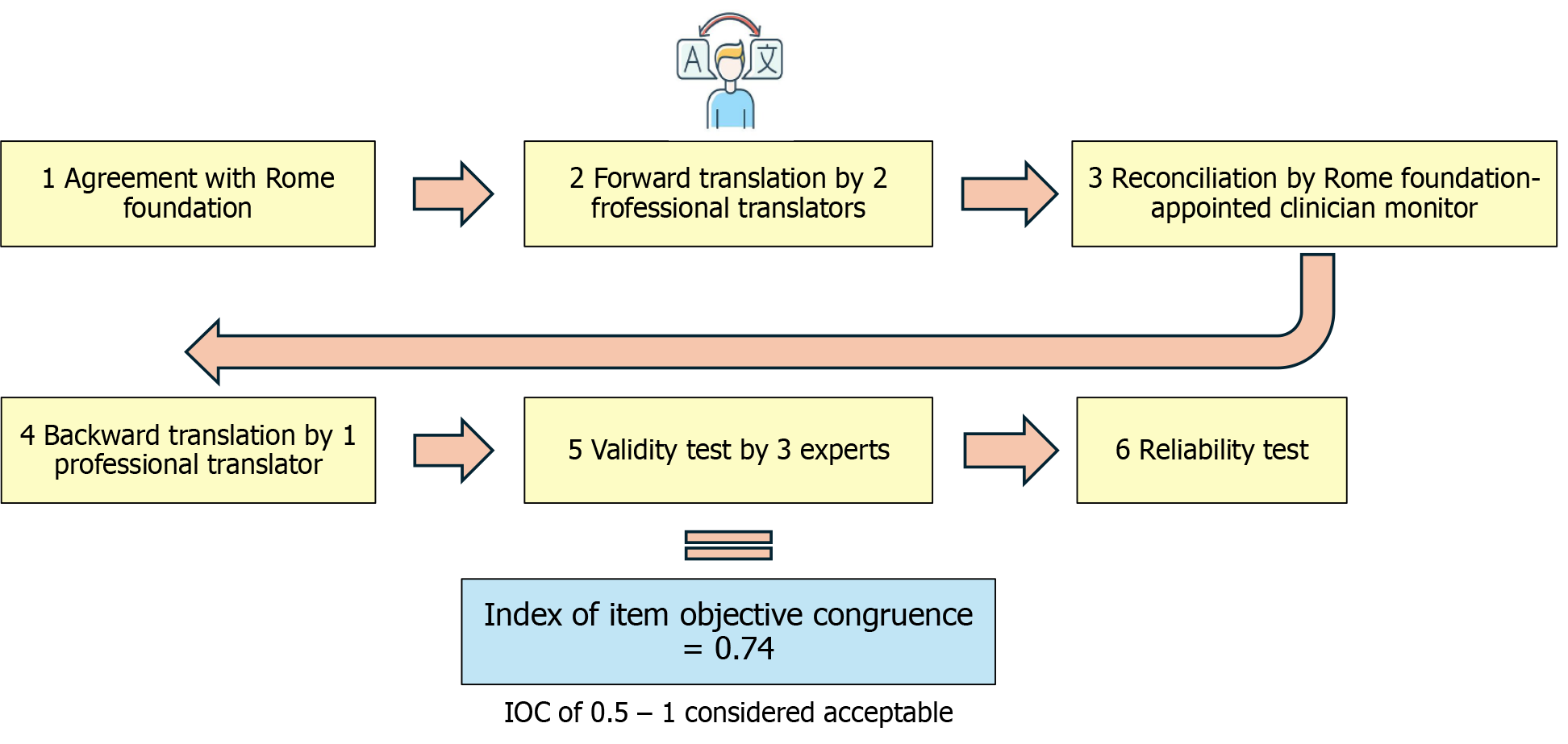
As part of the formal agreement with the Rome Foundation, the authors received the original English version of the Rome IV diagnostic questionnaire for FGIDs in infants and toddlers aged < 4 years. The original diagnostic questionnaire includes three sections: Section A addresses gastrointestinal issues in infants, section B focuses on vomiting, and section C covers bowel movements. To ensure accurate translation, the authors followed the translation guidelines of the Rome Foundation. First, two professional translators (Tassanee Keeratiratwattana and Vasin Boonpattanoporn) independently translated the diagnostic questionnaire into Thai. Next, the two Thai versions were merged into a single translation (Suporn Treepongkaruna), by a clinician monitor appointed by the Rome Foundation. Subsequently, a third professional translator (Thitima Ngoenmak) performed a thorough backward translation into English. The validity of the translated diagnostic questionnaire was evaluated by three experts (Suporn Treepongkaruna, Palittiya Sintusek, and Vasin Boonpattanaporn) using the item-objective congruence (IOC) index, which included the comparison of the translated diagnostic questionnaire with the original English version and the back-translation. Figure 1 summarizes the stepwise process of translation and validation. In addition to determination of linguistic accuracy, cultural relevance was assessed. Terms familiar to Thai caregivers, such as “grunt”, “bowel movement”, and “regurgitation” were selected to enhance comprehension without altering the intended meaning.
The reliability of the Thai version of the diagnostic questionnaire was evaluated using internal consistency and test-retest reliability assessments. A total of 65 caregivers of healthy neonates and toddlers were recruited from the outpatient clinic of King Chulalongkorn Memorial Hospital, Bangkok, Thailand. All caregivers completed the paper-based Thai version of the Rome IV diagnostic questionnaire for FGIDs in neonates and toddlers. The validity and internal consistency of each section were evaluated using intraclass correlation coefficient (ICC) and Cronbach’s alpha coefficient, respectively. For the assessment of test-retest reliability, the Thai version of the Rome IV diagnostic questionnaire, available both as a paper-based form and an electronic form (Google Form), was administered twice to all subjects at an interval ranging from 4 to 15 days. ICC was utilized to evaluate the correlation and stability of the responses. The present study was reviewed and approved by the Institutional Review Board, Faculty of Medicine, Chulalongkorn University, No. 0649/2023.
Statistical analysis was conducted using IBM SPSS Statistics 22. To assess questionnaire validity, the IOC was employed with a threshold of 0.5 to 1 considered acceptable[14]. For reliability testing, test-retest reliability was assessed using the ICC with the Two-Way Mixed-Effect Model, where ICC values of < 0.5, 0.5-0.74, 0.75-0.9, and > 0.9 were categorized as poor, moderate, good, and excellent, respectively[15]. Internal consistency within each domain was evaluated using Cronbach’s alpha coefficient, with values of < 0.5, 0.5-0.59, 0.6-0.69, 0.7-0.79, 0.8-0.89, and ≥ 0.9 considered unacceptable, poor, questionable, acceptable, good, and excellent, respectively[16]. The statistical methods of this study were reviewed by Yuda Chongpison from Research Affairs, Faculty of Medicine, Chulalongkorn University, Bangkok, 10330, Thailand.
The Thai version of the Rome IV diagnostic questionnaire for FGIDs in neonates and toddlers was administered twice to 65 caregivers, and 58 caregivers completed the questionnaire both times, with a median of 7 days (range: 4 days to 15 days) between the first and second responses. The interval between the first and second responses was shorter than 7 days in 40 caregivers. The mean age of the children of the caregivers who completed the diagnostic questionnaire was 6.3 months (range: 3.4 months to 8.9 months), and 52.8% were male. The IOC of the Thai version of the Rome IV diagnostic questionnaires was 0.74. The Cronbach’s alpha coefficients were 0.753, 0.712, and 0.750 for sections A (infant gastrointestinal issues), B (vomiting), and C (bowel movement), respectively. The ICC were 0.782 (0.658-0.865), 0.782 (0.658-0.865), and 0.807 (0.694-0.881) for sections A, B, and C, respectively. Table 1 shows the reliability indices for each section of the questionnaire, including the Cronbach’s alpha coefficients and ICCs (Table 1).
We demonstrate the successful development of the Thai version of the Rome IV diagnostic questionnaire for FGIDs in neonates and toddlers aged < 4 years, with our analyses highlighting its validity and good reliability, ensuring its suitability in evaluating FGIDs in this patient population. Its use allows for comparability with studies conducted in other countries utilizing the same symptom-based standardized tool. This tool will facilitate cross-cultural comparisons, contributing to a broader understanding of FGIDs in neonates and toddlers worldwide.
Indeed, one of the most challenging aspects of cross-cultural research is the adaptation of original diagnostic questionnaires to culturally relevant and comprehensible versions in other languages, while preserving the meaning and intent of the original instrument[17]. This process, known as translation and cultural adaptation, requires careful consideration of the linguistic, cultural, and conceptual differences between the source and target cultures. To achieve the successful adaptation of the original Rome IV diagnostic questionnaire for FGIDs in neonates and toddlers, we developed stan
In addition to engaging proficient translators and overseeing the work of clinicians designated by the Rome Founda
To the best of our knowledge, this is the first study to report the Cronbach’s alpha coefficients and the ICCs of the Thai version of the Rome IV diagnostic questionnaire for neonates and toddlers aged < 4 years. Although previous studies evaluated other translated versions of the Rome IV diagnostic questionnaires in older populations, such as the Thai version for children (Cronbach’s alpha 0.94-0.96, ICC 0.99)[13] and the Spanish version for adolescents (ICC 0.61-0.79)[18], no prior study has reported the Cronbach’s alpha coefficient or the ICC of a translated version of the Rome IV diagnostic questionnaire for neonates and toddlers aged < 4 years in any language. Beyond research applications, the newly developed Thai version of the Rome IV diagnostic questionnaire offers clinical value in primary care and general pediatric practice, by supporting the early diagnosis of FGIDs by standardizing caregiver symptom reporting, particularly in settings where symptom interpretation can vary. Clinicians can utilize this new tool as part of routine evaluation to guide referrals or early interventions.
The present study included only caregivers from a single tertiary hospital in Bangkok, which was a limitation of our study. Although most of the participating caregivers visited the hospital for vaccinations and check-up evaluations, the study cohort may not fully represent the broader Thai population, especially in rural or community-based settings. Therefore, the generalizability of our findings is limited. Future studies should incorporate multicenter data collection to validate our questionnaire across diverse healthcare settings and sociodemographic groups in Thailand. Additionally, the interval between the initial and follow-up assessments ranged from 4 days to 15 days, with a median of 7 days. Although this distribution reflects practical scheduling constraints, longer intervals may introduce changes in the condition of the child or the perception of the caregiver, which can impact the test-retest reliability. This limitation should be considered in the interpretation of our ICC data. To address this concern, we also conducted subgroup analysis including only the responses of caregivers with a test–retest interval of 4-7 days. In this subgroup, the ICCs were 0.888 (0.799-0.939), 0.787 (0.632-0.882), and 0.782 (0.625-0.878) for sections A, B, and C, respectively, which were comparable with the ICCs of the full cohort analysis and indicated good reliability. This finding suggested that the extended interval between the two responses in some caregivers did not meaningfully impact the reliability outcomes.
The Thai version of the Rome IV diagnostic questionnaires for FGIDs in neonates and toddlers exhibited good validity and reliability, supporting its value as a tool for the diagnosis of FGIDs in neonates and toddlers in Thailand. The study adhered to the Rome Foundation’s translation guidelines, ensuring quality through expert oversight and rigorous evaluation methods, including item-objective congruence and reliability testing using Cronbach’s alpha coefficient and ICC. This diagnostic questionnaire should be considered a diagnostic tool for Thai infants and toddlers with suspicious FGIDs in research and clinical practice settings.
| 1. | Vandenplas Y, Abkari A, Bellaiche M, Benninga M, Chouraqui JP, Çokura F, Harb T, Hegar B, Lifschitz C, Ludwig T, Miqdady M, de Morais MB, Osatakul S, Salvatore S, Shamir R, Staiano A, Szajewska H, Thapar N. Prevalence and Health Outcomes of Functional Gastrointestinal Symptoms in Infants From Birth to 12 Months of Age. J Pediatr Gastroenterol Nutr. 2015;61:531-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 2. | Zeevenhooven J, Koppen IJ, Benninga MA. The New Rome IV Criteria for Functional Gastrointestinal Disorders in Infants and Toddlers. Pediatr Gastroenterol Hepatol Nutr. 2017;20:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 3. | Hyman PE, Milla PJ, Benninga MA, Davidson GP, Fleisher DF, Taminiau J. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 2006;130:1519-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 328] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 4. | Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1077] [Article Influence: 56.7] [Reference Citation Analysis (6)] |
| 5. | Benninga MA, Faure C, Hyman PE, St James Roberts I, Schechter NL, Nurko S. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology. 2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 364] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 6. | Vernon-Roberts A, Alexander I, Day AS. Systematic Review of Pediatric Functional Gastrointestinal Disorders (Rome IV Criteria). J Clin Med. 2021;10:5087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Chew KS, Em JM, Koay ZL, Jalaludin MY, Ng RT, Lum LCS, Lee WS. Low prevalence of infantile functional gastrointestinal disorders (FGIDs) in a multi-ethnic Asian population. Pediatr Neonatol. 2021;62:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Játiva-Mariño E, Rivera-Valenzuela MG, Velasco-Benitez CA, Saps M. The prevalence of functional constipation in children was unchanged after the Rome IV criteria halved the diagnosis period in Rome III. Acta Paediatr. 2019;108:2274-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Steutel NF, Zeevenhooven J, Scarpato E, Vandenplas Y, Tabbers MM, Staiano A, Benninga MA. Prevalence of Functional Gastrointestinal Disorders in European Infants and Toddlers. J Pediatr. 2020;221:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 10. | Bloem MN, Baaleman DF, Thapar N, Roberts SE, Koppen IJN, Benninga MA. Prevalence of functional defecation disorders in European children: A systematic review and meta-analysis. J Pediatr Gastroenterol Nutr. 2025;80:580-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Velasco-Benítez CA, Collazos-Saa LI, García-Perdomo HA. A Systematic Review And Meta-Analysis In Schoolchildren And Adolescents With Functional Gastrointestinal Disorders According To Rome Iv Criteria. Arq Gastroenterol. 2022;59:304-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Ho L, Chen S, Ho FF, Wong CHL, Ching JYL, Cheong PK, Wu IXY, Liu X, Leung TH, Wu JCY, Chung VCH. Comparing diagnostic performance of Cantonese-Chinese version of Rome IV criteria and a short Reference Standard for functional dyspepsia in China. BMC Gastroenterol. 2022;22:432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Siajunboriboon S, Ngoenmak T, Tanpowpong P, Sarawit M, Treepongkaruna S. Validity and reliability of the Thai version of Rome IV diagnostic questionnaires for pediatric gastrointestinal disorders. J Med Assoc Thai. 2019;102 Suppl 10:1-4. |
| 14. | Roberts P, Priest H. Reliability and validity in research. Nurs Stand. 2006;20:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9979] [Cited by in RCA: 15898] [Article Influence: 1766.4] [Reference Citation Analysis (0)] |
| 16. | Tavakol M, Dennick R. Making sense of Cronbach's alpha. Int J Med Educ. 2011;2:53-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6227] [Cited by in RCA: 4680] [Article Influence: 334.3] [Reference Citation Analysis (0)] |
| 17. | Sperber AD. Translation and validation of study instruments for cross-cultural research. Gastroenterology. 2004;126:S124-S128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 466] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
| 18. | Baaleman DF, Velasco-Benítez CA, Méndez-Guzmán LM, Benninga MA, Saps M. Can We Rely on the Rome IV Questionnaire to Diagnose Children With Functional Gastrointestinal Disorders? J Neurogastroenterol Motil. 2021;27:626-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |