Published online Jan 26, 2024. doi: 10.12998/wjcc.v12.i3.623
Peer-review started: November 22, 2023
First decision: December 8, 2023
Revised: December 17, 2023
Accepted: January 4, 2024
Article in press: January 4, 2024
Published online: January 26, 2024
Processing time: 57 Days and 0.1 Hours
Pulmonary tuberculosis (PTB) is prevalent in immunocompromised populations, including patients with hematologic malignancies, human immunodeficiency virus infections, and chronic diseases. Effective treatment for acute promyelocytic leukemia (APL) combined with PTB is lacking. These patients show an extremely poor prognosis. Therefore, studies should establish efficient treatment options to improve patient survival and prognosis.
A 60-year-old male with pain in the right side of his chest and a fever for 4 d visited the outpatient department of our hospital. Peripheral blood smear revealed 54% blasts. Following bone marrow examinations, variant APL with TNRC18-RARA fusion gene was diagnosed. Chest computed tomography scan showed bilateral pneumonitis with bilateral pleural effusions, partial atelectasis in the lower lobes of both lungs, and the bronchoalveolar lavage fluid gene X-Pert test was positive, indicative of PTB. Carrimycin, ethambutol (EMB), and isoniazid (INH) were administered since he could not receive chemotherapy as the WBC count decreased continuously. After one week of treatment with carrimycin, the patient recovered from fever and received chemotherapy. Chemotherapy was very effective and his white blood cells counts got back to normal. After being given five months with rifampin, EMB and INH and chemotherapy, the patient showed complete remission from pneumonia and APL.
We report a case of PTB treated successfully with carrimycin with APL that requires chemotherapy.
Core Tip: Here, we report a case of a patient with acute promyelocytic leukemia (APL) combined with pulmonary tuberculosis (PTB). Addition of carrimycin in a patient with tuberculosis in remission thus enabling chemotherapy for leukemia. He could not tolerate the side effects of routine anti- tuberculosis drugs because of a low white blood cell count, so carrimycin was used for treatment for PTB. He achieved complete remission of APL and PTB after six months treatment. Further research is necessary to determine whether carrimycin is therapeutically useful in PTB.
- Citation: Yang FY, Shao L, Su J, Zhang ZM. Carrimycin in the treatment of acute promyelocytic leukemia combined with pulmonary tuberculosis: A case report. World J Clin Cases 2024; 12(3): 623-629
- URL: https://www.wjgnet.com/2307-8960/full/v12/i3/623.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i3.623
A subtype of acute myeloid leukemia (AML) is acute promyelocytic leukemia (APL). A heterogeneous disease of hematological malignancies known as AML is characterized by aberrant clonal proliferation, poor differentiation, and inhibition of myeloid hematopoietic stem cell apoptosis[1]. Patients with AML have qualitative and quantitative deficits in gra
A 60-year-old male presented with pain in his right chest and a fever for the last 4 d.
The patient presented with dull pain in the right chest aggravated by deep breathing and activity. His highest recorded temperature was 38.6℃, accompanied by generalized fatigue, muscle and joint pain, and occasional coughing with a small amount of white sputum. The patient was treated at home with self-administered antipyretic drugs after disease onset, with poor results. Before admission, the patient visited a local hospital where examinations revealed pancytopenia, pneumonia, and pleural effusion.
The patient had a history of hypertension for more than 10 years, which was well controlled with valsartan and amlodipine.
The patient had a history of smoking for more than 30 years (60 cigarettes per day) and drinking for more than 20 years (200 g of liquor per day). The patient’s father had died of esophageal cancer.
Physical symptoms were as follows: temperature, 36.8 °C; blood pressure, 132/75 mmHg; heart rate, 78 beats per min, and respiratory rate, 19 breaths per min. Physical examination revealed no palpable enlargement of the superficial lymph nodes throughout the body, no moist rales in either lungs, or hepatosplenomegaly.
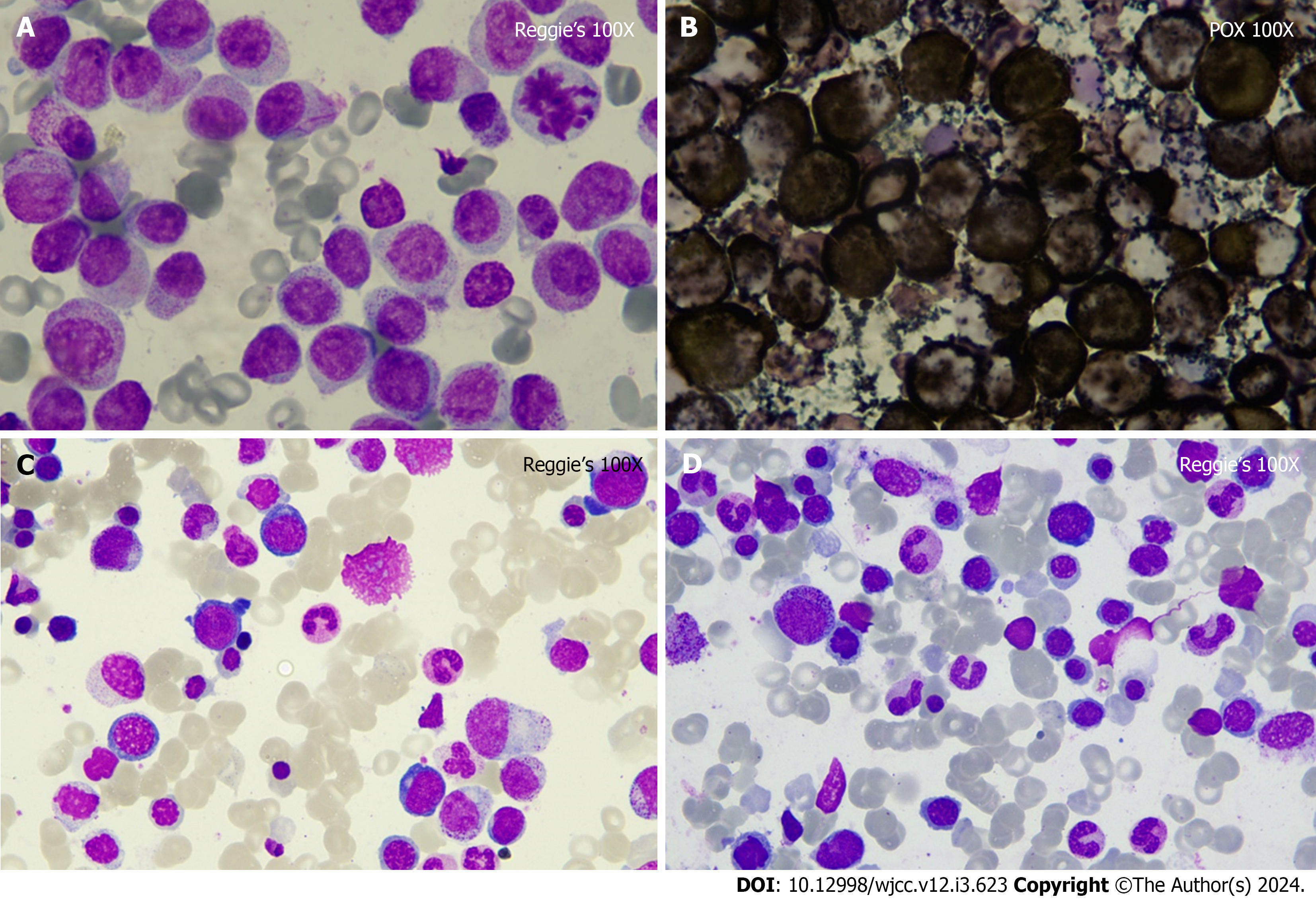
Pancytopenia (absolute monocyte count: 3.55 × 109/L; percentage monocyte: 53.30%; absolute neutrophil count: 1.85 × 109/L; hemoglobin: 76 g/L; platelet: 101 × 109/L) was identified during the initial examination. Peripheral blood smear revealed 54% blasts. C-reactive protein (190.39 mg/L), and D-dimer (29.64 mg/L) levels were increased. Bone marrow smear suggested a high probability of APL. Reggie’s stain showed promyelocyte accounted for about 55% and Peroxidate stain was positivity (Figure 1A and B). A bone marrow biopsy was subsequently performed which revealed a small number of cells with moderately enlarged cytosomes, deviated nuclei, fine chromatin, and inconspicuous nucleoli, requiring clinical and other tests to exclude AML. Flow cytometry of the specimens was consistent with the immu
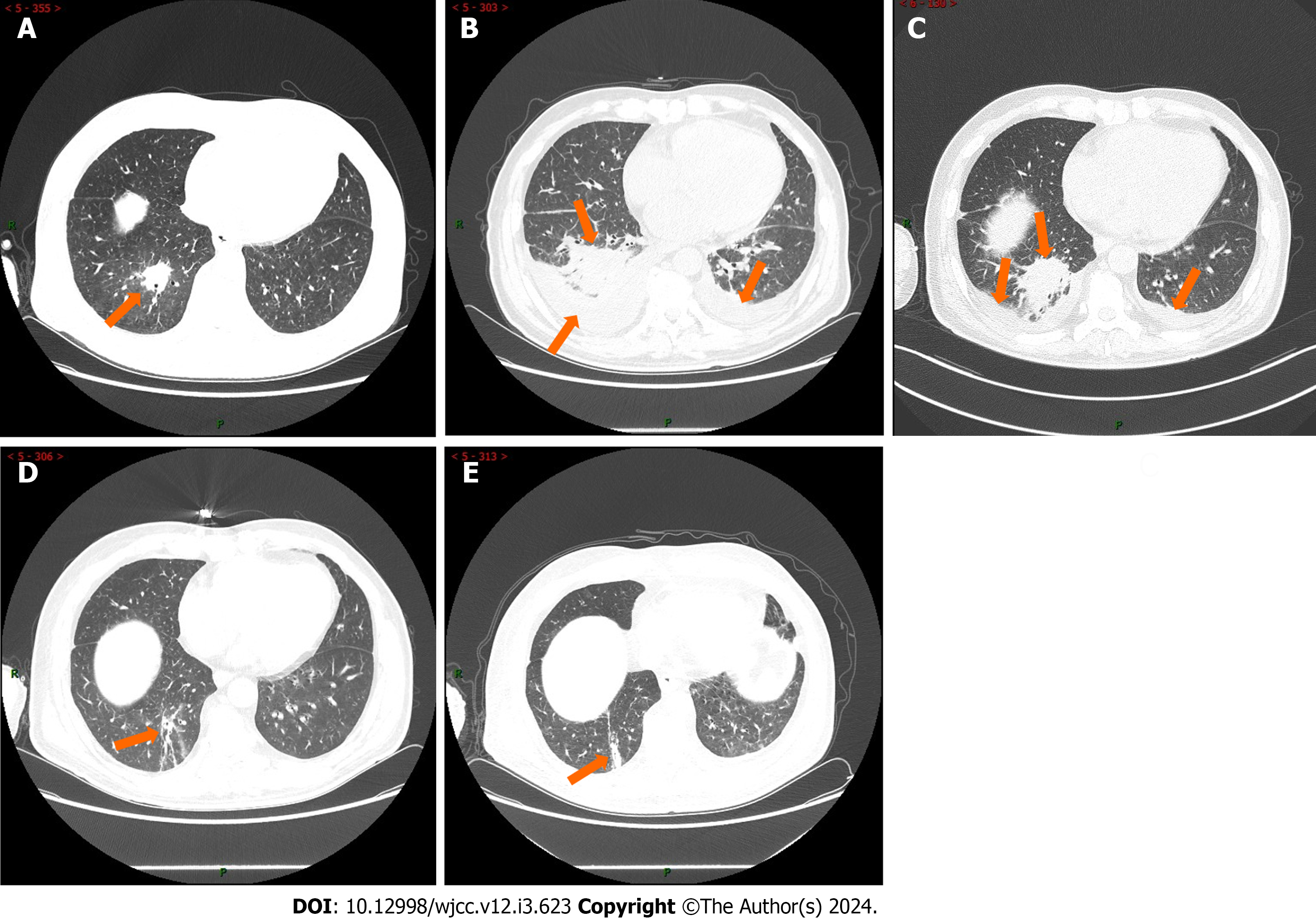
Chest computed tomography scans revealed bilateral pneumonitis with pleural effusion, partial atelectasis in the lower lobes of both lungs (Figure 2A). The patient's lung inflammation gradually deteriorated (Figure 2B). Multiple bronchoscopies were performed, and the BALF gene X-Pert test was positive.
The patient was finally diagnosed with APL combined with PTB.
Initially, the patient received treatment with ceftriaxone sodium, piperacillin-tazobactam, cefoperazone sodium, sulbactam sodium, meropenem, voriconazole, posaconazole, linezolid (LZD), caspofungin, and amphotericin B cholesteryl sulfate complex, which was ineffective, and he continued to experience fever recurrently (Figure 2A-C). During this period, the patient received three courses of chemotherapy for APL. After PTB diagnosis, he was ad
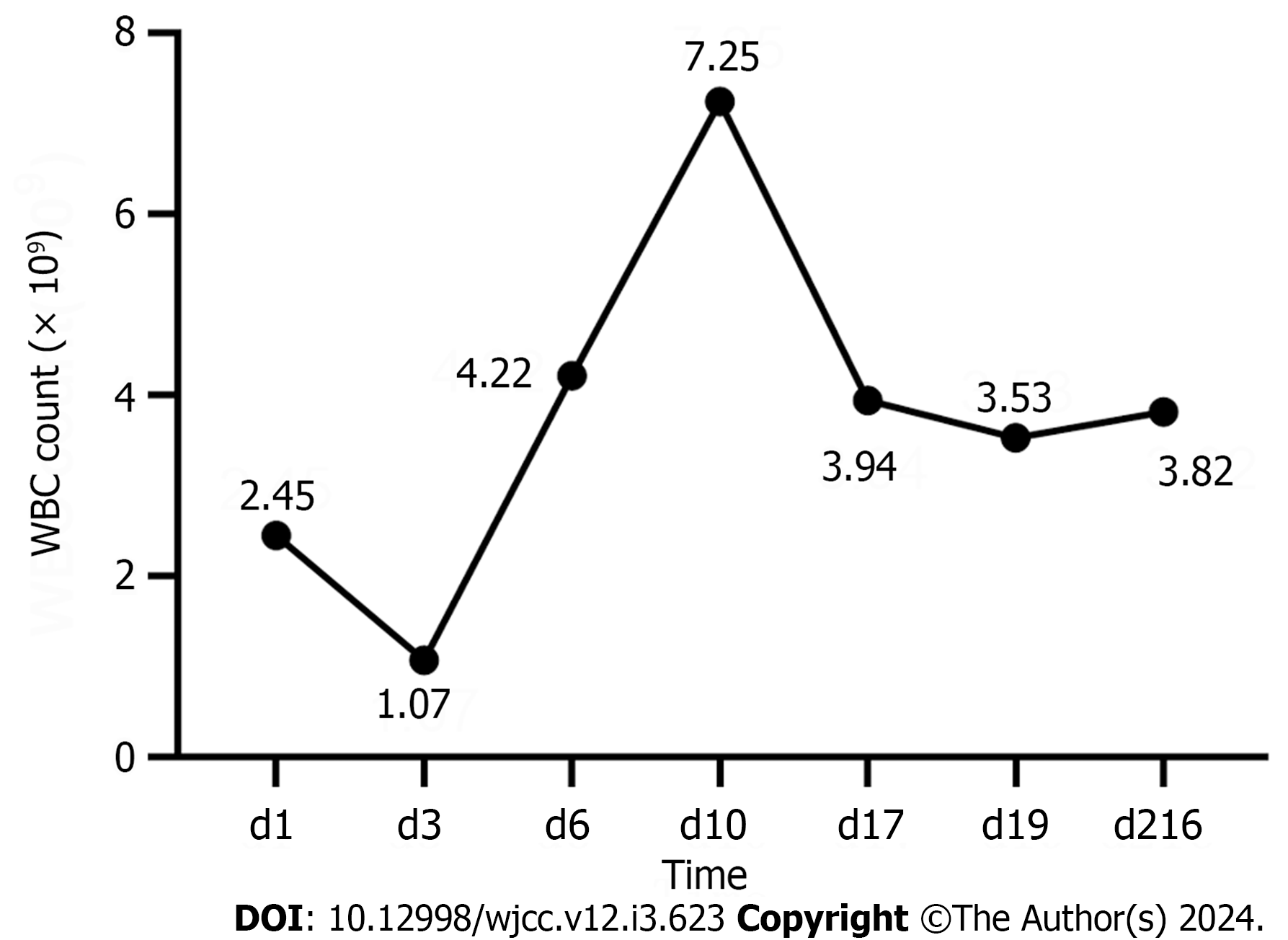
Carrimycin (0.4 g, qd) was administered for 24 d, and the lung infection improved dramatically. Chemotherapy for leukemia can be administered normally. The patient achieved complete remission after six months of treatment.
APL is a highly heterogeneous disease caused by the aberrant differentiation of myeloid lineage cells in the hematopoietic system[4]. Patients with APL have reduced autoimmune function and are susceptible to co-infections. The risk of developing tuberculosis is higher in patients with hematological malignancies. A direct result of immunosuppression brought on by the malignancy, a side effect of treatment, or a combination of both can increase susceptibility to PTB[5]. Herein, we report a case of APL combined with PTB. X-Pert is a rapid nucleic acid amplification test used to tuberculosis[6]. The patient’s BALF gene X-Pert test result was positive, confirming the diagnosis of tuberculosis. After a definitive diagnosis, the patient received both anti-tuberculosis and leukemia chemotherapy. However, anti-tuberculosis chemotherapy resulted in a persistent decrease in WBC count and was subsequently discontinued, causing recurrent fever, which made it impossible to properly administer leukemia chemotherapy. The patient was treated with carrimycin, INH, and EMB to control the lung infections so that leukemia chemotherapy could be administered. Surprisingly, the patient had no fever on the day carrimycin was added. The patient's WBC count normalized after five days of treatment, and the fourth cycle of leukemia chemotherapy with venetoclax combined with azacytidine was subsequently initiated. The patient achieved complete remission after six months of treatment.
APL and PTB have seldom been documented. Abdullah et al[7] described a case of APL combined with tuberculosis in a patient receiving the PETHEMA protocol consisting of ATRA plus Idarubicin and anti-TB treatment. Due to the interaction of several anti-tuberculosis drugs with chemotherapy, treating tuberculosis in patients with acute leukemia can be challenging. Susan Realegeno et al[8] reported a case of a patient with APL developing disseminated tuberculosis while undergoing APL therapy. The patient was treated with rifampin (RIF), pyrazinamide (PZA), moxifloxacin (MFX), and EMB, INH, levofloxacin, amikacin and LZD as anti-tuberculosis therapy. Conventional chemotherapy regimens for leukemia are myelosuppressive, making them ineffective for active PTB control[5]. Recurrent fever due to uncontrolled tuberculosis can limit the use of chemotherapeutic regimens for leukemia. RIF, INH, PZA, and EMB are well-known first-line medications. The selection of anti-tuberculosis drugs is limited owing to factors such as multidrug resistance and adverse effects of the drugs[9]. In our patient, carrimycin was added because of a persistent decrease in WBC count after the application of conventional anti-tuberculosis chemotherapy, which showed promising results for anti-tuberculosis treatment. Azacitidine has the potential to cause reactivation of tuberculosis[5].
Carrimycin is a new national class I drug that has been approved by the CFDA for treating acute tracheal bronchitis caused by Haemophilus influenzae and Streptococcus pneumoniae and acute sinusitis caused by Streptococcus pneumoniae, Haemophilus influenzae, Streptococcus pyogenes, Moraxella catarrhalis, and Staphylococcus[10]. Carrimycin exerts an inhibitory effect on inflammation[11]. Carrimycin considerably decreases interleukin (IL)-4 levels in the lungs and kidneys, especially in the liver and spleen. Furthermore, carrimycin significantly decreases IL-1β levels in the spleen, liver, and kidneys, especially in the small intestine and the lungs, showing an excellent anti-inflammatory effect[11]. Second, carrimycin is a novel macrolide antibiotic with excellent antibacterial effects that can inhibit bacterial protein synthesis by blocking the activity of peptidyl transferase in the 50s ribosome subunit to develop antibacterial effects[11]. Carrimycin has significant efficacy against multidrug-resistant bacteria that do not respond to conventional anti-infective treatment[12]. Carrimycin is a dual-acting immunoantibiotic with antibacterial and immunomodulatory properties. In terms of intrinsic immunity, carrimycin promotes neutrophil migration to the sites of inflammation and enhances phagocytosis by macrophages. In terms of adaptive immunity, carrimycin significantly increased the total T-cell population (CD3-positive cells), along with an increase in both CD4- and CD8-positive cells. Overall, carrimycin can kill pathogens directly while enhancing the host’s immune response and indirectly killing bacteria. The normal WBC count was achieved in the patient after the application of carrimycin, and we speculate that carrimycin has a WBC count-increasing effect, which enhances host immunity by flipping the host's immune switch, thus eradicating pathogens. Thus, carrimycin is a novel therapeutic option for patients with APL and TB. Carrimycin also shows broad-spectrum antiviral efficacy against human coronaviruses (HCoVs) by targeting the post-entry replication event and inhibiting HCoV RNA synthesis[10]. Moreover, it has potential antitumor activity through the inhibition of protein synthesis to regulate cell physiology, proliferation, and immunity[13]. Cui et al[14] found that the main component of carrimycin, isovalerylspiramycin I, suppresses tumorigenesis and metastasis. It is effective in the treatment of non-small cell lung cancer, head and neck tumors, and hepatocellular carcinoma[15]. Therefore, we speculated that carrimycin also exerts an inhibitory effect on hematological tumors.
Herein, we report a case of APL combined with PTB. Given that the patient's WBC count continued to decrease, we added carrimycin to the PTB treatment and achieved excellent therapeutic outcomes. This study provides a reference for the treatment of patients with APL and TB. The therapeutic efficacy of carrimycin in APL accompanied by PTB requires further investigation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Jong D, Netherlands S-Editor: Zhang H L-Editor: A P-Editor: Cai YX
| 1. | Ji L, Yang W, Xu XF, Xu YQ. A case report of complete remission of acute myeloid leukemia combined with DNMT3A, FLT3-TKD, and IDH2 gene mutations and active pulmonary tuberculosis treated with homeharringtonine + venetoclax + azacytidine. Front Med (Lausanne). 2023;10:1180757. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Logan C, Koura D, Taplitz R. Updates in infection risk and management in acute leukemia. Hematology Am Soc Hematol Educ Program. 2020;2020:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Parker VR, Bennet JA, Sanne IM. An incidental finding of chronic lymphocytic leukaemia in a patient with pulmonary tuberculosis. S Afr J Infect Dis. 2020;35:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 4. | Cao Z, Shu Y, Wang J, Wang C, Feng T, Yang L, Shao J, Zou L. Super enhancers: Pathogenic roles and potential therapeutic targets for acute myeloid leukemia (AML). Genes Dis. 2022;9:1466-1477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Pescatore J, Cohen A, Moturi K, Hernandez-Acosta R. Reactivation of Pulmonary Tuberculosis During Treatment of Chronic Myelomonocytic Leukemia. Cureus. 2021;13:e15491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Pillay S, Steingart KR, Davies GR, Chaplin M, De Vos M, Schumacher SG, Warren R, Theron G. Xpert MTB/XDR for detection of pulmonary tuberculosis and resistance to isoniazid, fluoroquinolones, ethionamide, and amikacin. Cochrane Database Syst Rev. 2022;5:CD014841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Abdullah AS, Adel AM, Hussein RM, Abdullah MA, Yousaf A, Mudawi D, Mohamed SF, Nashwan AJ, Soliman D, Ibrahim F, Yassin MA. Hypercalcemia and acute pancreatitis in a male patient with acute promyelocytic leukemia and pulmonary tuberculosis. Acta Biomed. 2018;89:23-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Realegeno S, Adeyiga O, Winston DJ, Beaird OE, Garner OB, Yang S. Utilization of whole genome sequencing for resolution of discrepant Mycobacterium tuberculosis drug susceptibility results: A case report. IDCases. 2021;26:e01308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Lan Z, Ahmad N, Baghaei P, Barkane L, Benedetti A, Brode SK, Brust JCM, Campbell JR, Chang VWL, Falzon D, Guglielmetti L, Isaakidis P, Kempker RR, Kipiani M, Kuksa L, Lange C, Laniado-Laborín R, Nahid P, Rodrigues D, Singla R, Udwadia ZF, Menzies D; Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment 2017. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med. 2020;8:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 10. | Yan H, Sun J, Wang K, Wang H, Wu S, Bao L, He W, Wang D, Zhu A, Zhang T, Gao R, Dong B, Li J, Yang L, Zhong M, Lv Q, Qin F, Zhuang Z, Huang X, Yang X, Li Y, Che Y, Jiang J. Repurposing carrimycin as an antiviral agent against human coronaviruses, including the currently pandemic SARS-CoV-2. Acta Pharm Sin B. 2021;11:2850-2858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Li XY, Luo YT, Wang YH, Yang ZX, Shang YZ, Guan QX. Anti-inflammatory effect and antihepatoma mechanism of carrimycin. World J Gastroenterol. 2023;29:2134-2152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Reference Citation Analysis (3)] |
| 12. | Zhu W, Tian Y, Xiang L, Cao L, He L. A Case of Multidrug-Resistant Klebsiella pneumoniae Treated with Carrimycin. Infect Drug Resist. 2023;16:2365-2369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Liang SY, Zhao TC, Zhou ZH, Ju WT, Liu Y, Tan YR, Zhu DW, Zhang ZY, Zhong LP. Anti-tumor effect of carrimycin on oral squamous cell carcinoma cells in vitro and in vivo. Transl Oncol. 2021;14:101074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Cui J, Zhou J, He W, Ye J, Westlake T, Medina R, Wang H, Thakur BL, Liu J, Xia M, He Z, Indig FE, Li A, Li Y, Weil RJ, Aladjem MI, Zhong L, Gilbert MR, Zhuang Z. Targeting selenoprotein H in the nucleolus suppresses tumors and metastases by Isovalerylspiramycin I. J Exp Clin Cancer Res. 2022;41:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 15. | Liu Z, Huang M, Hong Y, Wang S, Xu Y, Zhong C, Zhang J, Zhuang Z, Shan S, Ren T. Isovalerylspiramycin I suppresses non-small cell lung carcinoma growth through ROS-mediated inhibition of PI3K/AKT signaling pathway. Int J Biol Sci. 2022;18:3714-3730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |