Published online Aug 6, 2025. doi: 10.12998/wjcc.v13.i22.104258
Revised: March 30, 2025
Accepted: April 15, 2025
Published online: August 6, 2025
Processing time: 145 Days and 13.8 Hours
Epstein-Barr virus (EBV)-positive T-cell/natural killer (NK)-cell lymphoproliferative disorder is a rare but challenging condition that requires multidisciplinary teamwork, including co-management by infection medicine, radiotherapy, reha
The patient was a 33-year-old female who presented with an erythema-like lesion on the left upper extremity that became desquamated and then blistered and eventually became a giant ulcer with exposed nerves and muscles. The left wrist had a fixed posture, and pathology tests showed cutaneous EBV-positive NK/T-cell proliferative disease. We employed a multidisciplinary collaborative treat
Giant ulcers caused by cutaneous EBV-positive NK/T-cell proliferative disease can be treated using a multidisciplinary collaborative approach.
Core Tip: The holistic intervention treatment of cutaneous Epstein-Barr virus positive T/NK cell proliferative disease was carried out in a patient-centered multidisciplinary teamwork model, so that the patient's wounds could achieve healing.
- Citation: Guo YP, Wang ZX, Guo SL. Giant cutaneous ulcer in Epstein-Barr virus positive T-cell/NK-cell lymphoproliferative disorder: A case report. World J Clin Cases 2025; 13(22): 104258
- URL: https://www.wjgnet.com/2307-8960/full/v13/i22/104258.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i22.104258
Epstein-Barr virus (EBV), is classified as a γ-herpesvirus and possesses a double-stranded DNA genome. EBV primarily targets B lymphocytes, although it is also capable of infecting T lymphocytes, epithelial cells, and natural killer (NK) cells, leading to various associated diseases. Disruption of the balance between the host immune response and EBV can precipitate a range of EBV-associated lymphoproliferative disorders (LPDs) in B, T, or NK cells[1].
EBV-positive T-cell/NK-cell lymphoproliferative disorders (EBV+T/NK-LPD) comprise a heterogeneous group of conditions, including rare peripheral T-cell lymphomas that predominantly affect children and young adults and are associated with high mortality. Currently, hematopoietic stem cell transplantation is the only treatment shown to be effective in patients with EBV + T/NK-LPD[2]. The initial clinical signs of cutaneous EBV+T/NK-LPD are often nons
A 33-year-old female patient presented to Peking Union Medical College Hospital in January 2022 with progressive large skin ulcers on the left forearm with pain, oozing, and crusting for more than two years.
In December 2019, the patient had erythema-like skin lesions of the left upper limb that flaked without obvious triggers, which gradually transformed into blisters with pain. The skin lesions gradually expanded, ulcerated, and formed scabs, and she was transferred to several hospitals for treatment but the diagnosis was never confirmed, and the lesions aggravated. In May 2020, a pathological examination was performed at an outside hospital, and the lesion of the cutaneous lymphohematopoietic system was considered to be a tumor. Additionally, treatment with oral and topical traditional Chinese medicines did not alleviate the lesions. Subsequently, the skin lesions gradually expanded and spread to the right upper limb and back.
The patient was a young female who was previously fit and had no past history.
The patient had no personal and family history.
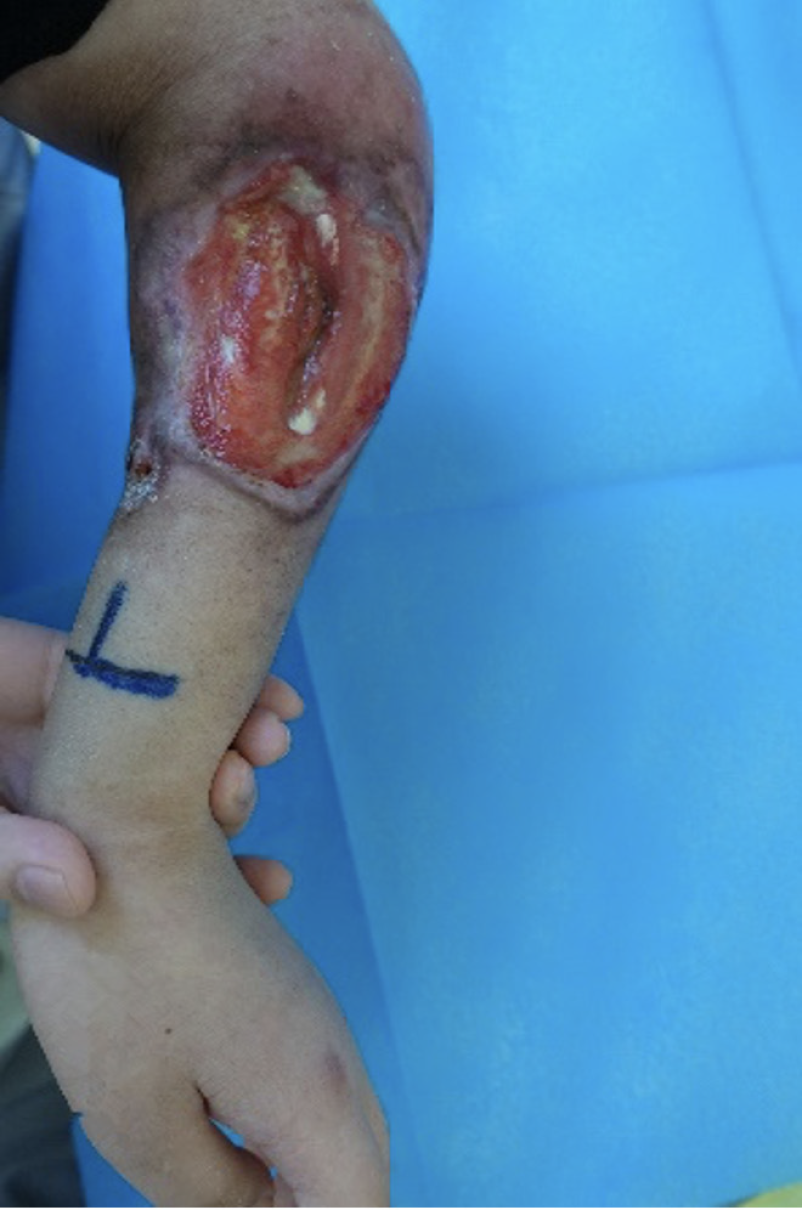
The patient visited Peking Union Medical College Hospital in January 2022. At the first evaluation, the skin lesions on the left upper limb were prominent, and the soft tissue of the left forearm was defective with a light red wound, accompanied by corrosive flesh and edematous granulation tissue, and three tendons, muscles, and nerves could be seen to be exposed. The size of the wound bed was 8 cm × 6.2 cm, and the tissue type was > 75% red tissue and < 25% yellow tissue (Figure 1). The exudate was saturated, highly viscous, purulent yellow, and had a grade 2 odor; the skin around the wound was red, pigmented, and dry, with an NRS score of 5. The mobility of the left shoulder and elbow joints was limited, and the wrist was in a fixed position.
The patient demonstrated a forward flexion and abduction of 160° in the left shoulder, with elbow flexion at 130° and extension to -30°. Rotation of the left forearm was limited to 20° forward and 30° backward. The right upper extremity showed normal range of motion.
Since the onset of the disease, the patient's food intake has decreased, and she has lost 5 kg of body weight in the last six months, with a nutritional risk score of 4 on the NRS2002 nutritional risk assessment, indicating that she is at nutritional risk.
After the initial wound assessment, multidisciplinary collaborative treatment was initiated owing to the complexity of the primary disease.
A biopsy was performed at the wound clinic, the specimen was embedded in paraffin and tissue pathology revealed changes at the epidermal interface. A patchy proliferative infiltrate consisting of small to moderately large heterogeneous lymphocytes was observed in the dermis and subcutaneous adipose tissue. These cells exhibited irregular nuclei and necrosis was observed. Immunohistochemically, the cells were CD3(+), CD56(+), CD30(+), CD4(+), CD8(+), CD20(-), CD30(+), CD4(+), CD8(+), CD20(-), T(+), and CD79a(-); EBER in situ hybridization positive.
Enhanced magnetic resonance imaging of the patient's left forearm revealed normal bone morphology and signal intensity. Effusion was observed in the elbow joint cavity. Swelling of the soft tissues of the radial and extensor sides of the forearm was also observed. Flake-like and striated areas of abnormal high-signal shadows were visible in the subcutaneous, muscular, and intermuscular spaces with marked enhancement of the lesions, some of which protruded above the skin surface. No normal skin was observed, suggesting the primary pathological involvement of the disease. Laboratory testing for immunological markers was conducted (Table 1).
| Laboratory testing | Result | Laboratory testing | Result |
| TCRB, % | - | LY | 9.3 |
| TCRG, % | - | NEUT | 85.4 |
| EBV-DNA | 48700 copies/mL | ALT | 69 U/L |
| ALP | 163 U/L | AST | 181 U/L |
| Transferrin | 1.69 g/L | Immunoglobulin M | 0.35 g/L |
| B-lymphocyte CD19% | 7.4 | B- lymphocyte CD19 | 64/µL |
| T- lymphocyte CD3 | 562/µL | CD4+ T-cells | 283/µL |
| CD8+ T-cells | 245/µL | CD4+CD28+ | 267/µL |
| CD8+CD28+ | 176/µL | CD8+CD38+ | 151/µL |
| NK% | 27.0 | Exudate culture identified | Enterobacter cloacae |
After a multidisciplinary consultation, the final diagnosis was peripheral T-cell lymphoma and EBV-positive lymphoproliferative disorder.
A multidisciplinary team comprising dermatology, radiotherapy, hematology, nutrition, rehabilitation experts, and wound stoma specialist nurses developed an integrated diagnostic and treatment model tailored to the specific needs of the patient, providing comprehensive systemic treatment. The regimen included:
(1) Immunotherapy: Human interferon α-2a (Interferon) administered as a 3 million IU intramuscular injection every other day; (2) Radiation therapy: A total dose of 30 Gy of localized radiation was administered over 15 sessions. Ultraviolet phototherapy sessions were also conducted. Ultraviolet light represents a specific range of the electromagnetic spectrum. UVA1 treatment is a recently developed new ultraviolet light treatment approach. UVA1 is commonly used in the treatment of dermatofibrosis diseases, atopic dermatitis, and cutaneous T cell lymphoma. UVA1 could fulfill a therapeutic role by inducing dermal T cell and fibroblast apoptosis and matrix metalloproteinase production, reducing inflammatory cytokines, and inhibiting collagen type I and III synthesis as well as that of other mechanisms[7-9]; (3) Anti-infection measures: Cefaclor extended-release tablets (0.375G, taken orally twice daily); (4) Hepatoprotective therapy: Polyenophosphatidylcholine capsules (456 mg) and bisabolol (25 mg) were taken orally thrice daily; (5) Nutrition: Since the onset of the disease, the patient's food intake had reduced, resulting in 5-kg of weight loss over the previous six months, and an NRS2002 score of 4 points, indicating nutritional risk. Strengthening nutritional support and oral nutritional supplementation at 400–600 kcal/day, thrice day; (6) Psychology: Psychological counseling; (7) Specialized wound care managed by specialist nurses; and (8) Rehabilitation therapy: Functional rehabilitation exercises for the left shoulder, elbow, and wrist joints. Rehabilitation training promotes the clinical symptom improvement of the patients and strengthens hand functions through the targeted instruction of active and passive upper limb activities, limb coordination, sensory function, and other training. Rehabilitation training includes neuromuscular electrical stimulation, intermediate frequency electrotherapy and functional training. Neuromuscular electrical stimulation: Combined with the injured nerves and muscle group damage of the patient to provide myoelectric-evoked treatment[10]. Medium-frequency electrotherapy: Placed at the relevant parts of the muscles. The intensity should be tolerated. Functional training: At the initial stage (1-2 weeks), conduct muscle strength training, including clenching the fist, finger extension, wrist extension and flexion, muscle contraction, etc.; at the middle stage (3-4 weeks), increase elbow flexion and extension activities, gently rotate the forearm, enhance muscle strength training intensity, and gradually increase the motion range of the wrist and elbow joints. In the later stage (6-8 weeks), increase the shoulder joint forward flexion and backward extension activities, and gradually increase mobility. Individualized assessment of muscle strength, joint mobility, adjust the intensity and difficulty of functional exercises, and encourage patients to carry out self-care activities within their abilities.
A specialist nurse conducted a comprehensive assessment of the wound, where the wound was dynamically evaluated, and a targeted dressing change program was developed. The “TIME” principle was followed, i.e., removal of necrotic tissue, control of infection and inflammation, exudate management, and promotion of granulation tissue growth.
The patient also experienced increased anxiety and depression. To mitigate these negative emotions during dressing changes, wound nurses adopted a calm and gentle demeanor, spoke softly, and provided frequent encouragement to bolster the patient's confidence in overcoming the illness. The patient's concerns about wound odor and limited limb mobility, which affected her appearance and resulted in social withdrawal and low self-esteem, were addressed by enhancing odor management during dressing changes. Additionally, wound nurses facilitated communication with patients’ families to encourage increased familial support.
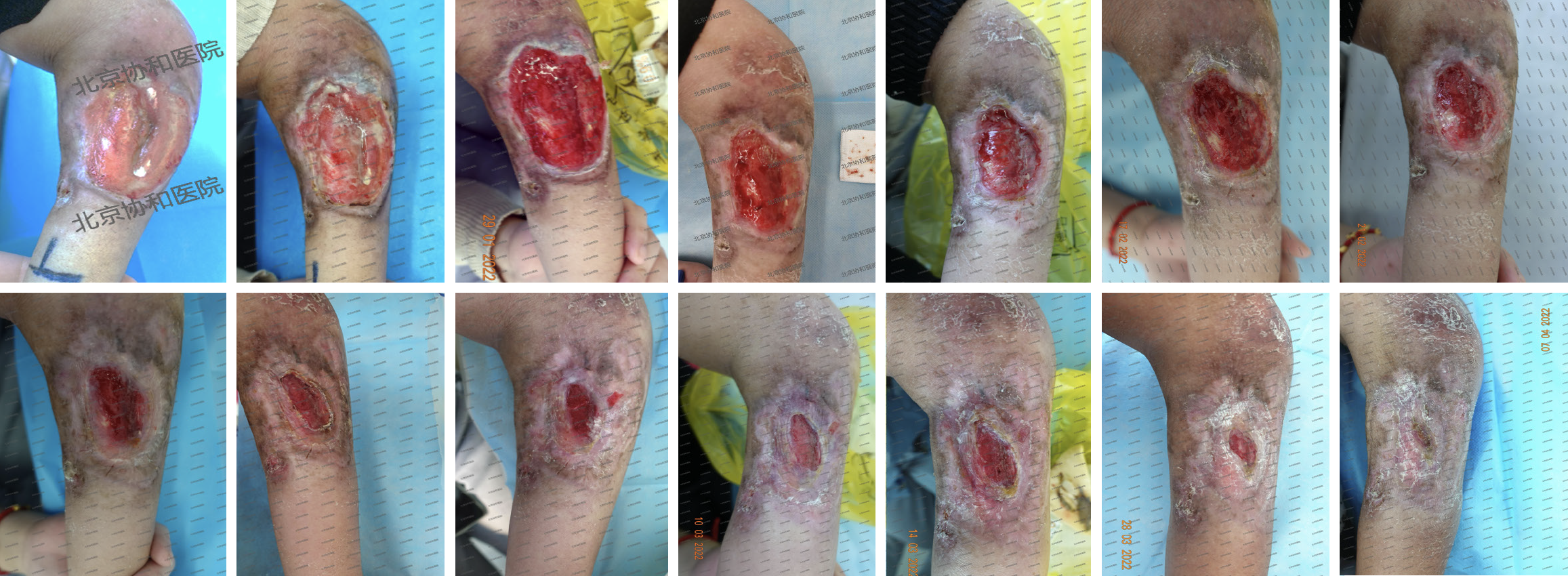
After 77 days of multidisciplinary teamwork intervention, the wound size ranged from 8 cm × 6.2 cm to completely heal. Healing means here that the exposed tendons and nerves at the wound are not necrotic, are completely encapsulated by granulation tissue, and the wound surface is covered by epithelial cells, forming a new epithelial layer. Swab cultures were performed on the wound of the patient. No bacteria could be cultured and no exudate or odor was present, all of which proved that the wound of the patient was no longer infected. Meanwhile, the upper extremity swelling of the patient had subsided, and the left shoulder, elbow, and wrist joints gradually regained function.
Genetic testing did not detect EBV DNA after hematopoietic stem cell transplantation at an outside institution in 2023, and the patient showed good regression.
In this case, the patient's wound progression was unsatisfactory even after two weeks of dressing change, which was considered to be related to the presence of a bacterial biofilm. A biofilm is a group of bacterial cells embedded in a matrix that has increased tolerance to antimicrobials and the host defense system[11]. Bacterial biofilms are present in about 80% of chronic wounds, as they can lead to local tissue hypoxia and increased resistance to antimicrobial agents, which can delay or arrest wound healing[12].
Therefore, for wound cleansing, the specialist nurse used surfactant and antimicrobial cleanser to clean the wound, as well as a wet dressing of moistened gauze for 30 min. Necrotic tissue, bacteria, and bacterial biofilms were removed from the wound surface using a sharp, conservative instrument. An antibacterial ointment combined with a silver-containing dressing was used as an inner dressing. As the wound surface exposed the tendons and nerves, a hydrogel dressing was used to cover them to avoid dry necrosis. The hereby-applied pro-tissue regenerative hydrogel is a hydrogel with a porous structure adapting it to naturally suited applications for loading a wide range of substances and releasing them slowly at specific locations. Wound healing requires interactions between various cells, including fibroblasts, endothelial cells, keratinocytes, etc., and is also regulated by endogenous growth factors, cytokines, and chemokines at the wound site[13]. Therefore, hydrogel topical delivery of exogenous cells or cytokines promotes wound tissue repair and regeneration. Meanwhile hydrogel dressings form a gel-like substance with a three-dimensional mesh structure after fully absorbing water and swelling, which can have a protective effect on exposed tendons and nerves[13]. According to the patient's wound exudate situation, the inner layer dressing was added with negative-pressure microtubular silk wound dressing that had a stronger exudate absorption effect. The outer layer of the dressing was covered with a borderless foam dressing and bandaged for compression. Pain management was also important during dressing changes. For our patient, a sprayable local anesthetic was administered for surface anesthesia prior to dressing change, as prescribed by the physician, to reduce pain during dressing change and improve patient comfort. The patient’s pain score (NRS) decreased from an initial score of 5 to 2. Depending on wound exudation, the treatment was changed every three days. After 13 wound dressing changes over a 45-day period, significant improvements were observed in the characteristics of the wound bed, transitioning from > 75% red tissue and less than 25% yellow tissue to 100% red tissue. The wound dimensions reduced to 3.0 cm × 1.5 cm, and the exudate level decreased from saturated to moist. Notably, odor intensity increased from Grade 1 to Grade 4. The tendons, nerves, and muscles were completely enveloped by neoplastic granulation tissue, allowing discontinuation of hydrogel usage (Figure 2).
The patient also experienced increased anxiety and depression. To mitigate these negative emotions during dressing changes, the wound nurses adopted a calm and, gentle demeanor, spoke softly, and provided frequent encouragement to bolster the patient's confidence in overcoming the illness. The patient's concerns about the wound odor and limited limb mobility, which affected her appearance and resulted in social withdrawal and low self-esteem, were addressed by enhancing odor management during dressing changes. Additionally, the wound nurses facilitated communication with the patient's family to encourage increased familial support.
Wound healing is a complex process involving tissue proliferation and remodeling. Aggressive nutritional support during the wound-healing process helps reverse the body's hypermetabolic state[14]. The NRS2002 score of 4 indicated that the patient was at nutritional risk, and to accelerate wound healing, the patient was encouraged to eat nutritious, protein-rich, and high-calorie foods, as well as oral enteral nutrients, 400–600 Kcal/day, to ensure adequate energy intake, and the patient was instructed to avoid spicy and stimulating foods.
Since the patient's wound originated from a primary disease, treatment should encompass not only local wound care but also management of the underlying condition. Throughout the 77 days of wound dressing change, the patient underwent systemic immunotherapy, localized radiation therapy, and ultraviolet phototherapy. Research has demonstrated that the first-episode remission rate for radiation therapy in patients with limited-stage disease exceeds 90%[15]. Furthermore, the patient’s EBV-DNA levels decreased from 48700 copies/mL to as low as 400 copies/mL following treatment, illustrating that control of the primary disease is a crucial factor in wound healing.
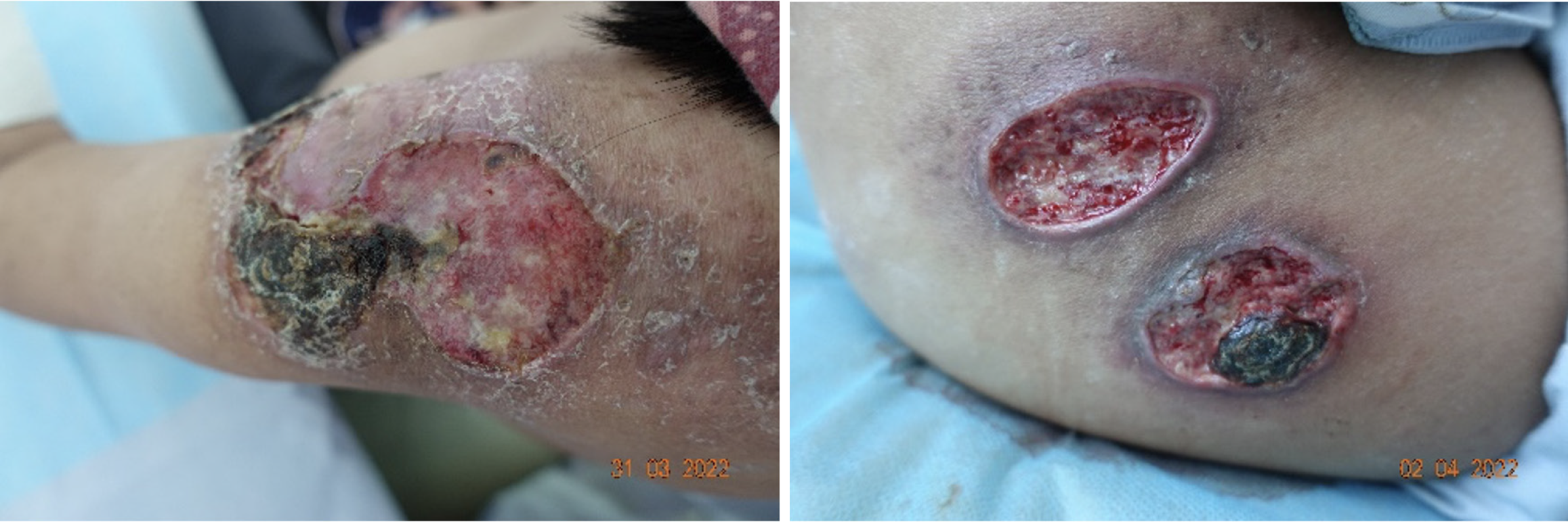
The treatment of EBV-positive T/NK cell lymphoproliferative disease typically requires autologous stem cell transplantation. However, as this approach was infeasible during the trauma treatment period, initial control of the disease was necessary. During treatment, the patient's primary disease recurred, with EBV-DNA levels increasing to 44000 copies/mL. Concurrently, multiple ulcers and black scabs developed on both shoulders and the left hip (Figure 3). This recurrence not only exacerbated the patient’s anxiety and depression but demonstrated the persistence of the skin condition until the primary disease was thoroughly managed. This underscores the complexities encountered when treating this condition.
In this case, the holistic intervention treatment of cutaneous EBV-positive T/NK cell proliferative disease involved a patient-centered multidisciplinary teamwork model, following the TIME principles of wound treatment. The wounds were dynamically assessed, different wet healing dressings were selected according to the patient's wound condition, and pain management, nutritional support, and psychosocial care, as well as immunotherapy and phototherapy were provided to facilitate wound healing. In this case, we demonstrated that wound healing involves systemic factors and requires multifaceted treatment to achieve satisfactory results.
The limitation of this article is that the treatment of the patient's subsequent ulcers that developed in both shoulders and the left hip is not described in specific detail, leaving the article slightly lacking in completeness.
The patient was very grateful to the medical staff for their careful treatment throughout the treatment period. The form of multidisciplinary treatment improved the accuracy of diagnosis and treatment and formulated a personalized treatment plan for her, which improved the treatment effect, shortened the treatment time, and led to a more adequate commu
| 1. | Kim WY, Montes-Mojarro IA, Fend F, Quintanilla-Martinez L. Epstein-Barr Virus-Associated T and NK-Cell Lymphoproliferative Diseases. Front Pediatr. 2019;7:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 2. | Ai J, Xie Z. Epstein-Barr Virus-Positive T/NK-Cell Lymphoproliferative Diseases in Chinese Mainland. Front Pediatr. 2018;6:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Zhang YL, Wei P, Xie JL, Zheng YY, Zhou XG. Cutaneous T/NK-cell EBV-infected lymphoproliferative disorders of lymphoid tissue and NK/T-cell lymphoma. Linchuang Yu Shiyan Binglixue Zazhi. 2018;34:1123-1125. [DOI] [Full Text] |
| 4. | Li S, Xie M, Luo W, Zhou Q, Li C, Liu Y, Hu A. Quality of Life and Its Influencing Factors in Chinese Patients with Chronic Wounds. Adv Skin Wound Care. 2022;35:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Yan R, Strandlund K, Ci H, Huang Y, Zhang Y, Zhang Y. Analysis of Factors Influencing Anxiety and Depression among Hospitalized Patients with Chronic Wounds. Adv Skin Wound Care. 2021;34:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Fearns N, Heller-Murphy S, Kelly J, Harbour J. Placing the patient at the centre of chronic wound care: A qualitative evidence synthesis. J Tissue Viability. 2017;26:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Ju M, Chen K, Chang B, Gu H. UVA1 irradiation inhibits fibroblast proliferation and alleviates pathological changes of scleroderma in a mouse model. J Biomed Res. 2012;26:135-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Keyal U, Bhatta AK, Wang XL. UVA1 a promising approach for scleroderma. Am J Transl Res. 2017;9:4280-4287. [PubMed] |
| 10. | Du WW. Effect of combined treatment of patients with brachial plexus nerve injury by electroacupuncture and rehabilitation training on the functional recovery of the upper limb. Shijie Zuixin Yixue Xinxi Wenzhai. 2020;20:52-53. |
| 11. | Thaarup IC, Iversen AKS, Lichtenberg M, Bjarnsholt T, Jakobsen TH. Biofilm Survival Strategies in Chronic Wounds. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Malone M, Bjarnsholt T, McBain AJ, James GA, Stoodley P, Leaper D, Tachi M, Schultz G, Swanson T, Wolcott RD. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care. 2017;26:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 255] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 13. | Fu CL, Zhang WJ, Wang RY, Lin HD. Research progress on the application of functional hydrogels in wound healing. Shengwu Guke Cailiao Yu Linchuang Yanjiu. 2025;22:64-69. [DOI] [Full Text] |
| 14. | Li Y, Jiang QX, Zhou X, Peng Q, Huang XL. Advances in dietary guidance programs for patients with chronic wounds. Zhonghua Xiandai Huli Zazhi. 2013;19:1727-1729. [DOI] [Full Text] |
| 15. | Bi XW, Li YX, Fang H, Jin J, Wang WH, Wang SL, Liu YP, Song YW, Ren H, Dai JR. High-dose and extended-field intensity modulated radiation therapy for early-stage NK/T-cell lymphoma of Waldeyer's ring: dosimetric analysis and clinical outcome. Int J Radiat Oncol Biol Phys. 2013;87:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |