Published online Sep 6, 2024. doi: 10.12998/wjcc.v12.i25.5791
Revised: May 8, 2024
Accepted: June 24, 2024
Published online: September 6, 2024
Processing time: 99 Days and 15.3 Hours
Solitary fibrous tumor (SFT) is a remarkably uncommon mesenchymal tumor. STAT6 level and a combination of clinical, pathological, and molecular features are required to arrive at a proper diagnosis.
In this report, we present an intriguing case involving a 43-year-old woman who initially exhibited symptoms of a bleeding retroperitoneal tumor, initially resembling a gastrointestinal stromal tumor, but later confirmed as an SFT. However, a year later, what was initially believed to be a recurrence of her SFT was instead identified as a desmoid tumor.
Distinguishing SFT from other tumors was pivotal. Correcting misdiagnoses of tumor type initially and of recurrence later was necessary for appropriate treatment of the correct desmoid type.
Core Tip: A 43-year-old woman initially exhibited symptoms of a bleeding retroperitoneal tumor, resembling a gastrointestinal stromal tumor, but was later confirmed as a solitary fibrous tumor (SFT). However, a year later, what was initially believed to be a recurrence of her SFT was instead identified as a desmoid tumor. Asymptomatic and slow-growing, SFTs are frequently incidentally discovered during imaging. Accurate diagnosis plays an essential role in the effective treatment and management of SFT. The key initial step in this process is differentiating SFT from its sister tumors. Biological and antiangiogenic therapies hold potential as adjuncts to surgery in treating SFT.
- Citation: Maalouf H, Aby Hadeer R, Ghattas S, Tabbikha O, Numan H, Wakim R. Solitary fibrous tumor: A case report of this multifaceted tumor. World J Clin Cases 2024; 12(25): 5791-5797
- URL: https://www.wjgnet.com/2307-8960/full/v12/i25/5791.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i25.5791
With over 150 histological subtypes of soft tissue sarcomas (STS) and mesenchymal tumors, solitary fibrous tumors (SFT) are an exceptionally rare mesenchymal tumor, comprising 3.7% of cases[1]. Initially described by Klemperer and Rabin in 1931, they delineated the morphological characteristics of SFT in a series of five cases of pleural neoplasms[2]. SFT was exclusively observed in the pleura or lungs until 1990[2]. Accurately classifying related cancers is crucial for proper diagnosis, prognosis, and patient management[1]. With a rarely metastasizing aptitude, WHO recently classified SFT as a fibroblastic neoplasm with intermediate behavior[3].
SFT is referred to as the "great stimulator" among soft-tissue neoplasms because it presents with numerous potential differential diagnoses[4]. Therefore, a combination of clinical, pathological, immunohistochemical, and molecular features are required to have an appropriate diagnosis[1]. A particular cytogenetic hallmark for SFT has emerged to help in this process, identified by the NAB2 (NGFI-A-binding protein 2)–STAT6 (signal transduction and activator of transcription 6) fusion oncogene[1,2].
In this instance, we present a compelling example of a 43-year-old woman who originally demonstrated a bleeding retroperitoneal tumor mimicking a gastrointestinal stromal tumor (GIST), which was subsequently diagnosed as SFT. However, a year later, what was initially suspected to be a recurrence of her SFT turned out to be another benign soft tissue tumor.
A 43-year-old female known to have B12 deficiency anemia following Roux-en-Y gastric bypass; presented with diffuse vague abdominal pain of 1-month duration.
The patient had no associated symptoms of fever, chills, nausea, vomiting, change in bowel habits, weight loss, or night sweats.
The patient had no previous relevant medical history.
The patient’s personal history included B12 deficiency anemia following Roux-en-Y gastric bypass. Her family history was negative for any tumors or other illnesses.
Physical exam showed a soft non-distended abdomen with mild epigastric tenderness. Laboratory tests were unremarkable.
None appropriate for this case.
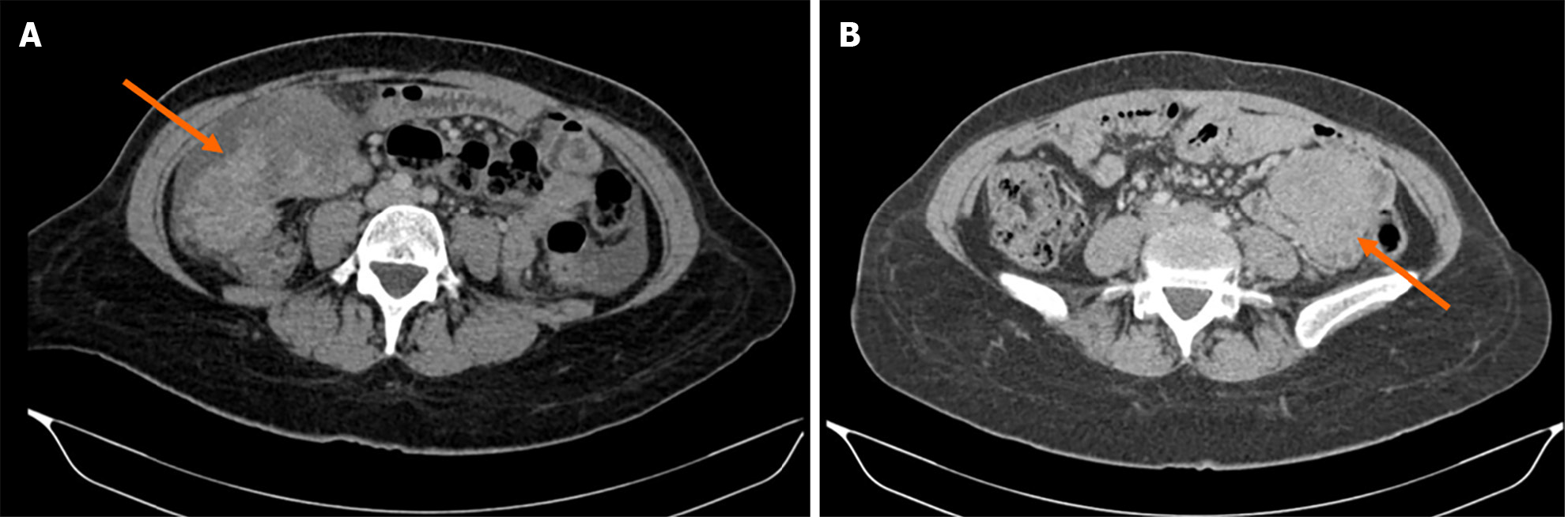
Abdomen and pelvis computed tomography (CT) showed a lobulated lesion over the right mid abdomen, in close contact with the ascending colon, measuring 10 cm × 8 cm × 6.5 cm (Figure 1A). It shows soft tissue attenuation density with heterogeneous enhancement. A small adjacent 7 mm lymph node is seen. A small amount of free fluid was found in the abdomen, predominantly in the bilateral para-colic gutters, and to lesser extent in the para-hepatic, para-splenic spaces, and pelvis.
Ultimately, following deliberation in a multidisciplinary expert consultation and considering the risk of bleeding, a prompt decision was made to proceed with surgical removal.
A gastroscopy and a colonoscopy were normal and excluded any intraluminal lesion. A CT-guided biopsy of the lesion showed a spindle cell tumor that can correspond to GIST or smooth muscle tumor.
Open surgery revealed two hundred milliliters of hemoperitoneum, no peritoneal deposits, and no distal metastasis. A large tumor adherent to the ascending and transverse colon at the hepatic pedicle was identified. It was also encased by the omentum and in close contact with the duodenum. R0 was achieved after dissection of the tumor from the second part of the duodenum and the hepatic pedicle and surrounding bowels. Histopathology showed a completely excised tumor with spindle-shaped cells with slightly irregular nuclei with foci of necrosis. CD-34 positive on immunostaining along with STAT6 positivity, but DOG1 and epithelial membrane antigen negative, compatible with a solitary fibrous tumor. Numerous vessels run through the lesion. No abnormal mitotic activity was seen. The excised lymph node was reactive and showed no malignancy.
Her postoperative stay was complicated by pneumonia and a pulmonary embolism. On day 2 postoperatively, she had respiratory distress and desaturation. Chest X-ray and a spiral chest CT revealed parenchymal consolidation involving the right lower lobe along with loss of volume of the right lung with a shift of the mediastinum to the ipsilateral side along with left-sided pulmonary embolism. The accompanying abdominal scan showed superior mesenteric vein (SMV) thrombosis. After being treated with therapeutic anticoagulation for her pulmonary embolism and SMV thrombosis, the patient improved drastically and was discharged home on day 6 postoperatively.
One year later, the patient had an abdomen pelvis CT that showed normal findings with no signs of recurrence. However, 2 years later, the CT scan showed an interval appearance of a 3.8 cm × 5 cm slightly enhanced soft tissue mass in the mesentery (Figure 1B). The tumor was shown to be in close contact with the small bowels. Mesenteric lymph nodes measuring up to 8 mm × 10 mm were also seen. A decision was made to perform tumor resection due to concerns about recurrence. No ascites or distant metastasis were found during laparotomy. A mesenteric lesion invading the small bowel was noted 80 cm from the ligament of Treitz for which en-bloc resection with partial enterectomy was done followed by end-to-end manual anastomosis. Histopathology showed a lesion made of spindle cells arranged in fascicles and sheets with many blood vessels. There was no evidence of necrosis or abnormal mitosis. Immunophenotyping was also done that showed DOG 1, CD117, smooth muscle actin, S100, and CD34 negative but beta-catenin nuclear positive compatible with mesenteric desmoid tumor. The intestine was unremarkable and 12 lymph nodes were identified and shown to be reactive in nature. Therefore, a spindle cell tumor was diagnosed.
SFTs can manifest across a broad age spectrum, with the highest occurrence observed in the fifth and sixth decades of life[1,2]. They can appear anatomically anywhere, with the pleura being the most frequent site, comprising 30% of instances. Additional common locations comprise the meninges (27%), abdomen (20%), torso (10%), limbs (8%), and head and neck (5%)[5]. SFTs located in the mediastinum, peritoneum, or retroperitoneum typically exhibit a more aggressive behavior than tumors located elsewhere in the body[6]. No specific risk factors are known for the development of SFTs[7]. Typically, asymptomatic SFTs are slow-growing tumors often incidentally discovered during imaging studies[8]. Symptoms may occasionally arise due to pressure effects on adjacent organs[1,2]. In our case, vague abdominal pain prompted evaluation, likely due to mass effect, as no other symptoms indicated this diagnosis.
Furthermore, some patients may develop paraneoplastic syndromes that can aid in diagnosis. Pierre Marie–Bamberger syndrome or hypertrophic osteoarthropathy is an uncommon nonspecific condition occasionally linked to SFT in the pleura. Common presentations include digital clubbing distally, synovial effusions, and periostitis believed of having an association with the upregulation of vascular endothelial growth factor. Moreover, less than 5% of patients with SFT may show a persistent hypoglycemic syndrome caused by the overproduction of insulin-like growth factor-2 from big pleural or peritoneal SFTs, referred to as Doege–Potter syndrome[9,10].
No specific radiologic findings are associated with SFTs, typically revealing a well-defined isodense mass to skeletal muscle on CT scans, often exhibiting contrast enhancement in highly vascularized tumors (65%)[11]. SFTs often display areas of low signal intensity on both T1- and T2-weighted MRI reflecting their content of collagen. While 18-fluorine-fluorodeoxyglucose positron-emission-tomography/CT can offer partial diagnostic value for suspected cases of SFT, the detection of multiple high-grade lesions should prompt consideration of malignant SFT[1]. During both instances of our patient's presentation, CT scans were utilized as the preferred imaging modality. While they aided in localizing the tumor and confirming its classification as an STS, they provided no additional information, and the diagnosis was not solely based on imaging. Given the tumor's nature and suspicion of bleeding, further testing or biopsy was not conducted before surgery to enhance tumor characterization. However, ideally, every STS should undergo a thorough biopsy and characterization for improved diagnosis and direct treatment.
Macroscopically, SFT exhibits well-defined, partially encapsulated features and a multinodular, firm, whitish-cut surface. In certain instances, myxoid change and hemorrhage may be observed. However, malignant and locally aggressive tumors might display irregular, infiltrative borders along with areas of necrosis[3]. Microscopically, SFTs represent tumors with variable cellularity, comprising cells that are oval-shaped to spindle-shaped that demonstrate either growth without a distinct pattern or a pattern resembling a woven mat situated within the surrounding stroma of varying collagen content, which contains thin-walled, large branching blood vessels resembling "staghorn" structures. Additionally, medium-sized blood vessels accompanied by perivascular fibrosis are frequently observed[5]. In cases of markedly cellular tumors, sheets of more primitive-appearing rounded cells are evident[2]. Clinical and histomorphological indicators indicative of malignancy include older individuals, tumors with increased size, amplified cellularity, higher mitotic activity (≥ 4 per 10 high-power fields or > 2 mitoses per 2 square millimeters), nuclear variability, tumor necrosis, and attained borders[2]. In the presented case, the tumor cells displayed spindle-shaped histology with slightly irregular nuclei and areas of necrosis. The lesion contained numerous vessels, yet abnormal mitotic activity was absent. These histological characteristics serve as strong indicators for diagnosing SFTs when combined with other features.
The combination of CD34, CD99, and BCL-2 is commonly employed in the diagnosis of SFT. These immunohistochemical (IHC) markers are sensitive, showing strong expression in approximately 90% of cases. However, their utility is limited due to their expression in other neoplasms that closely resemble SFT histologically[12]. Immunohistochemical staining for STAT6 has become a valuable indicator for detecting gene fusion NAB2-STAT6, showing high sensitivity and specificity, particularly in cases of malignancy[2]. Nevertheless, it can also be detected in several additional soft tissue cancers, such as well-differentiated liposarcoma (WDL) or dedifferentiated liposarcoma (DDL), myxoid liposarcoma, fibromyxoid sarcoma, synovial sarcoma, undifferentiated pleomorphic sarcoma, desmoid fibromatosis, neurofibroma, unclassifiable sarcoma, and ovarian fibroma[5,13]. A combination of radiological, histological findings and markers was used in our case to diagnose SFT given the challenge of depending on a single factor for diagnosis.
The common histological presentation of SFT alongside other soft tissue tumors discussed previously contributes to a considerable challenge in differential diagnosis. Histological variants of solitary fibrous tumors include lipomatous variants (mimicking adipocytes), giant cell-rich variants (more common in the head and neck region), rhabdomyosarcomatous tumors, and aggressive "de-differentiated" variants (usually with loss of CD34 and STAT6 expression)[1,2].
For extrameningeal solitary fibrous tumors, most pathologists rely on the DeMicco risk stratification scheme for metastatic potential. In the large series by DeMicco, tumors that rarely can exhibit strong expression of STAT6 include undifferentiated pleomorphic sarcoma, desmoid, well/dedifferentiated liposarcoma (particularly the latter), clear cell sarcoma, and “myxoid sarcoma”. The NAB2:STAT6 rearrangement results from an intrachromosomal inversion in chromosome 12. As the genes are in close proximity, the rearrangement may not be detected with conventional chromosome banding or by FISH but more reliably by reverse transcription polymerase chain reaction or Next Generation Sequencing[3,4].
This challenge underscores the importance of discerning lesions based on subtle histological variations, as well as the utilization of novel IHC markers specifically studied for this purpose. For instance, the presence of positive MDM2 and CD4 marker expression, observed in WDL and DDL but not in SFT, aids in ruling out an SFT diagnosis. Moreover, desmoid fibromatosis shares remarkably similar histological features with SFT, alongside STAT6 expression[1,2].
In our case, upon the new presentation, the diagnosis shifted to desmoid fibromatosis instead of recurrent SFT. Ultimately, the diagnosis of desmoid tumor can be confirmed through nuclear β-catenin positivity, along with positive staining for vimentin, smooth muscle actin, cyclooxygenase-2, and β-estrogen receptors, while showing negativity for desmin, CD34, S100, and tyrosine kinase receptor markers[14].
When treating these individuals, SFT patients must be treated at specialized sarcoma centers, where they can benefit from a multidisciplinary team comprising a medical oncologist, oncologic surgeons, radiation oncologist, radiologist and pathologist, all well versed in the complexities of this condition, should oversee their management[1]. The gold-standard treatment for localized disease is considered to be complete en-bloc surgical resection with negative margins (R0)[15]. Typically, this resection exhibits a 10-year overall survival (OS) rate ranging from 54% to 89%[1]. Patients with negative margins (R0) and lacking high-risk histologic features are advised to undergo observation since there is no evident indication of an OS advantage with adjuvant radiotherapy (RT). Given the complete, en-bloc resection in our presented case, surgery proved adequate, prompting a follow-up at 6 months followed by yearly assessments thereafter for a prolonged period.
For SFT cases categorized as intermediate to high risk with R1/R2 margins, re-evaluating the option of re-excision should be considered for eligible patients if achieving full resection with negligible morbidity is feasible. Adjuvant RT stands as a reasonable choice when the patient is unsuitable for additional resection or achieving R0 surgery is technically unfeasible[16,17]. Neoadjuvant RT may be considered in specific cases to enhance tumor resectability or when manageable wound complications are anticipated[1].
When SFTs are localized or deemed resectable, no substantiated evidence backing utilization of systemic treatments in either the neoadjuvant or adjuvant contexts were approved[18]. However, there is limited data on the response of SFT to conventional chemotherapy[1]. It is crucial to emphasize that adjuvant CT should never be employed as a means to compensate for insufficient surgery. Nonetheless, high risk SFT patients or extensive malignant cancers where achieving R0 surgery is unattainable, the consideration of neoadjuvant CT should be deliberated within a specialized multidisciplinary setting[1]. There is no evidence to suggest that multiagent CT enhances patient survival, and single-agent CT continues to be the standard approach[1].
Other treatment options include antiangiogenic agents such as sunitinib, sorafenib, and pazopanib[19,20], reflecting the highly vascularized nature of these tumors. Inhibiting angiogenesis can impede tumor cell proliferation. Additionally, overexpression of insulin like growth factor 1 is observed in SFT, with Figitumumab a human IgG2 anti-IGF-1 monoclonal antibody, has led to observable responses in certain patients with progressive SFTs[21].
Immunotherapy is also emerging as a potential treatment for SFTs. A translational study investigated the relationship between programmed cell death protein, programmed death ligand 1, and tumor-infiltrating lymphocyte expression and prognosis[22].
The prognosis of SFT is promising following complete surgery. However, recurrence may occur in 10%–25% of cases within 10 years[23]. In high-risk patients, the risk of metastatic recurrence within 5 years can reach 40%[24]. Delayed relapses, occurring more than 10 years and sometimes up to 20 years after the original presentation, are frequent, underscoring the importance of prolonged monitoring[23,24]. Common sites for metastasis include pleura, liver, and bones. Recurrence of SFTs is more likely in cases where the tumor has not been completely excised (R1/R2), or when there is seeding of tumor cells within the serosa like the pleura or peritoneum, or through distant hematogenous spread.
Sarcomas comprise a highly diverse group of tumors, exhibiting heterogeneity clinically and genomically. Accurate diagnosis is crucial for effectively treating and managing the rare STS tumor known as SFT. IHC serves as the most sensitive and specific method for diagnosing SFT, offering practicality and cost-effectiveness.
In our presented case, distinguishing SFT from its fellow tumors was pivotal. Initially, it was mistaken for GIST and later for recurrence but was ultimately identified as desmoid. At each stage, the correct diagnosis led to appropriate treatment.
SFTs demonstrate poor sensitivity to conventional chemotherapy, with appropriate surgery remaining the optimal curative approach. However, biological and antiangiogenic agents show promise, with STAT6 and IGF-1 overexpression rendering SFT a targetable sarcoma. While data on the effectiveness of immunotherapy are limited, ongoing research continues to uncover new insights into managing this multifaceted tumor.
We would like to acknowledge the efforts of the general surgery department at Mount Lebanon Hospital University Medical Center and the encouragement of the Faculty of Medicine at the University of Balamand for the completion of this work.
| 1. | de Bernardi A, Dufresne A, Mishellany F, Blay JY, Ray-Coquard I, Brahmi M. Novel Therapeutic Options for Solitary Fibrous Tumor: Antiangiogenic Therapy and Beyond. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Tariq MU, Din NU, Abdul-Ghafar J, Park YK. The many faces of solitary fibrous tumor; diversity of histological features, differential diagnosis and role of molecular studies and surrogate markers in avoiding misdiagnosis and predicting the behavior. Diagn Pathol. 2021;16:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 3. | Demicco EG, Fritchie KJ, Han A. Solitary fibrous tumour. In World Health Organisation Classification of Soft Tissue and Bone Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Eds.; IARC Press, Lyon, France; 2019; 104-108. |
| 4. | Demicco EG, Wagner MJ, Maki RG, Gupta V, Iofin I, Lazar AJ, Wang WL. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol. 2017;30:1433-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 5. | Ronchi A, Cozzolino I, Zito Marino F, Accardo M, Montella M, Panarese I, Roccuzzo G, Toni G, Franco R, De Chiara A. Extrapleural solitary fibrous tumor: A distinct entity from pleural solitary fibrous tumor. An update on clinical, molecular and diagnostic features. Ann Diagn Pathol. 2018;34:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Haas RL, Walraven I, Lecointe-Artzner E, van Houdt WJ, Strauss D, Schrage Y, Hayes AJ, Raut CP, Fairweather M, Baldini EH, Gronchi A, De Rosa L, Griffin AM, Ferguson PC, Wunder J, van de Sande MAJ, Krol ADG, Skoczylas J, Sangalli C, Stacchiotti S. Extrameningeal solitary fibrous tumors-surgery alone or surgery plus perioperative radiotherapy: A retrospective study from the global solitary fibrous tumor initiative in collaboration with the Sarcoma Patients EuroNet. Cancer. 2020;126:3002-3012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Thway K, Jordan S, Fisher C, Nicholson AG. Updates in the approach to intrathoracic sarcomas. Histopathology. 2015;67:755-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Zafar H, Takimoto CH, Weiss G. Doege-Potter syndrome: hypoglycemia associated with malignant solitary fibrous tumor. Med Oncol. 2003;20:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Steigen SE, Schaeffer DF, West RB, Nielsen TO. Expression of insulin-like growth factor 2 in mesenchymal neoplasms. Mod Pathol. 2009;22:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Wignall OJ, Moskovic EC, Thway K, Thomas JM. Solitary fibrous tumors of the soft tissues: review of the imaging and clinical features with histopathologic correlation. AJR Am J Roentgenol. 2010;195:W55-W62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Weiss SW, Goldblum JR, Folpe AL. Enzinger and Weiss's soft tissue tumors. Elsevier Health Sciences, 2019; 1133-1147. |
| 13. | Doyle LA, Tao D, Mariño-Enríquez A. STAT6 is amplified in a subset of dedifferentiated liposarcoma. Mod Pathol. 2014;27:1231-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Riedel RF, Agulnik M. Evolving strategies for management of desmoid tumor. Cancer. 2022;128:3027-3040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 15. | Cardillo G, Lococo F, Carleo F, Martelli M. Solitary fibrous tumors of the pleura. Curr Opin Pulm Med. 2012;18:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Bishop AJ, Zagars GK, Demicco EG, Wang WL, Feig BW, Guadagnolo BA. Soft Tissue Solitary Fibrous Tumor: Combined Surgery and Radiation Therapy Results in Excellent Local Control. Am J Clin Oncol. 2018;41:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Bonvalot S, Gronchi A, Le Péchoux C, Swallow CJ, Strauss D, Meeus P, van Coevorden F, Stoldt S, Stoeckle E, Rutkowski P, Rastrelli M, Raut CP, Hompes D, De Paoli A, Sangalli C, Honoré C, Chung P, Miah A, Blay JY, Fiore M, Stelmes JJ, Dei Tos AP, Baldini EH, Litière S, Marreaud S, Gelderblom H, Haas RL. Preoperative radiotherapy plus surgery vs surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21:1366-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 307] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 18. | Le Roith D. Tumor-induced hypoglycemia. N Engl J Med. 1999;341:757-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Maruzzo M, Martin-Liberal J, Messiou C, Miah A, Thway K, Alvarado R, Judson I, Benson C. Pazopanib as first line treatment for solitary fibrous tumours: the Royal Marsden Hospital experience. Clin Sarcoma Res. 2015;5:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Domont J, Massard C, Lassau N, Armand JP, Le Cesne A, Soria JC. Hemangiopericytoma and antiangiogenic therapy: clinical benefit of antiangiogenic therapy (sorafenib and sunitinib) in relapsed malignant haemangioperyctoma /solitary fibrous tumour. Invest New Drugs. 2010;28:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Stacchiotti S, Negri T, Palassini E, Conca E, Gronchi A, Morosi C, Messina A, Pastorino U, Pierotti MA, Casali PG, Pilotti S. Sunitinib malate and figitumumab in solitary fibrous tumor: patterns and molecular bases of tumor response. Mol Cancer Ther. 2010;9:1286-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Kamamoto D, Ohara K, Kitamura Y, Yoshida K, Kawakami Y, Sasaki H. Association between programmed cell death ligand-1 expression and extracranial metastasis in intracranial solitary fibrous tumor/hemangiopericytoma. J Neurooncol. 2018;139:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13:640-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 953] [Cited by in RCA: 847] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 24. | Baldi GG, Stacchiotti S, Mauro V, Dei Tos AP, Gronchi A, Pastorino U, Duranti L, Provenzano S, Marrari A, Libertini M, Pilotti S, Casali PG. Solitary fibrous tumor of all sites: outcome of late recurrences in 14 patients. Clin Sarcoma Res. 2013;3:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |