Published online Mar 26, 2024. doi: 10.12998/wjcc.v12.i9.1677
Peer-review started: December 4, 2023
First decision: January 9, 2024
Revised: February 3, 2024
Accepted: March 5, 2024
Article in press: March 5, 2024
Published online: March 26, 2024
Processing time: 112 Days and 8.1 Hours
Pancreatic ductal leaks complicated by endoscopic ultrasonography-guided tissue sampling (EUS-TS) can manifest as acute pancreatitis.
A 63-year-old man presented with persistent abdominal pain and weight loss. Di
Using the fanning method in EUS-TS can inadvertently cause damage to the pan
Core Tip: Endoscopic ultrasound guided tissue sampling is a crucial procedure for histological diagnosis of pancreatic lesions, but it may occasionally be accompanied by unforeseen complications. The Fanning method is a technique that can enhance diagnostic accuracy, but it also carries the risk of unintended ductal injury. Here, we report a case where the use of the Fanning method during endoscopic ultrasound guided tissue sampling resulted in leakage of the pancreatic duct. We successfully managed this complication by performing pancreatic duct stent via endoscopic retrograde cholangiopancreatography.
- Citation: Kim KH, Park CH, Cho E, Lee Y. Endoscopic ultrasound-guided tissue sampling induced pancreatic duct leak resolved by the placement of a pancreatic stent: A case report. World J Clin Cases 2024; 12(9): 1677-1684
- URL: https://www.wjgnet.com/2307-8960/full/v12/i9/1677.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i9.1677
Endoscopic ultrasound-guided tissue sampling (EUS-TS) has become the gold standard for histological diagnosis of solid pancreatic lesions[1,2]. Although EUS-TS is considered relatively safe, unexpected complications inevitably arise. Acute pancreatitis is one of the more severe complications of EUS-TS, as it can delay the surgical schedule of resectable lesions and can even make curative treatment impossible[3]. Pancreatic ductal leaks often complicate the situation, while recent treatment guidelines for acute pancreatitis, such as early feeding, can be counterproductive. EUS-TS puts patients at risk of developing pancreatic ductal leaks if normal pancreatic ducts are damaged during the procedure[4]. Fortunately, pancreatic ductal leaks can be adequately treated by placing a pancreatic stent to divert the leaking pancreatic juice into the duodenal lumen[5]. According to our review of English-language scientific literature, this is the first recorded case of a pancreatic ductal leak developing after timely EUS-TS treatment with pancreatic stent placement. Therefore, clinical suspicion is key to promptly diagnosing and treating pancreatic ductal leaks in patients who present with acute pancreatitis after EUS-TS. Herein, we present a case of acute pancreatitis caused by a pancreatic ductal leak after EUS-TS that was treated with a pancreatic stent.
A 63-year-old man visited the outpatient department with a weight loss.
The weight loss has been significant, with a decrease of 14 kg over the past two months, and upper abdominal pain was occurred 10 d ago.
He had been diagnosed with hypertension and diabetes mellitus four years earlier.
He was a chronic alcoholic and a current smoker.
The physical examination showed tenderness in the epigastric area without rebound tenderness.
His white blood cell count was 3500/mm3 (reference value: 6000–10000/mm3), lipase level was 120 U/L (reference value: 7–60 U/L), carbohydrate antigen 19-9 level was 5920 U/mL (reference value: < 37 U/mL), and carcinoembryonic antigen level was 23.7 ng/mL (reference value: 0–4.7 ng/mL). All other laboratory test results were within the normal ranges.
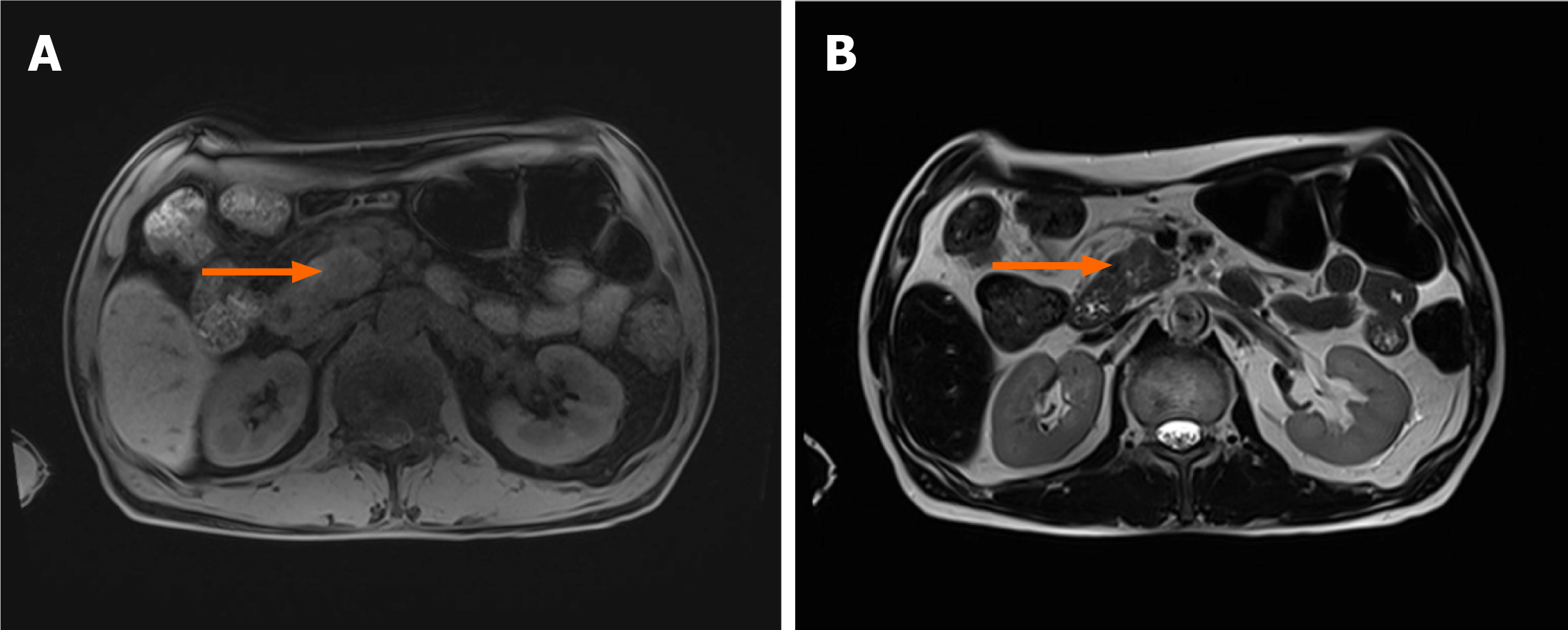
Magnetic resonance imaging of the pancreas showed an approximately 3 cm-sized, ill-defined space-occupying lesion in the inferior aspect of the head, with high signal intensity on T1- and T2-weighted imaging (Figure 1). The distal common bile duct and main pancreatic duct adjacent to the lesion were narrowed, the upstream main pancreatic duct was dilated, and the superior mesenteric artery was severely encased (Figure 2).
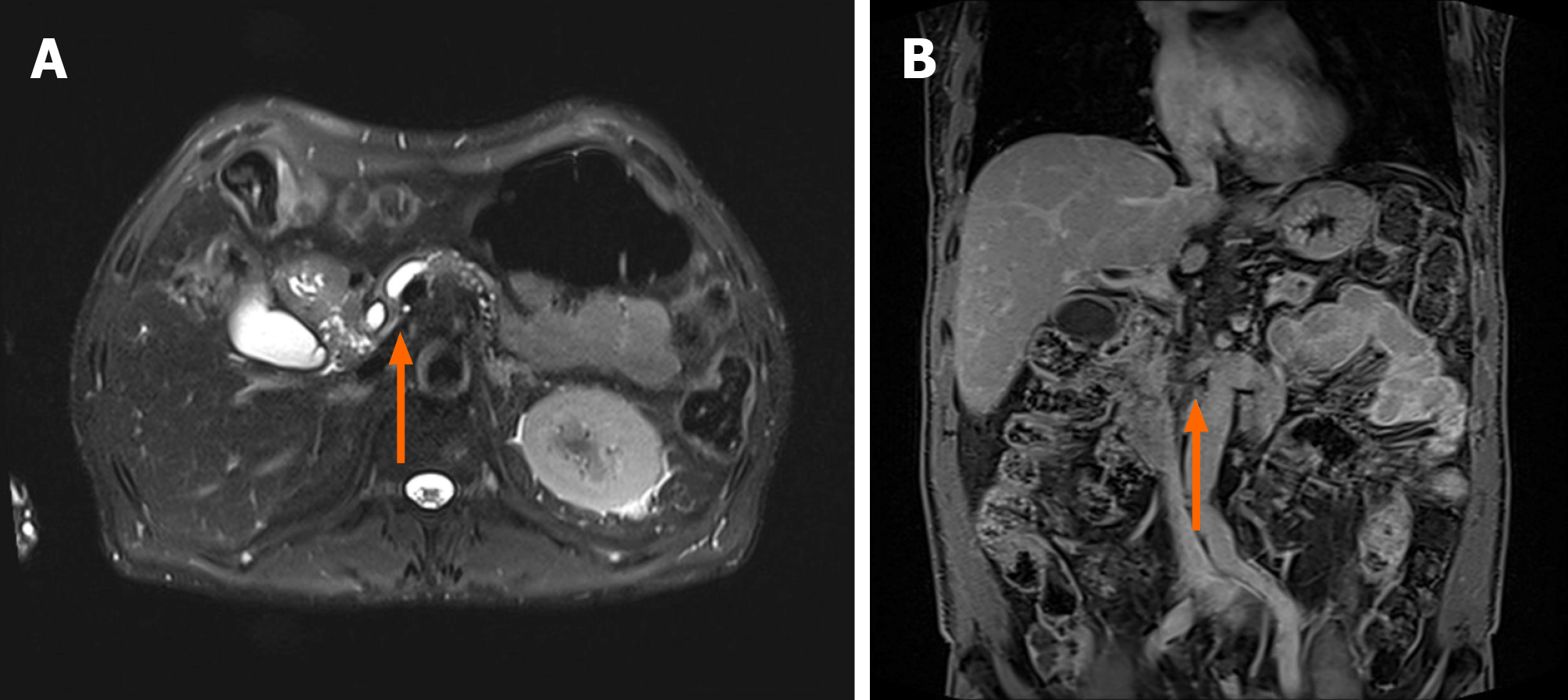
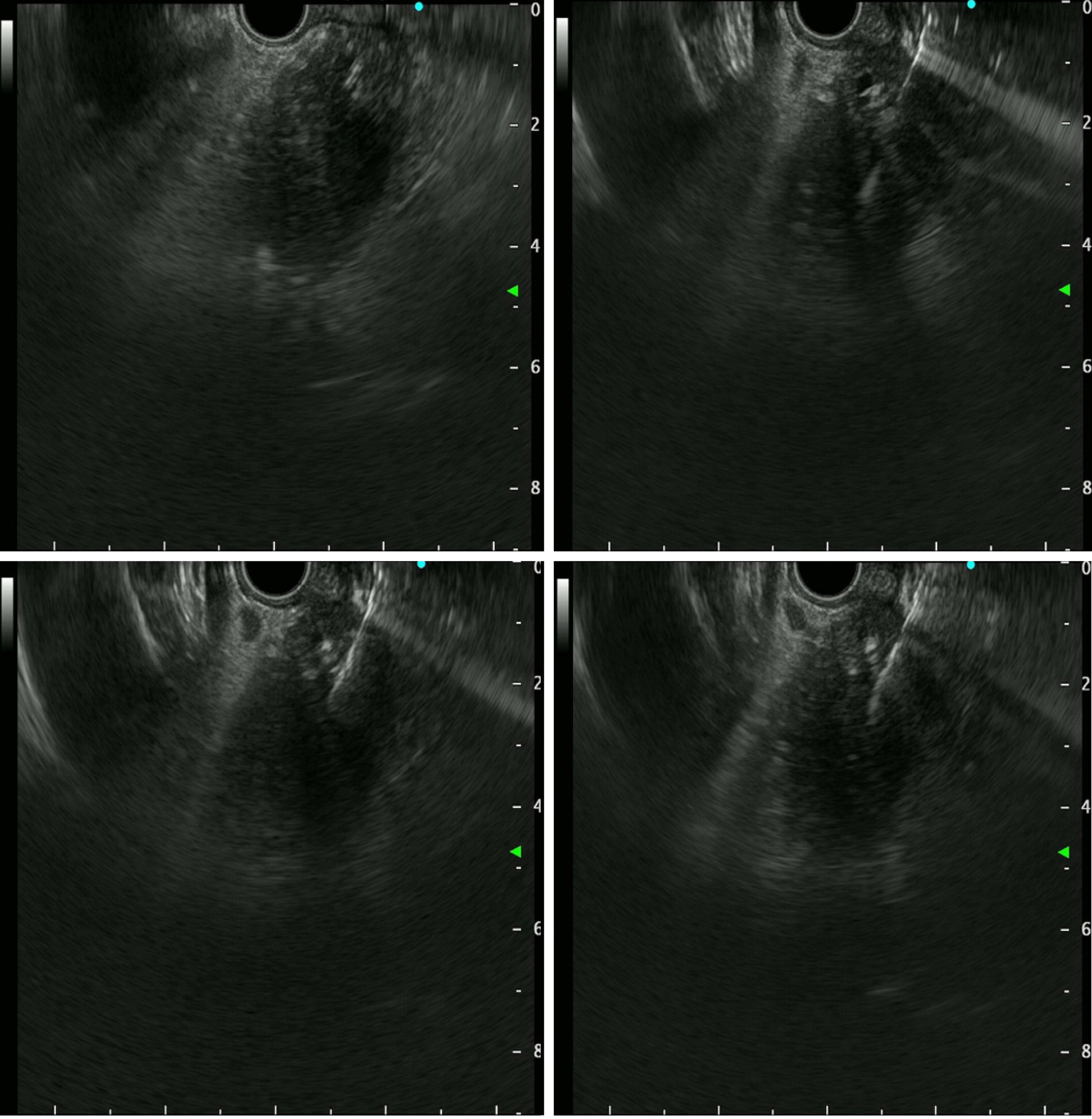
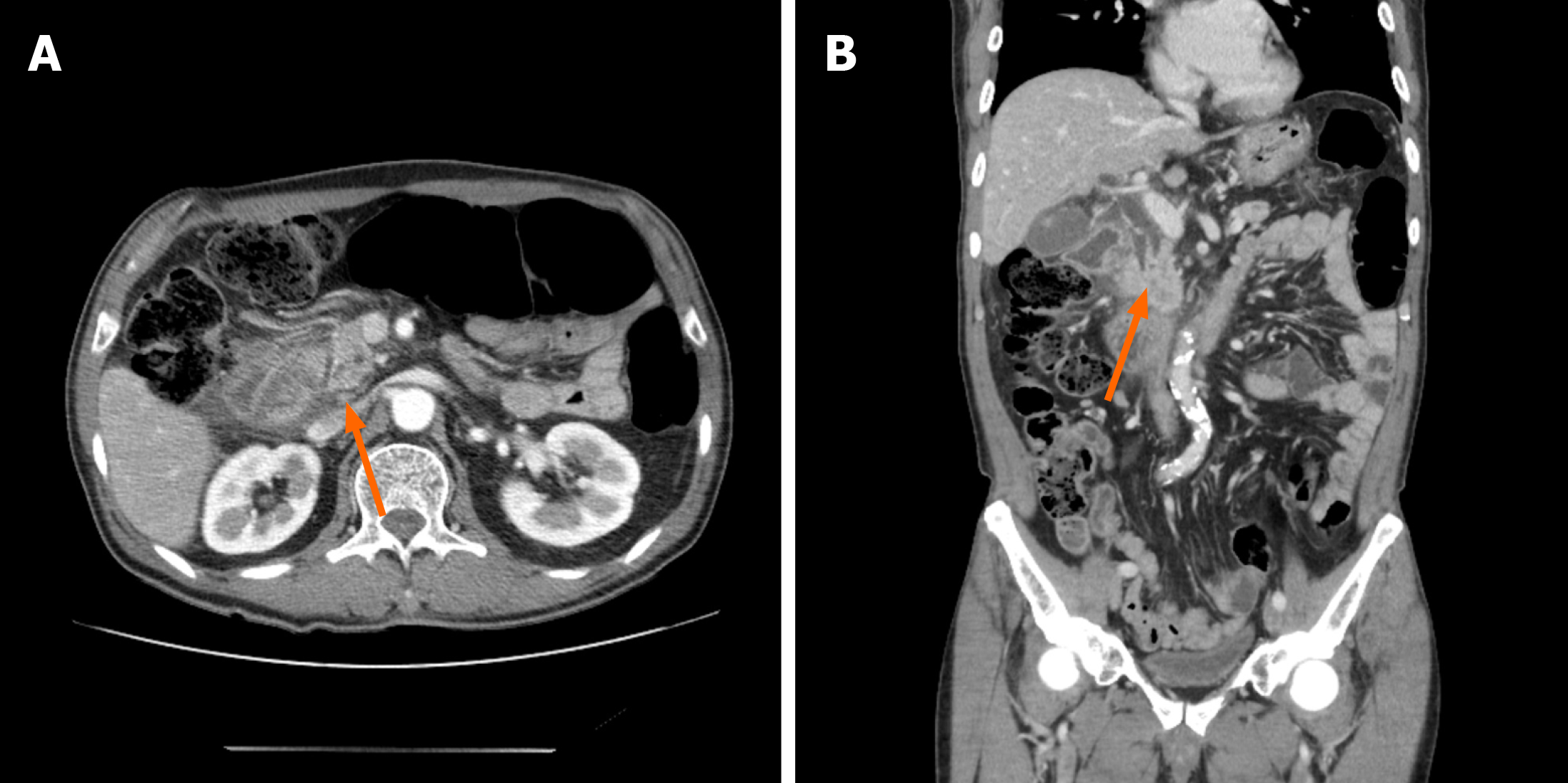
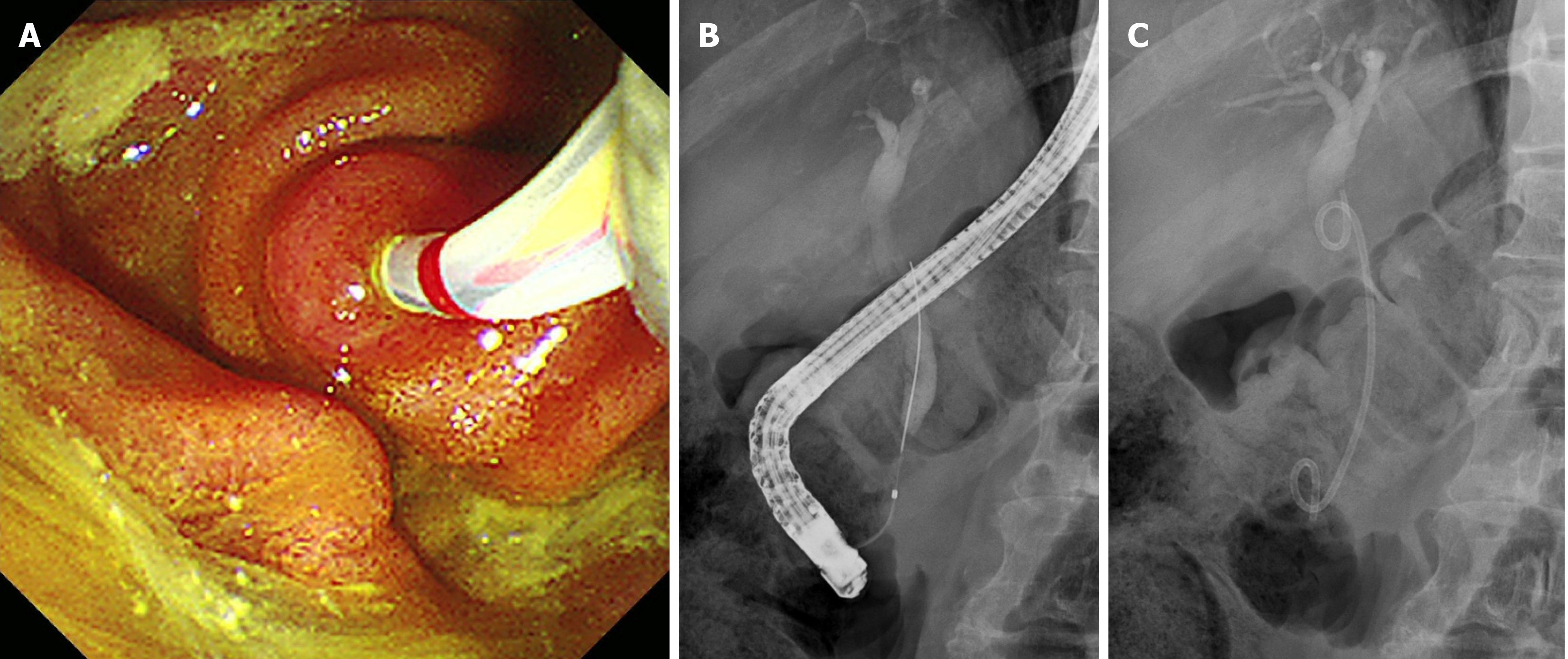
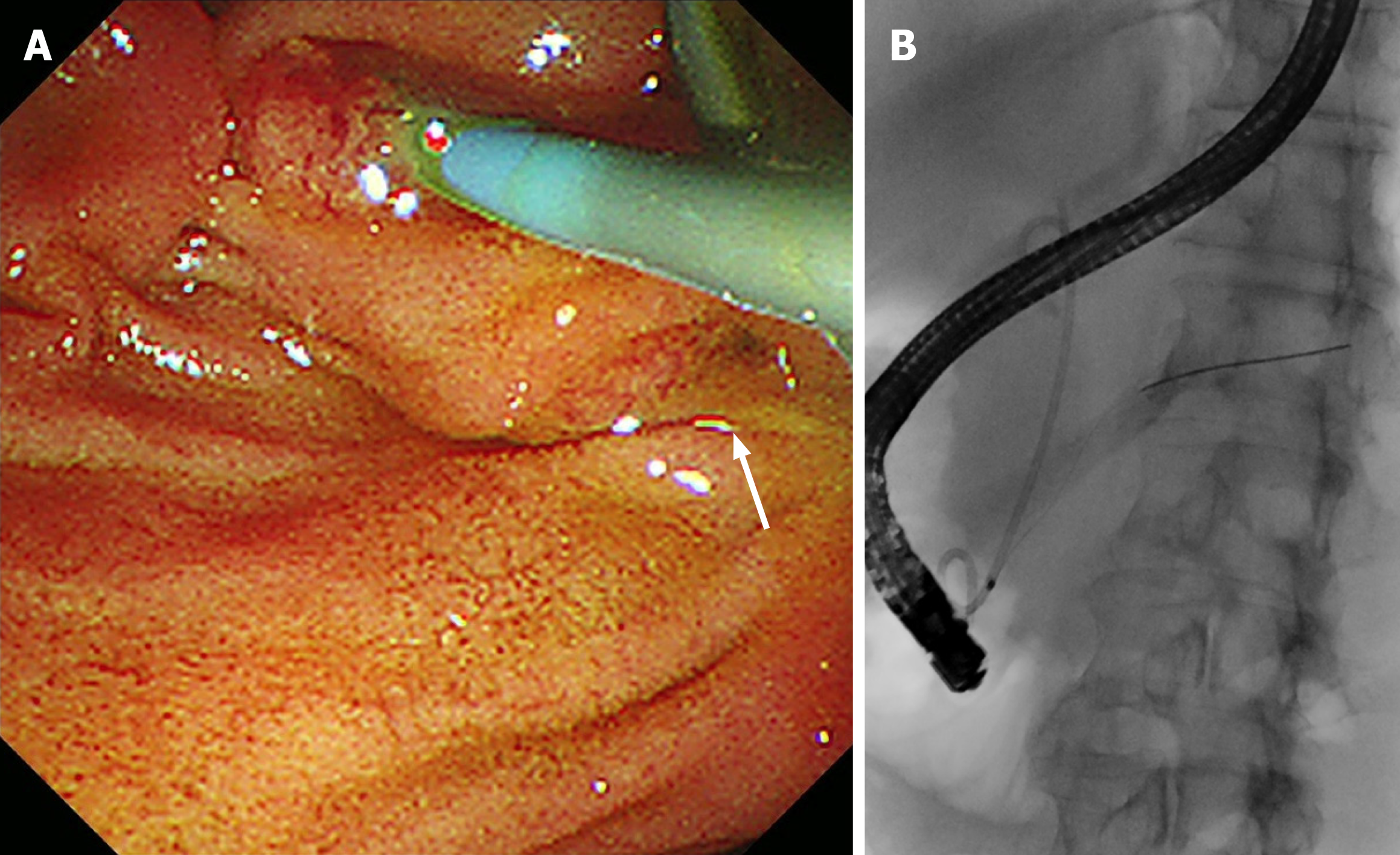
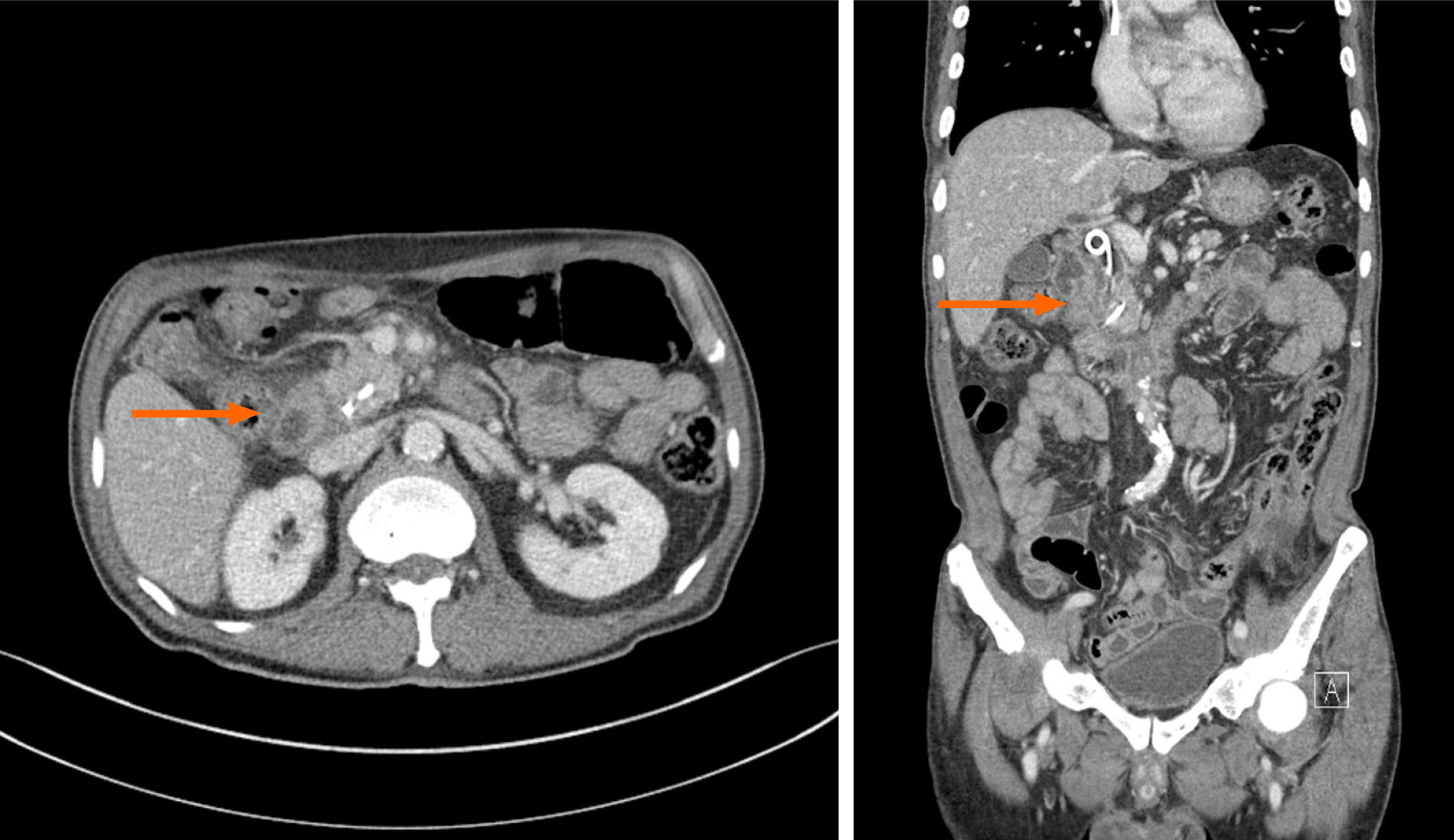
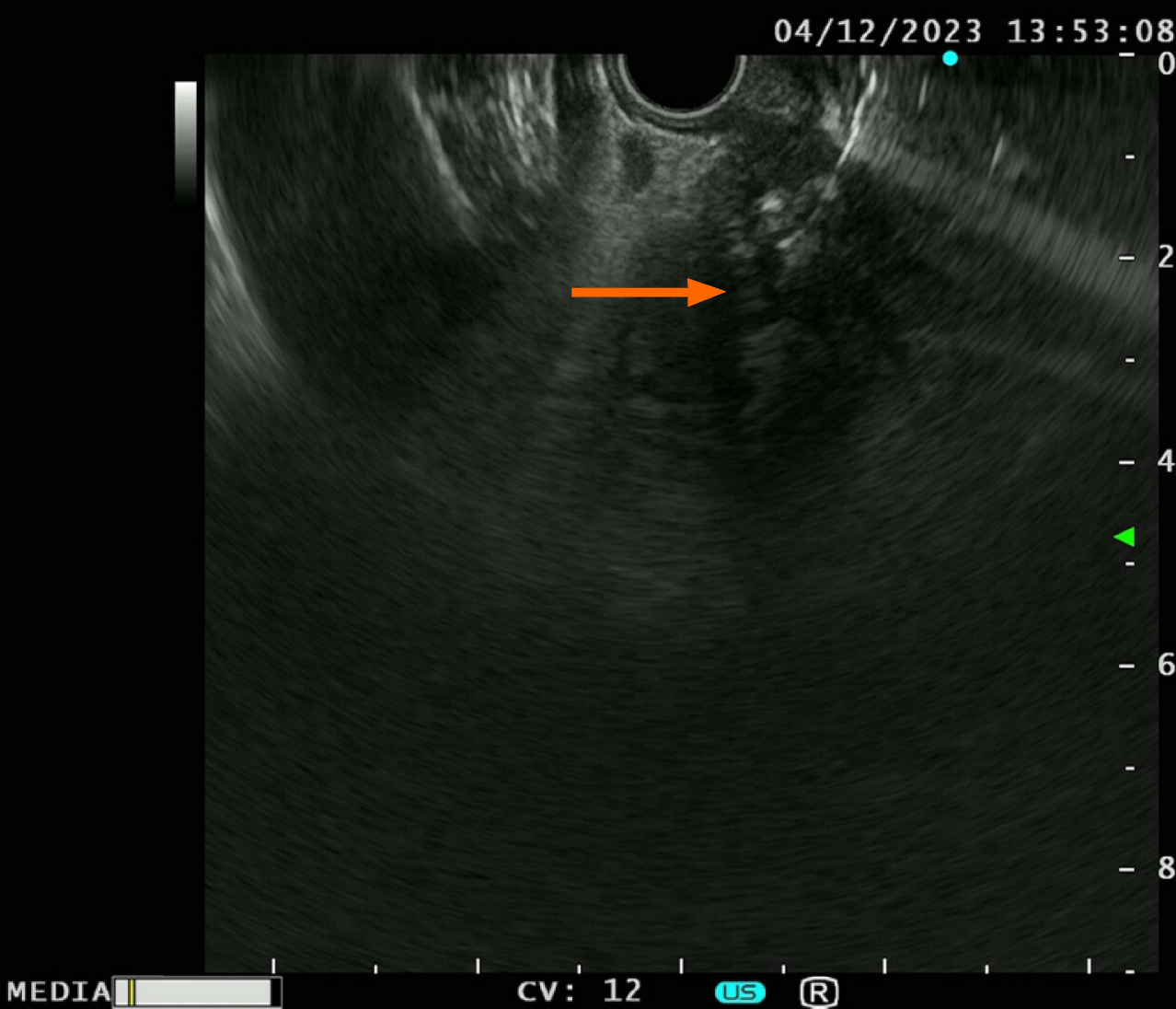
EUS-TS was performed for pathologic diagnosis, which demonstrated a 36.0 mm × 32.9 mm sized ill-defined, relatively heterogeneous, hypoechoic mass in the pancreatic head. EUS-TS was performed with a 22-gauge EZ shot (EZ shot 3 plus; Olympus, Tokyo, Japan) using the fanning method (Figure 3). Pathological examination confirmed a pancreatic ductal adenocarcinoma. The day after the procedure, the patient experienced severe abdominal pain in the right upper quadrant and epigastric areas. His follow-up amylase, lipase, and C-reactive protein levels were 265 U/L (reference range: 43–116 U/L), 1173 U/L (reference range: 7–60 U/L), and 27.7 mg/dL (reference range: 0–3 mg/dL), respectively. Computed tomography (CT) of the abdomen two days after EUS-TS revealed edematous wall thickening of the second portion of the duodenum with adjacent fluid collections and a suspicious leak from either the distal common bile duct or the main pancreatic duct in the head (Figure 4).
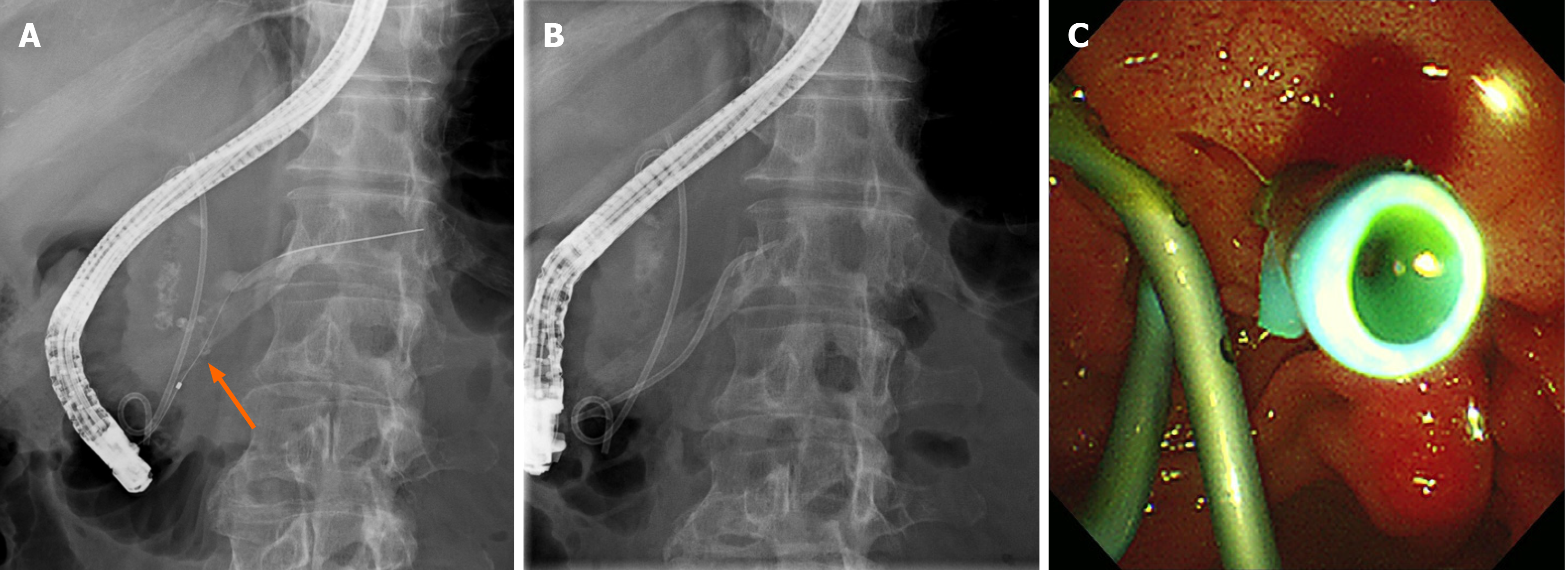
During the initial endoscopic retrograde cholangiopancreatography (ERCP), selective biliary cannulation could only be achieved through the ampulla of Vater. Although no definitive dye leak from the distal CBD was observed, a 7F 7 cm double pigtail plastic stent (ZSO-7-7; Cook Medical, Indiana, United States) was deployed over the stricture to allow for effective biliary drainage (Figure 5). Selective pancreatic cannulation was attempted several times without success. Despite biliary diversion, the patient continued to experience severe abdominal pain for four days. Follow-up abdominal CT showed no resolution of the suspected leak, suggesting a pancreatic ductal leak. During the second ERCP, a separate orifice was observed below the biliary orifice, which was not observed in the first ERCP. Selective pancreatic cannulation was easily achieved through the orifice (Figure 6). As the pancreatogram showed a dye leak in the head portion of the main pancreatic duct, a 5F 7 cm linear plastic stent was deployed to divert the pancreatic juice (Figure 7).
Immediately after insertion of the pancreatic stent, his abdominal pain markedly improved, and amylase and lipase levels normalized within a week. Two weeks after the pancreatic stent insertion procedure, follow-up abdominal CT imaging revealed the disappearance of adjacent fluid collection and edematous change of head of pancreas findings and healing of the duct leakage site (Figure 8). Based on these observations, neoadjuvant chemotherapy was initiated.
EUS-TS is a revolutionary technique that shifts the narrative surrounding the diagnosis of pancreatic lesions, such as cysts and solid lesions, and can target small pancreatic tumors. Before the advent of EUS-TS, ultrasonography-guided per
The patient in this case study was diagnosed with acute pancreatitis as he had suffered persistent severe pain over 24 h and had amylase and lipase levels more than three times the upper normal limit immediately after EUS-TS. Sequential abdominal CT imaging studies suggested a pancreatic ductal leak from the dilated main pancreatic duct, which a second ERCP confirmed. The patient recovered immediately after pancreatic stent placement to divert the leaking pancreatic juice from the intraperitoneal cavity to the duodenal lumen.
Acute pancreatitis (AP) is a rare EUS-TS complication. Recent research has demonstrated that a recent history of acute pancreatitis and undertaking EUS-TS through more than 5 mm of the normal parenchyma can lead to a much greater risk of acute pancreatitis[3]. However, a history of acute pancreatitis was not found in the current case, and the EUS-TS fine needle passed through less than 5 mm of the normal parenchyma. Another study reported that patients with a branch duct-type intraductal papillary mucin-secreting neoplasm are at risk of developing acute pancreatitis after EUS-TS[2]. However, unlike that study, the current case involved pancreatic ductal adenocarcinoma (PDAC).
In the present case, the fanning method was used to achieve a high diagnostic accuracy. The fanning method in EUS-TS involves sampling by cycles of in-and-out needle passes addressed in multiple directions within a solid lesion to obtain more target tissue[8]. While blood vessels were visualized and excluded by color Doppler during EUS-TS in the current case, pancreatic ductal structures without color Doppler might have a greater chance of being damaged by the fanning technique than the standard technique. A retrospective analysis of the EUS images in the current case revealed a sus
Although PDAC generally has a poor prognosis because it is frequently detected at an advanced stage, recent studies demonstrate that innovations in neoadjuvant chemotherapy have provided opportunities to proceed to curative surgery, thereby improving long-term outcomes in patients with borderline resectable and locally advanced PDAC[9]. Therefore, prompt treatment with neoadjuvant chemotherapy is critical for improving prognosis, while a delay in treatment could cause borderline resectable and locally advanced PDAC to become distant and metastatic. Acute pancreatitis initiates a hypercatabolic state caused by elevated protein catabolism and an inflammatory cytokine storm[10]. Recent guidelines dictate that patients with acute pancreatitis may benefit from early oral or enteral nutrition, which is in contrast to ma
In conclusion, pancreatic ductal leakage caused by EUS-TS can lead to acute pancreatitis. Therefore, imaging studies should be immediately performed after EUS-TS to rule out pancreatic ductal leaks in patients with clinical signs and symptoms of AP. If a pancreatic ductal leak is suspected, ERCP must confirm the leak. Pancreatic stent placement may allow patients to immediately recover from acute pancreatitis and shorten the waiting time for curative therapy. When using the fanning method during EUS-TS, ductal structures without color Doppler imaging should be excluded to pre
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nagaya M, Japan; Tang W, China S-Editor: Gong ZM L-Editor: A P-Editor: Xu ZH
| 1. | Reddymasu S, Oropeza-Vail MM, Williamson S, Jafri F, Olyaee M. Pancreatic leak after endoscopic ultrasound guided fine needle aspiration managed by transpapillary pancreatic duct stenting. JOP. 2011;12:489-490. [PubMed] |
| 2. | Siddiqui AA, Shahid H, Shah A, Khurana T, Huntington W, Ghumman SS, Loren DE, Kowalski TE, Laique S, Hayat U, Eloubeidi MA. High risk of acute pancreatitis after endoscopic ultrasound-guided fine needle aspiration of side branch intraductal papillary mucinous neoplasms. Endosc Ultrasound. 2015;4:109-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Ribeiro A, Goel A. The Risk Factors for Acute Pancreatitis after Endoscopic Ultrasound Guided Biopsy. Korean J Gastroenterol. 2018;72:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Larsen M, Kozarek R. Management of pancreatic ductal leaks and fistulae. J Gastroenterol Hepatol. 2014;29:1360-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Tian G, Ye Z, Zhao Q, Jiang T. Complication incidence of EUS-guided pancreas biopsy: A systematic review and meta-analysis of 11 thousand population from 78 cohort studies. Asian J Surg. 2020;43:1049-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Ikarashi S, Tsuchiya A, Hayashi K, Terai S. Delayed pancreatic ductal leakage after EUS-FNA for autoimmune pancreatitis. Endosc Ultrasound. 2019;8:277-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Versteijne E, van Dam JL, Suker M, Janssen QP, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Tienhoven G, van Eijck CHJ; Dutch Pancreatic Cancer Group. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol. 2022;40:1220-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 415] [Article Influence: 138.3] [Reference Citation Analysis (0)] |
| 10. | Rinninella E, Annetta MG, Serricchio ML, Dal Lago AA, Miggiano GA, Mele MC. Nutritional support in acute pancreatitis: from physiopathology to practice. An evidence-based approach. Eur Rev Med Pharmacol Sci. 2017;21:421-432. [PubMed] |