Published online Feb 16, 2024. doi: 10.12998/wjcc.v12.i5.973
Peer-review started: October 27, 2023
First decision: December 7, 2023
Revised: December 13, 2023
Accepted: January 18, 2024
Article in press: January 18, 2024
Published online: February 16, 2024
Processing time: 95 Days and 16.9 Hours
Venoarterial (VA) extracorporeal membrane oxygenation (ECMO), an effective short-term circulatory support method for refractory cardiogenic shock, is widely applied. However, retrospective analyses have shown that VA-ECMO-assisted cases were associated with a relatively high mortality rate of approximately 60%. Embolization in important organs caused by complications of left ventricular thrombosis (LVT) during VA-ECMO is also an important reason. Although the incidence of LVT during VA-ECMO is not high, the consequences of embolization are disastrous.
A 37-year-old female patient was admitted to hospital because of fever for 4 d and palpitations for 3 d. After excluding the diagnosis of coronary heart disease, we established a diagnosis of “clinically explosive myocarditis”. The patient still had unstable hemodynamics after drug treatment supported by VA-ECMO, with heparin for anticoagulation. On day 4 of ECMO support, a left ventricular thro
LVT with high mobility during VA-ECMO may cause embolism in important organs. Therefore, a "wait and see" strategy should be avoided.
Core Tip: Embolism in vital organs (brain, mesenteric artery, etc.) caused by detachment of a left ventricular thrombosis (LVT) can lead to catastrophic consequences. We report a case of explosive myocarditis in which a LVT was attached to the papillary muscle root of the mitral valve, which resulted in massive cerebral emboli. Although a "wait and see" strategy can be adopted considering the autolytic rate of LVT and the fatal complications associated with thrombolysis and surgical thrombectomy, more aggressive treatment methods should be adopted for left ventricular thrombi with high mobility, such as transcatheter left ventricular thrombolysis or surgical thrombectomy.
- Citation: Bai YB, Zhao F, Wu ZH, Shi GN, Jiang N. Left ventricular thrombosis caused cerebral embolism during venoarterial extracorporeal membrane oxygenation support: A case report. World J Clin Cases 2024; 12(5): 973-979
- URL: https://www.wjgnet.com/2307-8960/full/v12/i5/973.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i5.973
Venoarterial (VA) extracorporeal membrane oxygenation (ECMO) has been widely performed in short-term circulation support for refractory cardiogenic shock, due to its low cost and mature catheterization and management compared with other mechanical circulation assist devices[1]. In the past decades, the number of VA-ECMO applications both in China and abroad has markedly increased[2,3]. However, the clinical results of VA-ECMO application are disappointing, with the overall mortality rate in patients with refractory cardiogenic shock supported by VA-ECMO reported to be 60%[4]. Although the underlying diseases leading to cardiogenic shock are serious and are the main cause of failure, some complications during the application of VA-ECMO (e.g., fetal hemorrhage, thromboembolism of vital organs, severe hemolysis, infection, etc.) can also lead to failure. Embolism of vital organs has become one of the most frightening complications during VA-ECMO support. Thrombosis may occur in the circuit, oxygenator, pump and ventricle, with the incidence reported to range from 3% to 12%. Despite the low incidence of LVT, brain embolism caused by detachment of the thrombus can lead to catastrophic consequences[5]. It is reported that the mortality of cardioembolic stroke is higher compared with other ischemic stroke subtypes[6,7].
We report a case of explosive myocarditis in which a left ventricular thrombus attached to the papillary muscle root of the mitral valve resulted in massive cerebral emboli during VA-ECMO support.
The 37-year-old female patient was admitted to hospital mainly due to fever for 4 d and palpitations for 3 d.
The patient developed a fever 4 d before admission and continued to have intermittent fever after symptomatic treatment. Three days before admission, the patient had palpitations accompanied by chest tightness and fatigue and was admitted to the emergency department of our hospital.
The patient had a history of hyperthyroidism and was treated with iodine-131, and was currently treated with oral levothyroxine tablets for hypothyroidism.
The patient denied any family history of cardiac disease.
On physical examination, vital signs were as follows: Body temperature, 37.1oC; blood pressure, 90/71 mmHg; heart rate, 95 bpm and respiratory rate, 14 breaths/min. Cardiac auscultation revealed arrhythmia, decreased heart sound, and no heart murmur heard in auscultation areas.
Myocardium zymogram showed the following: Creatine kinase 466 U/L, creatine kinase isoenzyme 41 U/L, Troponin T 2.66 μg/L and N-terminal pro B-type natriuretic peptide 6599 pg/mL. Thyroid function tests demonstrated free triiodothyronine 2.13 pmol/L, free tetraiodothyronine 15.91 pmol/L and thyroid stimulating hormone 3.88 μIU/mL.
No abnormalities were found in routine blood and urine analyses.
Cardiac ultrasound in the emergency room revealed the following: Left atrium (LA) 32 mm, left ventricle (LV) 47 mm, right atrium 38 mm, right ventricle 16 mm, pulmonary arterial pressure 30 mmHg, LV ejection fraction (LVEF) 62%, and the contraction and diastolic function of the left heart were normal.
Re-examination with bedside ultrasound showed: LA 33 mm, LV 46 mm, LVEF 35-39%, left ventricular wall thickening, extensive myocardial motility reduction, and reduced left heart function.
Combined with the patient’s medical history and laboratory examinations, the final diagnosis was explosive myocarditis and arrhythmia.
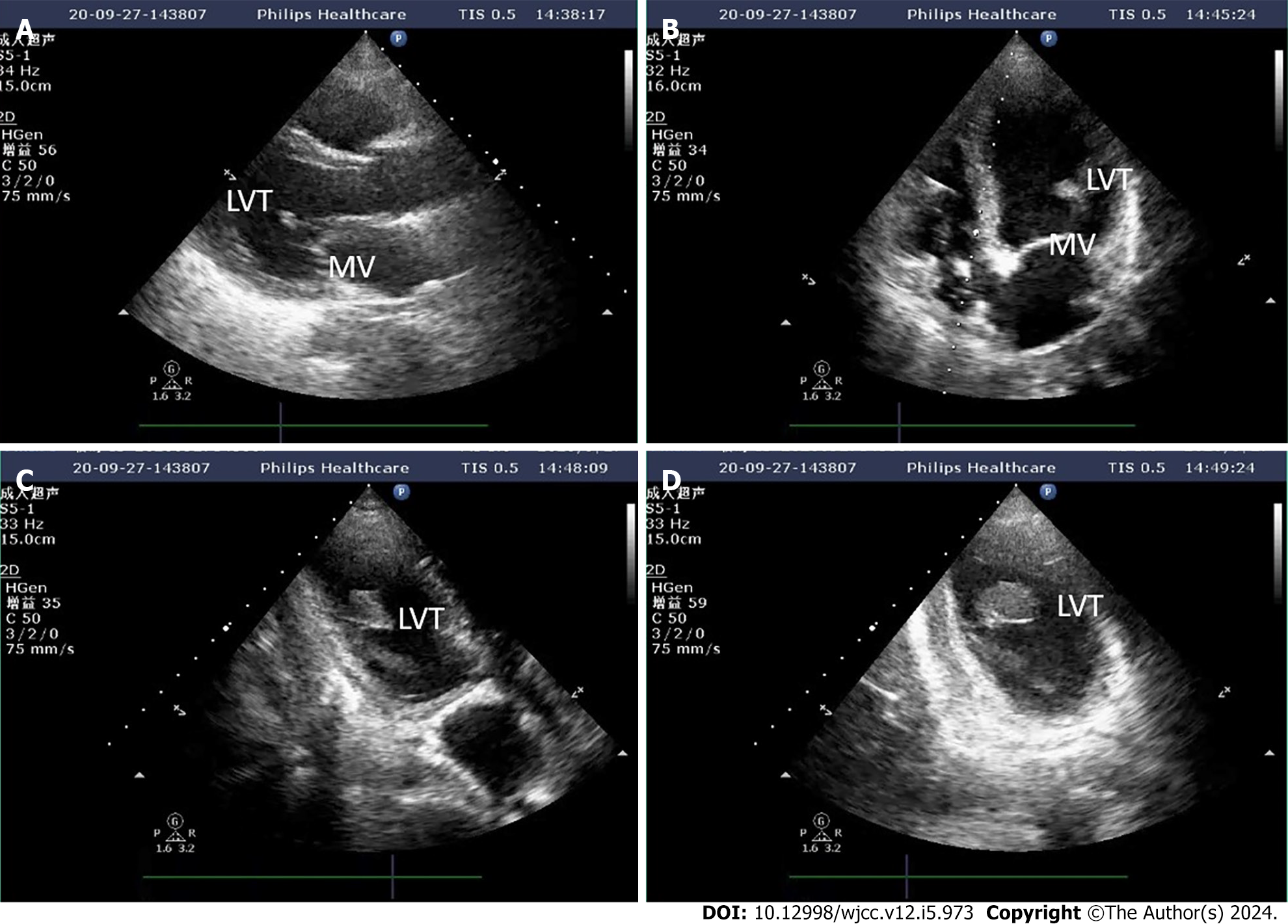
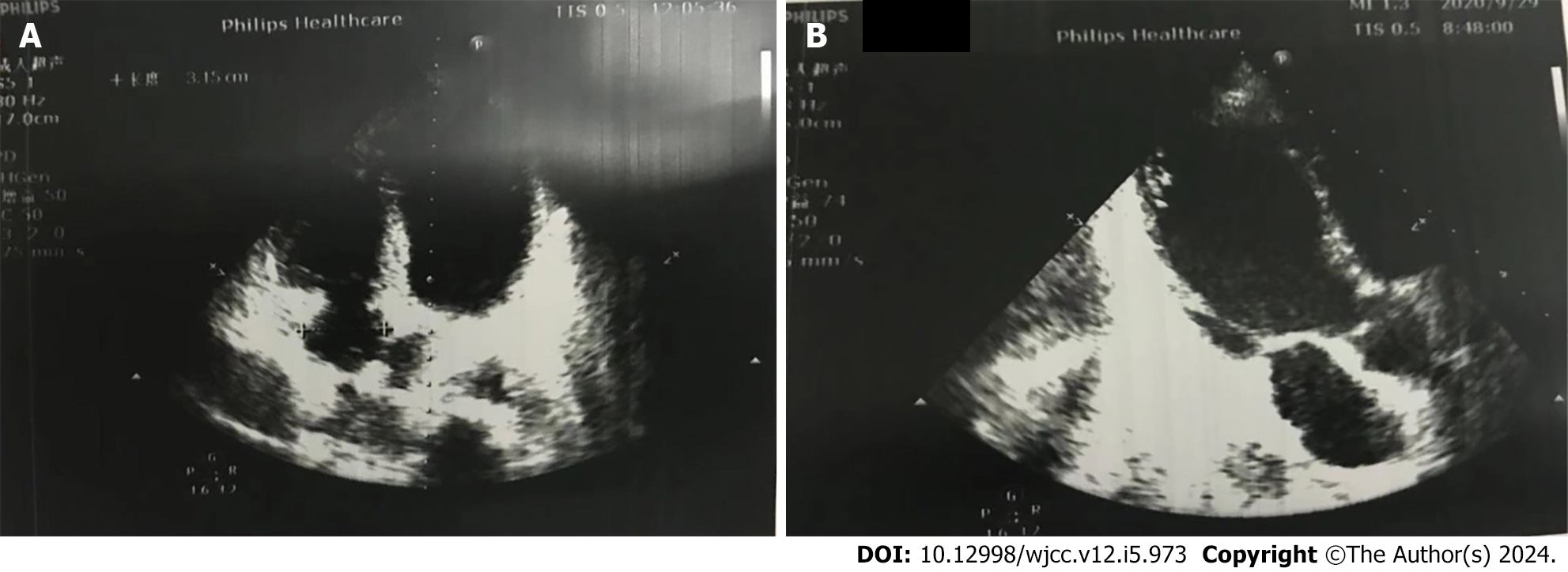
After admission, the patient was given myocardial nutrition, volume supplementation, dopamine cardiac strengthening, and norepinephrine vasopressor therapy. The patient's condition progressed rapidly, and 13 h after admission, she developed a third-degree atrioventricular (AV) block with a ventricular rate of approximately 60 bpm, blood pressure of 75/52 mmHg, and distal dampness. Rehydration fluids and high-dose vasoactive drugs continued to maintain circulation (vasoactive drug score: 30), and blood gas analysis showed metabolic acidosis combined with respiratory alkalosis. Blood lactic acid level was 2.5 mmol/L. With the assistance of an emergency endotracheal intubation ventilator, percutaneous ECMO implantation was performed at the bedside, using VA-mode, and a flow rate of 3.5 L/min. Heparin anticoagulation was administered during ECMO to maintain activated coagulation time 180-200 s; chest X-ray and echocardiography were monitored daily. Following implantation of ECMO, the patient had a heart rhythm of third-degree AV block, with a ventricular rate of about 50, occasional ventricular tachycardia and ventricular fibrillation. The cardiologist was contacted for temporary pacemaker support. Echocardiography results after 4 d of ECMO support showed a moderate intensity echoic mass of 2.4 cm 1.5 cm thought to be a left ventricular thrombus attached to the papillary muscle root of the mitral valve with a flow rate of 0.5 m/s (Figure 1); chest X-ray showed increased pulmonary edema. At this time, the patient was considered to have developed a hemodynamic change specific to peripheral VA-ECMO support of left ventricular dilation. Accordingly, the following therapeutic strategies were applied: the auxiliary flow was reduced to 3 L/min, maintaining the negative balance of the inflow and outflow, epinephrine was added to strengthen the heart, positive end-expiratory pressure was increased to improve right ventricular drainage, and Intra-Aortic Balloon Pump support was given to promote aortic valve opening. The patient's cardiac function gradually improved, and the pulmonary edema gradually subsided. On the 7th day of ECMO support, bedside ultrasound showed: LA 32 mm, LV 55 mm, LVEF 30%, the thrombus sound shadow in the heart was not obvious (Figure 2), and there was no abnormality in the patient's neurological examination at this time.
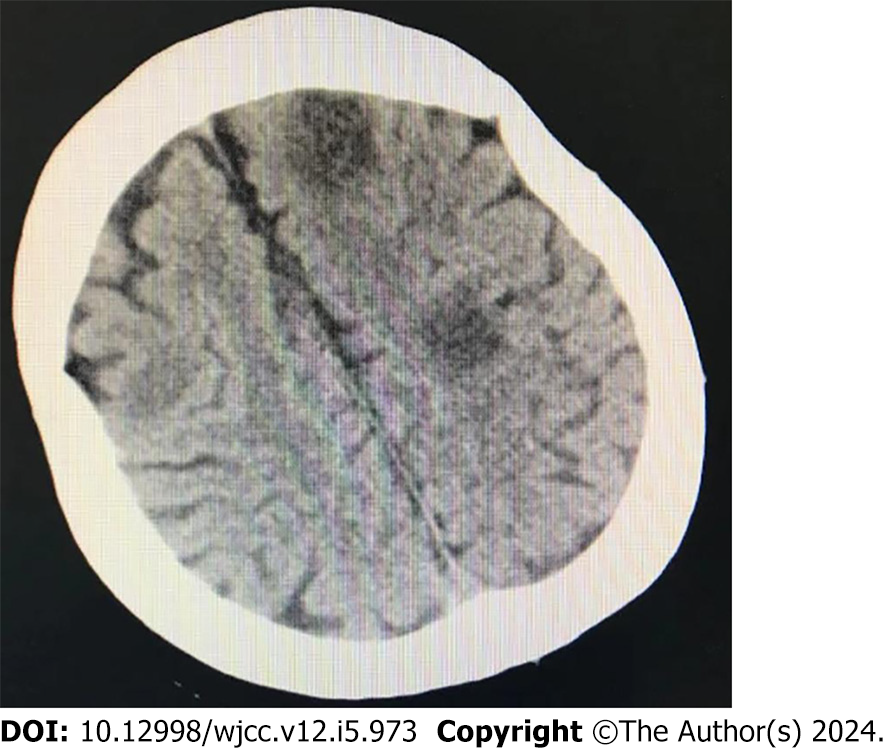
On the 9th day of ECMO support, the autonomic rhythm had recovered, cardiac function continued to improve, pulmonary edema was further reduced, and no abnormalities were found in the neurological examination; thus, ECMO support was removed. The patient then gradually recovered. Twelve days after admission, the patient suddenly lost consciousness, and computed tomography showed multiple cerebral emboli (Figure 3). The patient's family members gave up further treatment and the patient was discharged from hospital.
There are limited data on LVT in patients requiring VA-ECMO. One report showed a series of patients (n = 11) who developed LVT due to ischemic cardiomyopathy with cardiogenic shock, which accounted for 3.1% of the center’s total VA-ECMO experience[8]. LVT is a serious complication of VA-ECMO. Embolization of vital organs such as the brain, kidneys, and mesentery caused by thrombectomy can have fatal consequences, leading to the failure of ECMO support[9,10]. The pathophysiology of LVT formation during VA-ECMO support is complex and is the result of multiple factors. Severely impaired cardiac function, left ventricular dilation induced by VA-ECMO, and left ventricular blood stasis are the dominant factors associated with thrombosis. The hypercoagulable state of patients and the inadequacy of current anticoagulation therapy also play an important role in thrombosis.
Although transthoracic echocardiography (TTE) has been widely used in the diagnosis of LVT, it is greatly affected by the patient’s acoustic window (small intercostal space, large body size, chest deformities, or lung disease) and position[11]. In this case, the initial ultrasound images suggested that the thrombus was attached to the mitral valve, but after repeated multi-sectional examinations, the thrombus was eventually found to be attached to the root of the papillary muscle. A possible reason for this is that transthoracic ultrasound is a two-dimensional image and the patient was in the supine position, so judgement of the overall morphology of the thrombus was poor.
Currently, there are no guidelines or expert consensus recommendations for ECMO support for LVT treatment in patients[12,13]. Some therapeutic options reported include improving anticoagulant strength, surgical thrombectomy and thrombolytic therapy. Heparin: According to most reports[14,15], anticoagulation with heparin can reduce the incidence of LVT, but has no effect on thrombolysis. Surgery: Surgical resection of the LVT is an option when undergoing other open-heart surgeries or transitioning from peripheral VA-ECMO intubation to central intubation[16]. However, the risk-benefit ratio should also be considered, as most patients with LVT have a severely reduced LVEF, which has higher perioperative complications and mortality if patients undergo thrombectomy. Therefore, in the absence of other indications for emergency surgery, surgical thrombectomy should be carefully considered, as the risks for patients far outweigh the benefits[17]. Thrombolysis: Multiple studies have shown that fibrinolytic solvents can dissolve LVT, but the risk of this treatment is high (thrombosis can lead to embolism[18,19]). In one study, four patients with LVT were given intravenous fibrinolytic drugs, and after 8 to 12 h, the size of the thrombus was significantly reduced, and the thrombus disappeared completely in 2 of these patients; but the mobility of the thrombus also increased significantly. The remaining 2 patients in the study developed a severe systemic thromboembolic event. Simultaneous administration of thrombolytics increases the risk of bleeding[15].
In addition to thrombosis, bleeding at the puncture site or surgical site is also a common cause of death in VA-ECMO patients, and thrombolysis for VA-ECMO patients is a challenge[20]. Sangalli et al[21] reported a new approach for LVT, in which the patients’ LVT was completely dissolved 24 h after a catheterized injection of tenecteplase into the LV, and only moderate bleeding occurred. However, although a case report can provide us with new ideas for the treatment of LVT during VA-ECMO support, the therapeutic effect and related complications need to be studied in large-scale clinical trials.
It is reported that approximately 20%-40% of LVTs resolve spontaneously without anticoagulation with restoration of cardiac function[22,23]. Velangi et al[24] showed that the morphology, size and mobility of LVT can change, and there was no obvious correlation between the morphological characteristics and the occurrence of thromboembolism. Lemaître et al[17] believed that a "wait-and-see" strategy seems to be a safe and reasonable management plan for LVT in patients with heart failure. Therefore, we selected active conservative treatment measures: (1) To improve the strength of anticoagulation; and (2) to promote the development of aortic valves and improve blood stasis by giving positive inotropic drugs and reducing support flow. At the same time, we adopted a “wait-and-see” strategy and insisted on daily TTE monitoring.
In this case, the patient still had thromboembolism even though ultrasound suggested thrombolysis. This may be due to poor sensitivity of conventional ultrasound to LVT, and the thrombus was not found during routine examination. Therefore, such patients should be examined by magnetic resonance imaging (MRI) after ECMO removal[25] to exclude the existence of thrombosis, and regular anticoagulation should be given according to relevant guidelines if thrombosis is found during the examination[26].
This study had the following limitations: First, an MRI examination was not performed after the removal of ECMO support to confirm the complete disappearance of the LVT. Second, no laboratory tests for hematologic diseases was conducted to rule out stroke, as Arboix et al[27] reported that hematological disorders are an easily overlooked cause of acute stroke.
Future research should focus on the overall prognosis and treatment of patients with LVT during ECMO support, and develop relevant treatment guidelines or expert consensus to improve the outcome of ECMO support.
The occurrence of LVT during VA-ECMO is the result of multiple factors and has a high mortality rate. Management of LVT is a major challenge for clinicians. Although a "wait and see" strategy can be adopted considering the autolytic rate of LVT and the fatal complications associated with thrombolysis and surgical thrombectomy, more aggressive treatment methods for left ventricular thrombi with high mobility should be attempted, such as transcatheter left ventricular thrombolysis or surgical thrombectomy. In clinical practice, we should pay attention to the patient monitoring and management during operation, and actively prevent and treat left ventricular blood stasis. Continuous improvement of devices to improve biocompatibility and reduce the activation of coagulation and inflammatory reactions is required. Only when each element of ECMO is optimized can the prognosis of patients ultimately be improved.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arboix A, Spain S-Editor: Lin C L-Editor: A P-Editor: Yu HG
| 1. | Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ Heart Fail. 2018;11:e004905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 353] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 2. | Whitman GJ. Extracorporeal membrane oxygenation for the treatment of postcardiotomy shock. J Thorac Cardiovasc Surg. 2017;153:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Chinese Society of Extracorporeal Circulation. White book of Chinese cardiovascular surgery and extracorporeal circulation in 2019. Zhongguo Tiwai Xunhuan Zazhi. 2020;18:193-196. |
| 4. | Becher PM, Schrage B, Sinning CR, Schmack B, Fluschnik N, Schwarzl M, Waldeyer C, Lindner D, Seiffert M, Neumann JT, Bernhardt AM, Zeymer U, Thiele H, Reichenspurner H, Blankenberg S, Twerenbold R, Westermann D. Venoarterial Extracorporeal Membrane Oxygenation for Cardiopulmonary Support. Circulation. 2018;138:2298-2300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 5. | Williams B, Bernstein W. Review of Venoarterial Extracorporeal Membrane Oxygenation and Development of Intracardiac Thrombosis in Adult Cardiothoracic Patients. J Extra Corpor Technol. 2016;48:162-167. [PubMed] [DOI] [Full Text] |
| 6. | Kamel H, Healey JS. Cardioembolic Stroke. Circ Res. 2017;120:514-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 289] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 7. | Arboix A, Alió J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr Cardiol Rev. 2010;6:150-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 270] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 8. | Weber C, Deppe AC, Sabashnikov A, Slottosch I, Kuhn E, Eghbalzadeh K, Scherner M, Choi YH, Madershahian N, Wahlers T. Left ventricular thrombus formation in patients undergoing femoral veno-arterial extracorporeal membrane oxygenation. Perfusion. 2018;33:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Huerter M, Govostis D, Ellenby M, Smith-Singares E. Acute Bowel Ischemia Associated with Left Ventricular Thrombus and Arteriovenous Extracorporeal Membrane Oxygenation. J Extra Corpor Technol. 2018;50:58-60. [PubMed] [DOI] [Full Text] |
| 10. | Takei Y, Ejima Y, Toyama H, Takei K, Ota T, Yamauchi M. A case of a giant cell myocarditis that developed massive left ventricular thrombus during percutaneous cardiopulmonary support. JA Clin Rep. 2016;2:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Weinsaft JW, Kim HW, Shah DJ, Klem I, Crowley AL, Brosnan R, James OG, Patel MR, Heitner J, Parker M, Velazquez EJ, Steenbergen C, Judd RM, Kim RJ. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol. 2008;52:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Habash F, Vallurupalli S. Challenges in management of left ventricular thrombus. Ther Adv Cardiovasc Dis. 2017;11:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Cruz Rodriguez JB, Okajima K, Greenberg BH. Management of left ventricular thrombus: a narrative review. Ann Transl Med. 2021;9:520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Kontny F, Dale J, Abildgaard U, Pedersen TR. Randomized trial of low molecular weight heparin (dalteparin) in prevention of left ventricular thrombus formation and arterial embolism after acute anterior myocardial infarction: the Fragmin in Acute Myocardial Infarction (FRAMI) Study. J Am Coll Cardiol. 1997;30:962-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Delewi R, Zijlstra F, Piek JJ. Left ventricular thrombus formation after acute myocardial infarction. Heart. 2012;98:1743-1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 16. | Bolcal C, Kadan M, Kubat E, Erol G, Doğancı S. Surgical treatment of a left ventricular apical thrombus via robotic surgery. J Card Surg. 2019;34:216-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Lemaître AI, Picard F, Maurin V, Faure M, Dos Santos P, Girerd N. Clinical profile and midterm prognosis of left ventricular thrombus in heart failure. ESC Heart Fail. 2021;8:1333-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Garcia A, Gander JW, Gross ER, Reichstein A, Sheth SS, Stolar CJ, Middlesworth W. The use of recombinant tissue-type plasminogen activator in a newborn with an intracardiac thrombus developed during extracorporeal membrane oxygenation. J Pediatr Surg. 2011;46:2021-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | McCarthy CP, Murphy S, Venkateswaran RV, Singh A, Chang LL, Joice MG, Rivero JM, Vaduganathan M, Januzzi JL Jr, Bhatt DL. Left Ventricular Thrombus: Contemporary Etiologies, Treatment Strategies, and Outcomes. J Am Coll Cardiol. 2019;73:2007-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Murphy DA, Hockings LE, Andrews RK, Aubron C, Gardiner EE, Pellegrino VA, Davis AK. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev. 2015;29:90-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 287] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 21. | Sangalli F, Greco G, Galbiati L, Formica F, Calcinati S, Avalli L. Regional thrombolysis with tenecteplase during extracorporeal membrane oxygenation: a new approach for left ventricular thrombosis. J Card Surg. 2015;30:541-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Spirito P, Bellotti P, Chiarella F, Domenicucci S, Sementa A, Vecchio C. Prognostic significance and natural history of left ventricular thrombi in patients with acute anterior myocardial infarction: a two-dimensional echocardiographic study. Circulation. 1985;72:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Stratton JR, Nemanich JW, Johannessen KA, Resnick AD. Fate of left ventricular thrombi in patients with remote myocardial infarction or idiopathic cardiomyopathy. Circulation. 1988;78:1388-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 95] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Velangi PS, Choo C, Chen KA, Kazmirczak F, Nijjar PS, Farzaneh-Far A, Okasha O, Akçakaya M, Weinsaft JW, Shenoy C. Long-Term Embolic Outcomes After Detection of Left Ventricular Thrombus by Late Gadolinium Enhancement Cardiovascular Magnetic Resonance Imaging: A Matched Cohort Study. Circ Cardiovasc Imaging. 2019;12:e009723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Delewi R, Nijveldt R, Hirsch A, Marcu CB, Robbers L, Hassell ME, de Bruin RH, Vleugels J, van der Laan AM, Bouma BJ, Tio RA, Tijssen JG, van Rossum AC, Zijlstra F, Piek JJ. Left ventricular thrombus formation after acute myocardial infarction as assessed by cardiovascular magnetic resonance imaging. Eur J Radiol. 2012;81:3900-3904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Stevenson WG, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362-e425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 1134] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 27. | Arboix A, Jiménez C, Massons J, Parra O, Besses C. Hematological disorders: a commonly unrecognized cause of acute stroke. Expert Rev Hematol. 2016;9:891-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |