Published online Aug 16, 2025. doi: 10.12998/wjcc.v13.i23.104807
Revised: February 5, 2025
Accepted: April 24, 2025
Published online: August 16, 2025
Processing time: 153 Days and 8.6 Hours
Severe intraabdominal adhesions and ventral hernias pose significant technical challenges in bariatric surgery, especially in patients with a history of complex abdominal procedures.
This report describes a case involving a 30-year-old morbidly obese man who previously underwent a right lobe hepatectomy for living donor liver transplan
This case highlights the need for personalized surgical planning and precise techniques in bariatric surgery for patients with past abdominal operations.
Core Tip: This case demonstrates the necessity of individualized surgical planning in bariatric procedures for patients with prior abdominal surgeries. The successful outcome emphasizes the role of precise intraoperative techniques and careful patient selection in managing complex surgical cases.
- Citation: Cicek E, Karatepe YK, Kantarcı TR, Sahin TT. Demanding sleeve gastrectomy procedure in a patient with severe intraabdominal adhesions: A case report and review of the literature. World J Clin Cases 2025; 13(23): 104807
- URL: https://www.wjgnet.com/2307-8960/full/v13/i23/104807.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i23.104807
Previous abdominal surgeries often cause intraabdominal adhesions. This has been shown to have adverse effects on consequent surgeries and perioperative care of the patients[1,2]. In laparoscopic surgery, studies have shown that previous abdominal surgeries prolong the duration of the operation, increase postoperative complication rates, and increase the necessity to convert to open surgery[3-5]. However, none of them reported any difference in the success rates of the operations.
Morbid obesity incidence is increasing worldwide, and it has become a global public health problem[6]. Bariatric surgery is the gold-standard treatment option that reduces the time-dependent mortality of obese patients and provides a means for sustained weight loss and control[7]. The results of a study by Major et al[8] have shown that previous abdominal surgeries in patients with morbid obesity do not affect the excess body weight loss and complication rates following sleeve gastrectomy (LSG) and Rou-en-Y gastric bypass (RYGB). However, the duration of the operation and the length of hospitalization were longer in patients who had previous abdominal operations.
Liver transplantation is a lifesaving procedure that is the gold standard treatment for end-stage liver disease and primary liver cancers[9]. In many countries including Japan, Korea, India, and Turkiye, deceased donor liver donation is not enough, and living donor liver transplantation is the only means of providing the necessary liver grafts to the patients on the waiting list[10]. The majority of donor hepatectomies are performed through laparotomy using the Makuuchi incision which results in big incisions. Studies indicate that wound size and suture length directly affect the risk of incisional hernia[11,12]. The reported incidence of incisional hernia among living donors following living donor nephrectomy is nearly 10%[13]. However, there are not enough studies evaluating the prevalence of incisional hernia among living liver donors. The rates of incisional hernia following liver transplantation are reported to range between 7%-20%. However, most of these studies were performed on the recipients[14]. Living donor hepatectomies involve hepatectomy of the right lobe of the liver which causes left lobe hypertrophy and severe abdominal adhesions[15,16]. This causes a significant problem in subsequent surgeries in the upper abdomen.
We herein present the case of a young male patient, who, two years after a living donor hepatectomy, was admitted for a giant incisional hernia and morbid obesity. His complex surgical history, including severe abdominal adhesions, necessitated a demanding sleeve gastrectomy.
A 30-year-old male patient was admitted to our department for incisional hernia and morbid obesity. The main complaint of the patient was an incisional hernia. The incisional hernia was extensive, and there was a loss of abdominal domain. The patient needed to lose weight before performing a safe incisional hernia repair.
The medical history showed that the patient had donated the right lobe of his liver to his father 10 years ago. Right donor hepatectomy was performed through a Makuuchi incision.
Over the past ten years, the patient has progressively gained weight due to psychological stress and binge eating episodes. Before donor hepatectomy, his weight was 91 kg, his height was 184 cm, and his body mass index (BMI) was 26.9 kg/m2. The patient underwent reoperation due to intraabdominal bleeding on the 2nd postoperative day after donor hepatectomy and was then discharged after 38 days of hospitalization. His time in the intensive care unit was extended due to the reoperation. He weighed 56 kg, and his BMI was 16.5 kg/m2 at the time of his discharge.
The patient had a gradual weight gain in the past 10 years. He underwent two incisional herniorrhaphy operations in 2016 and 2020. He weighed 110 kg when a massive earthquake occurred in his hometown. The patient had to move out of his hometown, which contributed to his psychological stress. The binge eating episodes increased, and his body weight increased. At the time of our evaluation, his body weight was 136 kg, his height was 184 cm, and his BMI was 40.7 kg/m2.
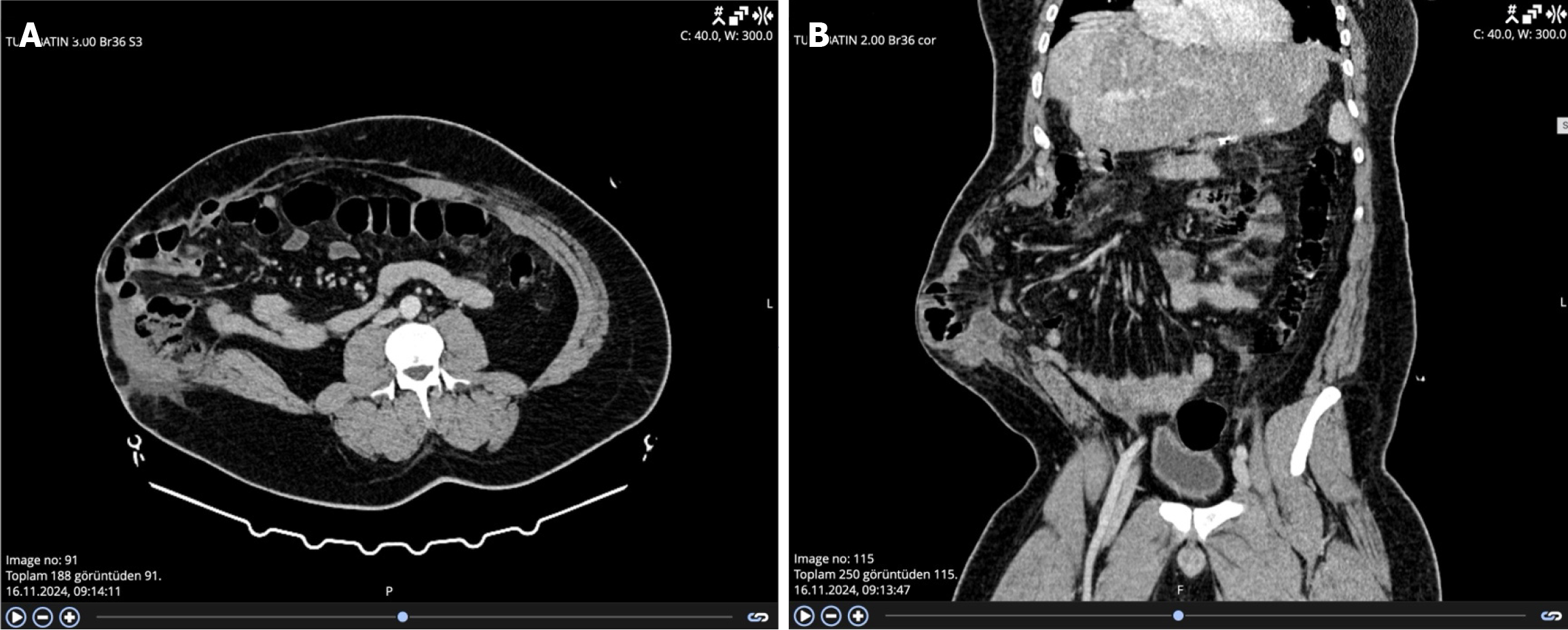
Our physical examination showed that the donor hepatectomy was performed through a Makuuchi incision, and the fascial defect was 19 cm × 15 cm in size. The loss of domain was extensive because most of the abdominal organs herniated into the incisional hernia sac.
The laboratory tests were normal.
Computed tomography scans showed a 19 cm × 15 cm fascial defect (Figure 1). The abdominal and visceral fat caused a tight abdomen, making the incisional hernia repair very difficult due to the loss of domain. We informed the patient about the risks of bariatric surgery and proposed a staged approach, including sleeve gastrectomy followed by an incisional hernia repair after he lost a considerable amount of weight.
The patient was diagnosed with giant incisional hernia and morbid obesity.
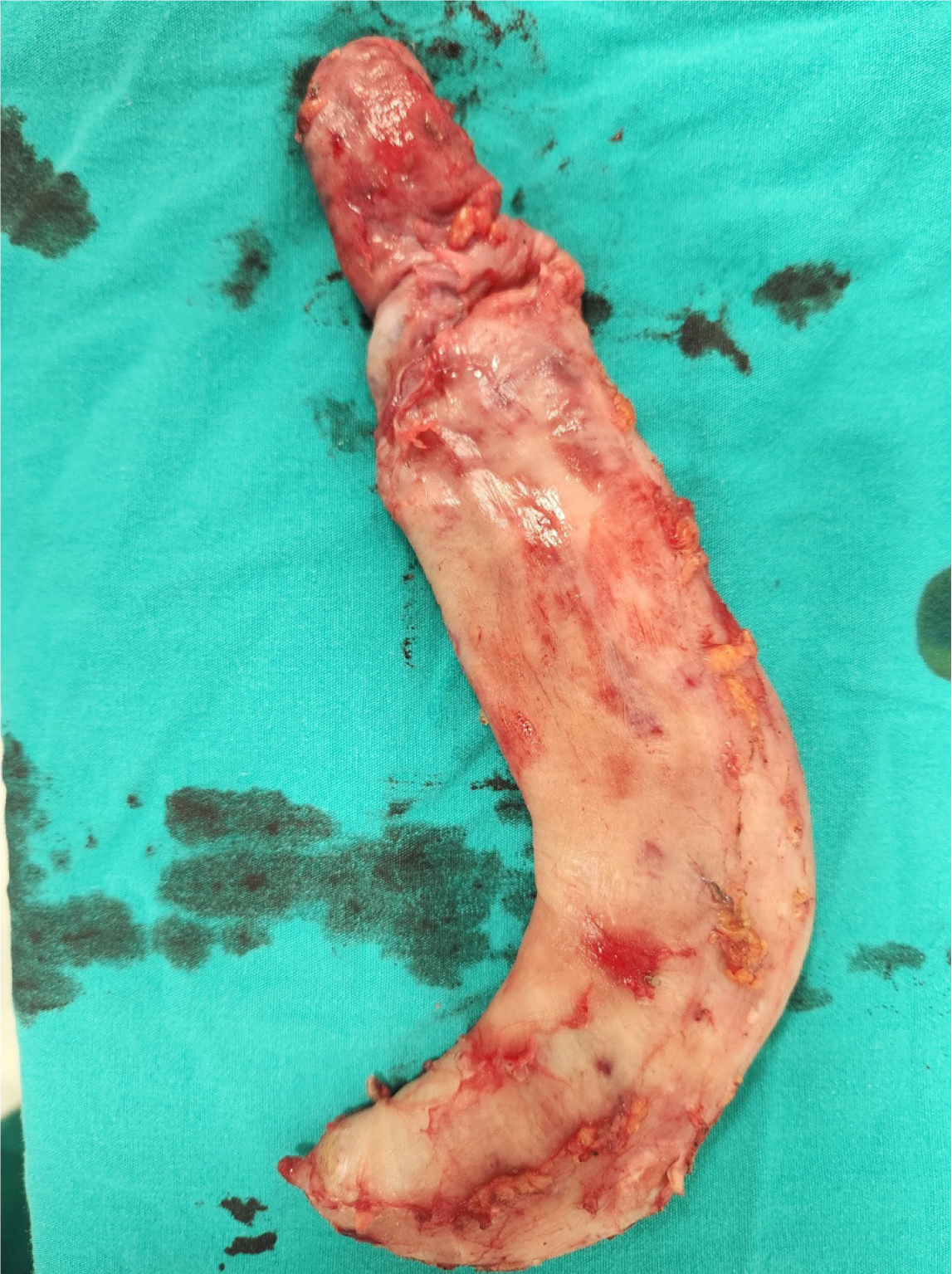
A sleeve gastrectomy was performed on the patient.
On postoperative day 1, the patient experienced trocar site bleeding at the 10-mm port site where the drain was inserted. This was managed conservatively, and the patient was discharged on postoperative day 5. At the 30-day follow-up, the patient had lost 27 kg.
LSG is one of the most frequently performed bariatric procedures. It is effective in treating obesity and associated diseases[17]. However, LSG is more demanding in patients with upper abdominal surgery and associated adhesions[18]. This also has significant implications for patients undergoing RYGB. Approximately 0.020% of planned RYGB procedures are converted to LSG due to massive adhesions. Furthermore, Major et al[8] reported that patients with previous abdominal surgeries undergo LSG more frequently compared to those without.
Donor hepatectomy following LSG is also reported frequently[19,20]. Bariatric surgery, such as sleeve gastrectomy or gastric bypass, is increasingly used to address fatty liver disease in potential living liver donors prior to hepatectomy[20-23]. It also reduces perioperative morbidity for living liver donors[20]. Similarly, concomitant sleeve gastrectomy and hepatic resection have been proposed for patients with hepatocellular carcinoma associated with non-alcoholic fatty liver disease and metabolic syndrome[24].
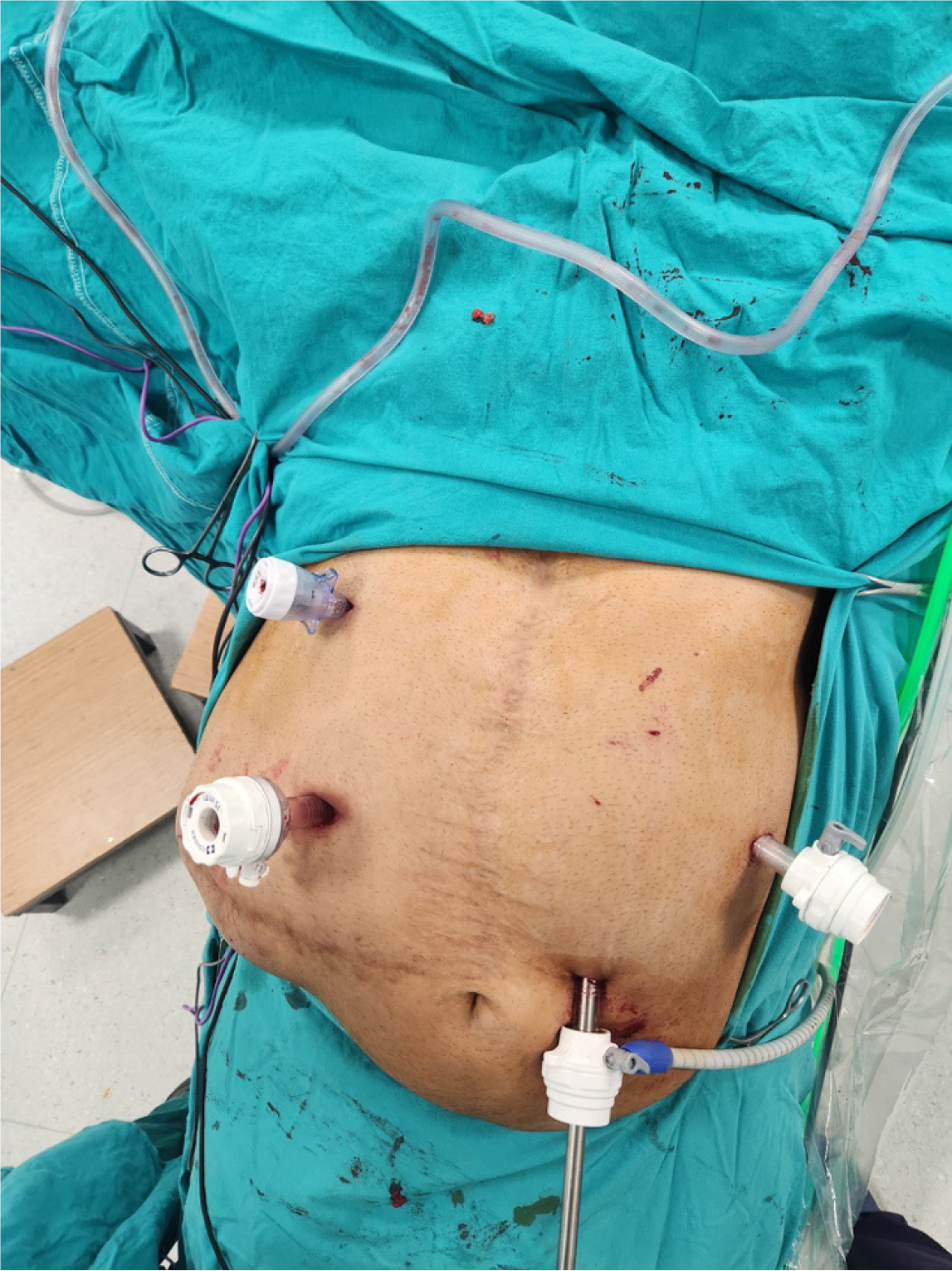
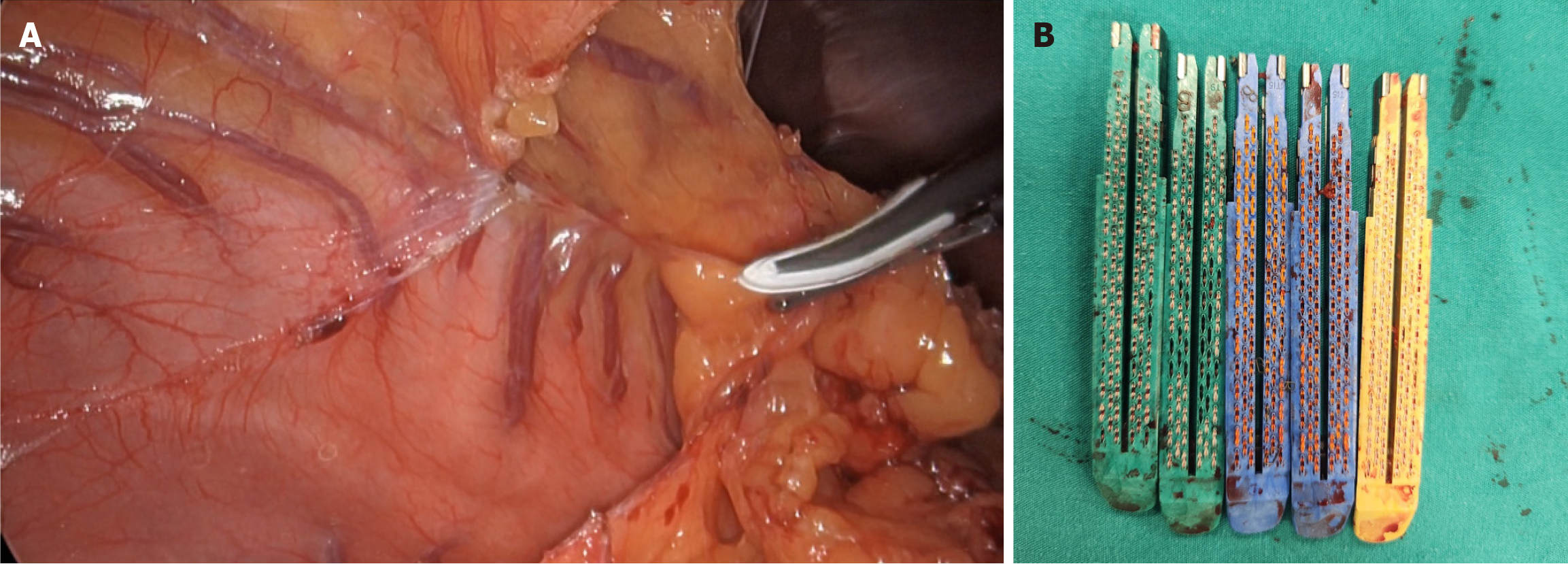
Treatment of ventral hernia in morbidly obese patients is very challenging[25]. There is an increased risk of wound site complications such as seromas, hematomas, and surgical site infections leading ultimately to an increased risk of recurrences[25]. Therefore, performing bariatric surgery before any ventral hernia repair in selected patients may increase the success rate of the operation[25]. To minimize the risk of complications, we selected a relatively adhesion-free area in the left upper quadrant for initial abdominal insufflation. Following insufflation, a 10-mm trocar was carefully inserted for the camera, and adhesiolysis was performed to create sufficient space for the placement of additional working trocars (Figure 2). Importantly, the Veress needle was removed under direct vision to ensure that no injuries occurred during its insertion. We believe that this technique is particularly useful for laparoscopic procedures in patients with a history of multiple abdominal surgeries and extensive adhesions (Figure 3).
Repairing ventral hernias with loss of domain is challenging, particularly in obese patients who are more prone to both primary and incisional ventral hernias due to increased intraabdominal pressure and comorbidities that can impair wound healing[26]. Many surgical techniques have been proposed to repair giant ventral hernias[27-30]. In contrast to staged procedures, single-step abdominal wall reconstruction techniques frequently involve the use of acellular dermal matrix mesh to provide immediate fascial reinforcement and support[27,28]. On the other hand, various multiple-step procedures have been proposed for complex ventral hernias, often involving a staged approach that includes preope
Complex ventral hernia repair following liver transplantation has been reported in the literature, with several studies describing the use of various surgical techniques, including component separation and mesh reinforcement[31-33]. While complex ventral hernia repair following liver transplantation has been reported, these studies have focused exclusively on hernia repair in liver recipients. Furthermore, laparoscopic ventral hernia repair has been shown to be safe and effective in recipients reported from a high-volume center[33]. Despite the increasing prevalence of complex ventral hernias, there is a paucity of data regarding their surgical management in living liver donors. This case report presents a unique approach to the management of a complex ventral hernia with loss of domain in a living liver donor, utilizing bariatric surgery as the initial step in a multi-step procedure (Figure 4).
In conclusion, this case demonstrates the feasibility and safety of sleeve gastrectomy as the initial step in a multi-step approach to complex ventral hernia repair in a living liver donor with a loss of domain. While technically demanding, this approach can be successful with careful preoperative planning and meticulous surgical technique by a team experienced in minimally invasive surgery.
| 1. | Aytac E, Stocchi L, De Long J, Costedio MM, Gorgun E, Kessler H, Remzi FH. Impact of previous midline laparotomy on the outcomes of laparoscopic intestinal resections: a case-matched study. Surg Endosc. 2015;29:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | van Goor H. Consequences and complications of peritoneal adhesions. Colorectal Dis. 2007;9 Suppl 2:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Lee SY, Kim CH, Kim YJ, Kim HR. Laparoscopic surgery for colorectal cancer patients who underwent previous abdominal surgery. Surg Endosc. 2016;30:5472-5480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Pędziwiatr M, Matłok M, Kulawik J, Major P, Budzyński P, Zub-Pokrowiecka A, Budzyński A. Laparoscopic adrenalectomy by the lateral transperitoneal approach in patients with a history of previous abdominal surgery. Wideochir Inne Tech Maloinwazyjne. 2013;8:146-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Law WL, Lee YM, Chu KW. Previous abdominal operations do not affect the outcomes of laparoscopic colorectal surgery. Surg Endosc. 2005;19:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2536] [Cited by in RCA: 2760] [Article Influence: 230.0] [Reference Citation Analysis (0)] |
| 7. | Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM; Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3684] [Cited by in RCA: 3152] [Article Influence: 175.1] [Reference Citation Analysis (1)] |
| 8. | Major P, Droś J, Kacprzyk A, Pędziwiatr M, Małczak P, Wysocki M, Janik M, Walędziak M, Paśnik K, Hady HR, Dadan J, Proczko-Stepaniak M, Kaska Ł, Lech P, Michalik M, Duchnik M, Kaseja K, Pastuszka M, Stepuch P, Budzyński A. Does previous abdominal surgery affect the course and outcomes of laparoscopic bariatric surgery? Surg Obes Relat Dis. 2018;14:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Karakayali H, Haberal M. The history and activities of transplantation in Turkey. Transplant Proc. 2005;37:2905-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Akbulut S, Yilmaz S. Liver transplantation in Turkey: historical review and future perspectives. Transplant Rev (Orlando). 2015;29:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 11. | Sekhar S, Ekka NM, Nair R, Pratap V, Mundu M, Kumar A. Effect of Suture Length on the Incidence of Incisional Hernia and Surgical Site Infection in Patients Undergoing Midline Laparotomy: A Systematic Review and Meta-Analysis. Cureus. 2023;15:e34840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Garmpis N, Spartalis E, Schizas D, Patsouras D, Damaskos C, Spartalis M, Garmpi A, Nikiteas NI, Dimitroulis D. Incisional Hernias Post Liver Transplantation: Current Evidence of Epidemiology, Risk Factors and Laparoscopic Versus Open Repair. A Review of the Literature. In Vivo. 2019;33:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | DuBray BJ, Tompson JJ, Shaffer D, Hale DA, Rega SA, Feurer ID, Forbes RC. Incisional Hernia Development after Live Donor Nephrectomy: Impact of Surgical Technique. Kidney360. 2023;4:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Huang R, Ding X, Fu H, Cai Q. Potential mechanisms of sleeve gastrectomy for reducing weight and improving metabolism in patients with obesity. Surg Obes Relat Dis. 2019;15:1861-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 15. | Satilmis B, Akbulut S, Sahin TT, Dalda Y, Tuncer A, Kucukakcali Z, Ogut Z, Yilmaz S. Assessment of Liver Regeneration in Patients Who Have Undergone Living Donor Hepatectomy for Living Donor Liver Transplantation. Vaccines (Basel). 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Heisterkamp J, Marsman HA, Eker H, Metselaar HJ, Tilanus HW, Kazemier G. A J-shaped subcostal incision reduces the incidence of abdominal wall complications in liver transplantation. Liver Transpl. 2008;14:1655-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Xia Q, Campbell JA, Ahmad H, Si L, de Graaff B, Palmer AJ. Bariatric surgery is a cost-saving treatment for obesity-A comprehensive meta-analysis and updated systematic review of health economic evaluations of bariatric surgery. Obes Rev. 2020;21:e12932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 18. | Osseis M, Lazzati A, Salloum C, Gavara CG, Compagnon P, Feray C, Lim C, Azoulay D. Sleeve Gastrectomy After Liver Transplantation: Feasibility and Outcomes. Obes Surg. 2018;28:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Obed A, Bashir A, Jarrad A. First right lobe living-donor hepatectomy after sleeve gastrectomy. BMC Surg. 2018;18:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Garcia D, Riveros S, Ochoa G, Rebolledo P, Achurra P, Briceño E, Viñuela E, Arab JP, Jarufe N, Fernandes E, Martinez J, Dib M. Right Lobe Liver Donation After Bariatric Surgery. A Case Series of 4 Living Donors. Transplant Proc. 2022;54:2212-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Luo RB, Suzuki T, Hooker JC, Covarrubias Y, Schlein A, Liu S, Schwimmer JB, Reeder SB, Funk LM, Greenberg JA, Campos GM, Sandler BJ, Horgan S, Sirlin CB, Jacobsen GR. How bariatric surgery affects liver volume and fat density in NAFLD patients. Surg Endosc. 2018;32:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Moretto M, Kupski C, da Silva VD, Padoin AV, Mottin CC. Effect of bariatric surgery on liver fibrosis. Obes Surg. 2012;22:1044-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 352] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 24. | Libia A, D'ugo S, Marchese T, Spampinato M. Liver resection and simultaneous sleeve gastrectomy for metabolic syndrome-related HCC: the LI.RE.S.S. National multicenter trial. HPB. 2024;26:S192. [DOI] [Full Text] |
| 25. | Gianchandani R, Moneva E, Manuel Sánchez J, Díaz C, Concepción V, Ángel Barrera M. Laparoscopic sleeve gastrectomy as a bridge to repair recurrent giant eventration in patients with morbid obesity, in relation to 3 cases. BMI Journal. 2020;2750-2753 Available from: https://www.bmi-journal.com/articulos/download/714/en. |
| 26. | Restrepo-rodas G, Barajas-gamboa JS, Guzman Fuentes JL, Meza Muñoz C, Al-baqain S, Vargas-cordova R, Guerrón AD. Management of abdominal wall hernias in bariatric patients: a narrative review. Ann Laparosc Endosc Surg. 2024;9:36-36. [DOI] [Full Text] |
| 27. | Azar FK, Crawford TC, Poruk KE, Farrow N, Cornell P, Nadra O, Azoury SC, Soares KC, Cooney CM, Eckhauser FE. Ventral hernia repair in patients with abdominal loss of domain: an observational study of one institution's experience. Hernia. 2017;21:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Lipman J, Medalie D, Rosen MJ. Staged repair of massive incisional hernias with loss of abdominal domain: a novel approach. Am J Surg. 2008;195:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Bueno-Lledó J, Torregrosa Gallud A, Jiménez Rosellón R, Carbonell Tatay F, García Pastor P, Bonafé Diana S, Iserte Hernández J. Preoperative preparation of «loss of domain» hernia. Progressive pneumoperitoneum and botulinum toxin type A. Cir Esp. 2017;95:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Borbély Y, Zerkowski J, Altmeier J, Eschenburg A, Kröll D, Nett P. Complex hernias with loss of domain in morbidly obese patients: role of laparoscopic sleeve gastrectomy in a multi-step approach. Surg Obes Relat Dis. 2017;13:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Ayuso S, Elhage SA, George MB, Anderson M, Levi DM, Heniford BT, Augenstein VA. Management of incisional hernias in liver transplant patients. IJAWHS. 2021;4:95-102. [RCA] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 32. | Cos H, Ahmed O, Garcia-Aroz S, Vachharajani N, Shenoy S, Wellen JR, Doyle MM, Chapman WC, Khan AS. Incisional hernia after liver transplantation: Risk factors, management strategies and long-term outcomes of a cohort study. Int J Surg. 2020;78:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Han GR, Johnson ER, Jogerst KM, Calderon E, Hewitt WR, Pearson DG, Harold KL. Outcomes of a Large Series of Laparoscopic Ventral Hernia Repairs after Liver Transplantation. Am Surg. 2023;89:5520-5526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |