Published online Jul 6, 2024. doi: 10.12998/wjcc.v12.i19.3961
Revised: May 8, 2024
Accepted: May 20, 2024
Published online: July 6, 2024
Processing time: 122 Days and 22.2 Hours
Juvenile hemochromatosis (JH) is an early-onset, rare autosomal recessive disorder of iron overload observed worldwide that leads to damage in multiple organs. Pathogenic mutations in the hemojuvelin (HJV) gene are the major cause of JH.
A 34-year-old male Chinese patient presented with liver fibrosis, diabetes, hypogonadotropic hypogonadism, hypophysis hypothyroidism, and skin hyperpigmentation. Biochemical test revealed a markedly elevated serum ferritin level of 4329 μg/L and a transferrin saturation rate of 95.4%. Targeted exome sequencing and Sanger sequencing revealed that the proband had a novel mutation c.863G>A (p.R288Q) in the HJV gene which was transmitted from his father, and two known mutations, c.18G>C (p.Q6H) and c.962_963delGCinsAA (p.C321*) in cis, which were inherited from his mother. The p.R288W mutation was previously reported to be pathogenic for hemochromatosis, which strongly supported the pathogenicity of p.R288Q reported for the first time in this case. After 72 wk of intensive phlebotomy therapy, the patient achieved a reduction in serum ferritin to 160.5 μg/L. The patient's clinical symptoms demonstrated a notable improvement.
This study highlights the importance of screening for hemochromatosis in patients with diabetes and hypogonadotropic hypogonadism. It also suggests that long-term active phlebotomy could efficiently improve the prognosis in severe JH.
Core Tip: Juvenile hemochromatosis (JH) is an early-onset, rare autosomal recessive disorder of iron overload that causes damage to multiple organs. Pathogenic mutations in the hemojuvelin (HJV) gene are the major cause of JH. We presented a 34-year-old male Chinese patient with a novel HJV mutation c.863G>A (p.R288Q) and two known mutations in cis, c.18G>C (p.Q6H) and c.962_963delGCinsAA (p.C321*). The proband presented with diabetes, hypogonadotropic hypogonadism, hypophysis hypothyroidism, hyperpigmentation, liver fibrosis, a markedly elevated serum ferritin level of 4329 μg/L and a transferrin saturation rate of 95.4%. His condition improved after 72 wk of intensive phlebotomy therapy.
- Citation: Xie LD, Kong XM, Shen JX, Wang TL, Ma J, Zhang YF, Chen XP. Novel compound heterozygous mutations in the hemojuvelin gene in a juvenile hemochromatosis patient: A case report. World J Clin Cases 2024; 12(19): 3961-3970
- URL: https://www.wjgnet.com/2307-8960/full/v12/i19/3961.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i19.3961
Hereditary hemochromatosis (HH) is a genetic disorder characterized by disturbed iron regulation. Juvenile hemochromatosis (JH), also known as type 2 HH, is the most severe and early-onset form of HH[1]. JH patients commonly exhibit multiorgan manifestations resulting from iron overload before 30 years of age, including cardiomyopathy, liver cirrhosis, arthritis, and skin pigmentation, as well as a series of endocrine disorders involving diabetes, hypogonadotropic hypogonadism, and hypothyroidism. Biallelic pathogenic mutations in the hemojuvelin gene [hemojuvelin (HJV) gene; also known as HFE2A; OMIM 602390], which encodes a coreceptor of bone morphogenetic proteins (BMPs) that regulate hepcidin levels, cause approximately 90% of the cases of JH in patients in a recessive inheritance pattern[2].
HJV-HH is a very rare disease worldwide. Diagnosis, management, and determination of patient prognosis in the clinic are very challenging. As it is sporadic, the genotypic spectrum varies across races and is still under intensive exploration. For instance, the substitution G320V has been frequently in Caucasians (mainly in the North European population), but has never been reported in patients originating from East Asian populations. In addition to the mutation profile, its phenotypes, therapeutic strategies, and long-term outcomes are poorly understood in East Asians, including in Chinese Han individuals.
Here, we report the case of a Chinese patient with JH caused by novel compound heterozygous mutations in the HJV gene with detailed clinical manifestations and long-term follow-up management after diagnosis.
A 34-year-old male patient who had been suffering from polydipsia and polyuria for 8 years and sexual hypoactivity for 2 years was admitted to our hospital.
Eight years prior, the patient was diagnosed with diabetes by symptoms of dry mouth and polyuria, and his fasting glucose level was determined to be 17.6 mmol/L at the local hospital. Since that time, he had been regularly treated with premixed insulin since then, but his blood glucose control was poor. He noted the gradual onset of fatigue, decreased libido, and erectile dysfunction two years prior.
The patient had a free previous medical history.
The patient had no history of consuming alcohol or smoking. He had not received blood transfusions or iron-supplementary nutrition. His mother had a cerebral infarction and heart disease. He had a younger brother who died from an unexplained hematologic disease at age 11. The patient was married and had an 8-year-old son. His wife, his son and his father were healthy. The parents, offspring and wife of the patient showed normal iron loading.
On physical examination, the patient was normotensive, had a body mass index of 23.3 kg/m2, and had normal pubic hair distribution. His skin was tanned, with no spider angiomas or palmar erythema. Abdominal examination revealed mild hepatomegaly and splenomegaly. The testicles were estimated to be 11 mL (left) and 13 mL (right), and the prostate examination was normal.
The initial laboratory test showed thrombocytes at a concentration of 67 × 109/L, an aspartate aminotransferase level of 56 U/L and an alanine aminotransferase level of 75 U/L. The hepatitis virus serological test was negative. The steamed buns meal test revealed an absolute deficiency of C peptide (C-p) similar to type 1 diabetes, but tests for glutamate decarboxylase antibody, insulin antibody and islet cell antibody were all negative. In addition, hormonal tests revealed a thyroid-stimulating hormone level of 2.29 mIU/L with 7.9 ng/L free T4, 0.58 IU/L follicle-stimulating hormone, 1.02 IU/L luteinizing hormone, 0.87 nmol/L testosterone, and 220.8 μg/L cortisol (8 am) (Table 1). The gonadotropin-releasing hormone stimulating test (gonadorelin 100 μg) also revealed no responsiveness of the pituitary (Table 1). Surprisingly, additional laboratory tests revealed transferrin oversaturation (95.4%) with a notable increase in the serum ferritin concentration of 4329 μg/L (Table 1).
| Laboratory parameters | Results | Normal range |
| White blood cells (109/L) | 3.92 | 3.5-9.5 |
| Neutrophils | 42.4 | 40-75 |
| Lymphocytes | 49.5 | 20-50 |
| Red blood cells (1012/L) | 4.3 | 4.3-5.8 |
| Hemoglobin (g/L) | 129 | 130-175 |
| Platelets (109/L) | 67 | 125-350 |
| Steamed buns meal test | ||
| Fasting glucose (mmol/L) | 8.45 | 3.61-6.11 |
| 0.5 h glucose (mmol/L) | 9.11 | |
| 1 h glucose (mmol/L) | 7.48 | |
| 2 h glucose (mmol/L) | 7.14 | |
| C-p (fasting) (μg/L) | 0.59 | 1.1-4.4 |
| C-p (0.5 h) (μg/L) | 0.63 | |
| C-p (1 h) (μg/L) | 0.5 | |
| C-p (2 h) (μg/L) | 0.37 | |
| HbA1c | 9.9 | |
| GAD, IAA, ICA | Negative | Negative |
| Free T4 (ng/L) | 7.9 | 9.3-17 |
| Free T3 (ng/L) | 2.37 | 2.0-4.4 |
| Total T4 (μg/L) | 46.6 | 51-141 |
| Total T3 (μg/L) | 0.795 | 0.8-2.0 |
| Thyroid-stimulating hormone (mIU/L) | 2.29 | 0.27-4.2 |
| Testosterone (nmol/L) | 0.87 | 6.07-27.10 |
| FSH (IU/L) | 0.58 | 1.27-19.26 |
| LH (IU/L) | 1.02 | 1.24-8.62 |
| The GnRH stimulating test | ||
| FSH (-15 min) (IU/L) | 0.59 | |
| FSH (0 h) (IU/L) | 0.63 | |
| FSH (0.5 h) (IU/L) | 0.72 | |
| FSH (1 h) (IU/L) | 0.63 | |
| FSH (1.5 h) (IU/L) | 0.68 | |
| FSH (2 h) (IU/L) | 0.67 | |
| LH (-15 min) (IU/L) | 1.07 | |
| LH (0 h) (IU/L) | 1.09 | |
| LH (0.5 h) (IU/L) | 1.79 | |
| LH (1 h) (IU/L) | 2.07 | |
| LH (1.5 h) (IU/L) | 1.98 | |
| LH (2 h) (IU/L) | 1.91 | |
| Prolactin (mIU/L) | 148.12 | 55.968-278.356 |
| Estradiol (pmol/L) | < 73 | < 73-252.49 |
| Iron (μmol/L) | 41.4 | 11-30 |
| Transferrin saturation value | 95.4 | 20-55 |
| Ferritin (μg/L) | 4329 | 23.9-336.2 |
| Alanine aminotransferase (U/L) | 75 | 0-40 |
| Aspartate aminotransferase (U/L) | 56 | 0-42 |
| Total bilirubin (μmol/L) | 13.24 | 5.00-21.00 |
| Direct bilirubin (μmol/L) | 3.57 | 0.00-7.00 |
| Total protein (g/L) | 62 | 60-80 |
| Albumin (g/L) | 40 | 35-55 |
| Gamma-glutamyl transferase (U/L) | 32 | 0-52 |
| Alkaline phosphatase (U/L) | 131 | 40-150 |
| Prothrombin activity | 83 | 80-120 |
| Hepatitis B surface antigens (IU/ml) | 0.0 | < 0.05 |
| Anti-HCV (S/CO) | 0.06 | < 1 |
| Cortisol (μg/L) (8 am) | 220.8 | 62-194 |
| Adrenocorticotropic hormone (ng/L) (8 am) | 38.27 | 7.2-63.3 |
| Urinary free cortisol (μg/24 h) | 93.06 | 36-137 |
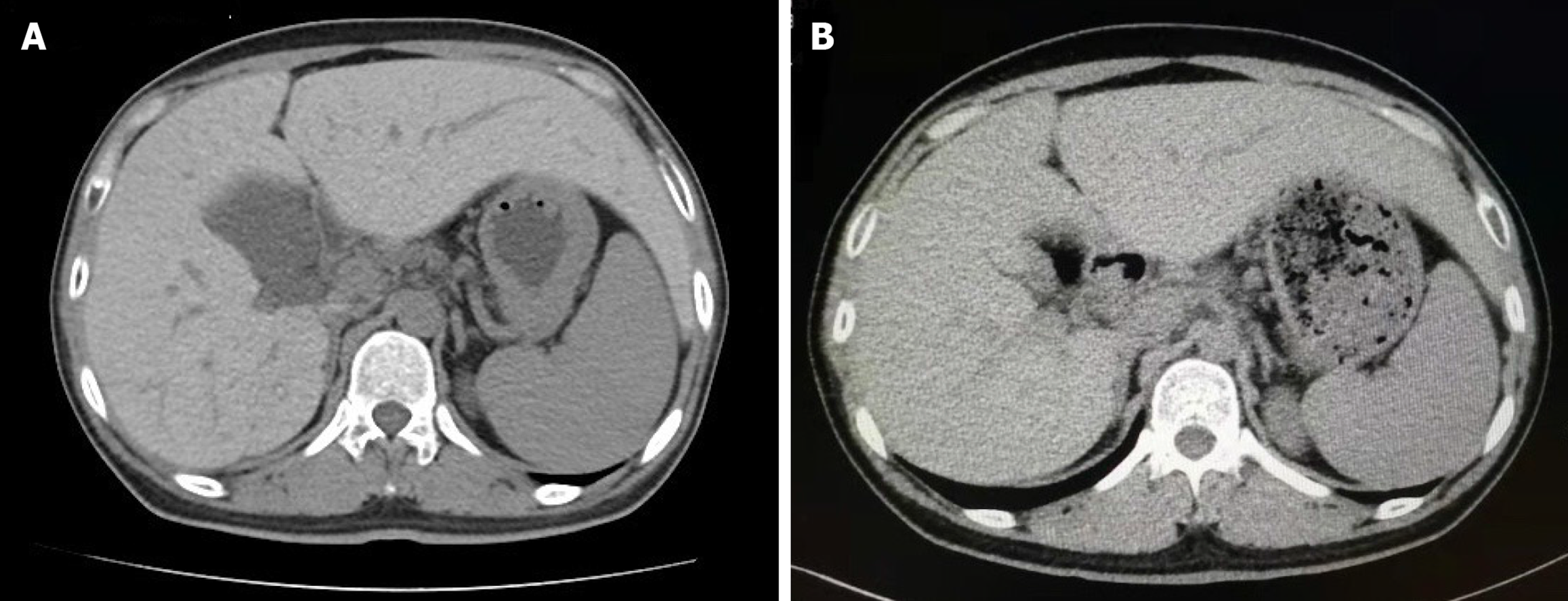
The patient’s pituitary magnetic resonance imaging (MRI) showed no abnormalities. An abdominal computed tomography (CT) scan revealed enlargement of the left lobe of the liver and splenomegaly. The density of the liver in the CT scan was obviously greater than normal. The liver attenuation value on CT had increased to 104 Hounsfield unit (HU) (Figure 1A).
The combination of diabetes, skin pigmentation, hypogonadotropic hypogonadism, hypophysis hypothyroidism, elevated liver enzymes, iron indices and CT imaging of liver raised the suspicion of hemochromatosis. The patient’s bone marrow biopsy did not show ineffective erythropoiesis due to thalassemia, sideroblastic anemia, and hemolysis. Therefore, we ruled out secondary hemochromatosis. A liver biopsy was carried out for diagnostic purposes and staging of the liver injury. As shown in Figure 2A, histological examination of the specimen revealed mild to moderate portal fibrosis. Iron staining revealed heavy iron deposition (grade 4 iron stores[3]) not only in most hepatocytes but also in some Kupffer cells, biliary epithelial cells in the portal area and endothelial cells. Iron deposition was maximal in hepatocytes in zone 1 of the hepatic acinus (Figure 2B). The histopathological findings strongly supported the diagnosis of HH.
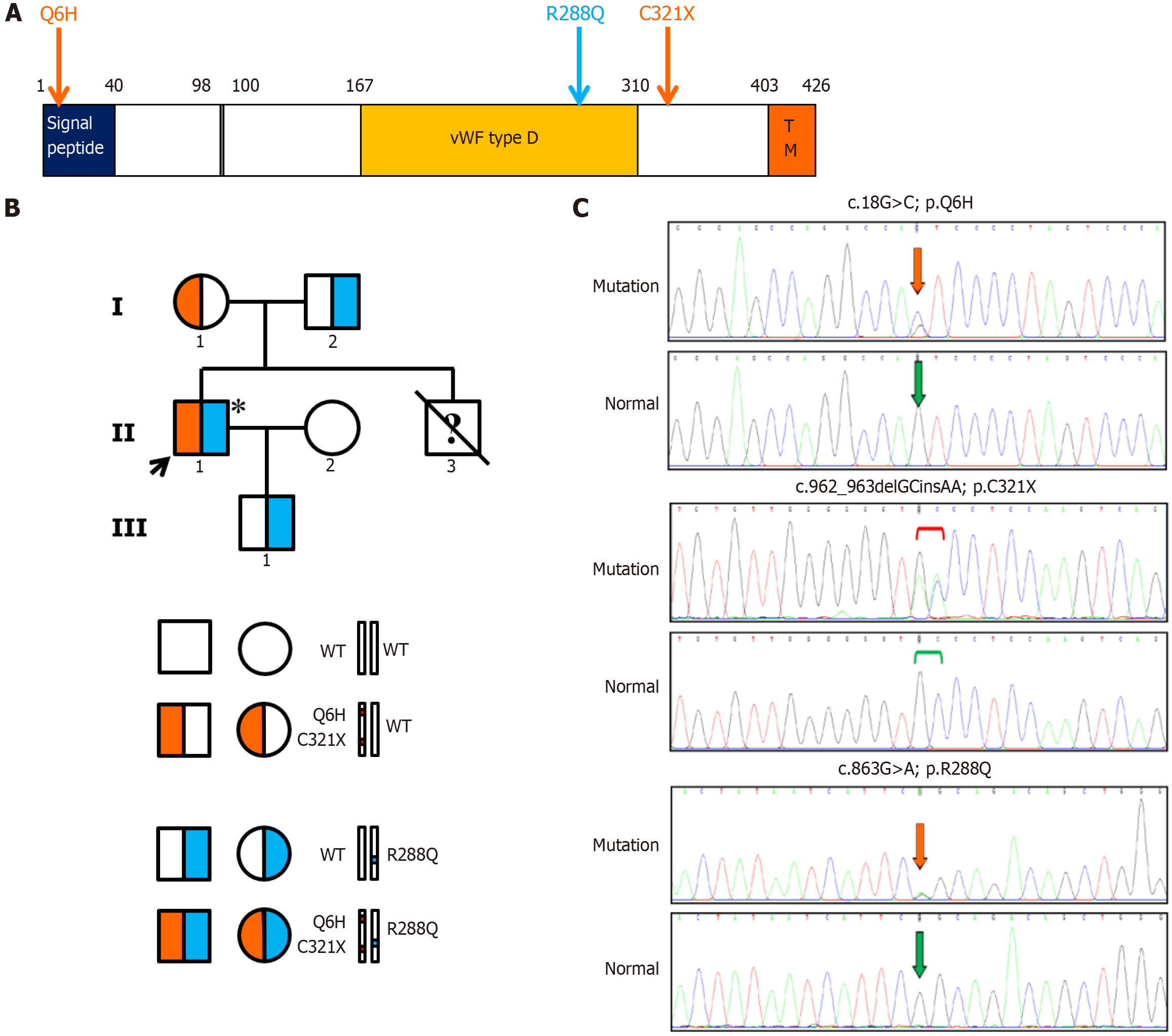
Three heterozygous mutations in the HJV gene were identified, including two missense mutations (c.18G>C, p.Q6H; c.863G>A, p.R288Q) and a nonsense mutation (c.962_963delGCinsAA, p.C321*). The mother of the proband was heterozygous for c.18G>C and c.962_963delGCinsAA in cis. The father and son of the proband were heterozygous for a novel missense mutation c.863G>A identified by a comprehensive literature search and the Human Gene Mutation Database. The transmission of the mutations in the pedigree is shown in Figure 3. The proband was a compound heterozygote for these mutations. The frequency of the missense mutation c.18G>C was only reported in the Exome Aggregation Consortium (ExAC; Cambridge, MA; http://exac.broadinstitute.org/) database as 0.00002. It was predicted to be damaging in Sorting Intolerant from Tolerant and benign in both Mutation Taster and PolyPhen-2. The nonsense mutation c.962_963delGCinsAA terminates the translation of the HJV protein and was not detected in any of the databases. The novel missense mutation c.863G>A was predicted to be possibly damaging at the protein level by all of the bioinformatic tools. Moreover, it was only previously detected in the ExAC database with a frequency of 0.000008.
The final diagnosis of the present case was JH due to the novel compound heterozygous mutations in the HJV gene.
The patient was managed by periodical therapeutic phlebotomy, testosterone and levothyroxine sodium replacement therapies and daily insulin therapy. A 500 mL (1 unit) therapeutic phlebotomy per week was initiated and continued for the first 24 wk. However, the outcome was unsatisfactory, and the ferritin serum levels were even slightly enhanced. Thus, the protocol was switched to 500 mL biweekly for more than 36 wk. At week 60, his serum ferritin concentration decreased to 423.8 μg/L. Then, the phlebotomy volume was adjusted to 500 mL once a week since then.
After 72 wk of phlebotomy, the patient’s serum ferritin concentration was 160.5 μg/L, which was very close to the target level (50-100 μg/L). The hemoglobin and blood counts were stable during this process. Moreover, the CT value of the liver was 68 HU (Figure 1B), suggesting stable iron depletion in the liver. In addition, the patient's symptoms of fatigue and skin pigmentation were obviously relieved. His thyroid function was normal after daily levothyroxine sodium replacement therapy, and liver function was currently adequate. His blood glucose was controlled with insulin therapy, despite an absolute deficiency of the C-p. His hypogonadism was not ameliorated.
The HJV gene, mapped to chromosome 1q21, encodes hemojuvelin. Full-length membrane-anchored HJV upregulates the expression of hepcidin through the BMP-Smad signaling pathway by acting as a BMP coreceptor[2]. Pathogenic mutations in HJV can cause failure in the modulation of the BMP pathway, resulting in excessive iron deposition. In the present case, compound heterozygous mutations in the HJV gene were identified, including a novel missense mutation R288Q, along with (Q6H; C321*), and no mutations in other HH genes were found.
The spectrum of HJV gene mutations varies across different races, family origins and geographical regions[1]. According to a comprehensive literature search of the PubMed and Chinese databases, only eight unrelated Chinese hemochromatosis probands with biallelic HJV mutations and four with monoallelic mutations have been reported (Table 2)[4-8]. (Q6H; C321*) is the most frequently reported mutation, which was present in six of these patients but was never observed in individuals of other ethnicities, suggesting that it is a hotspot mutation restricted to Chinese Han individuals. C321* produces a truncated hemojuvelin lacking the glycosylphosphatidylinositol anchor motif and is pathogenic based on the experimental findings[9]. Q6H, located in the signal peptide of hemojuvelin, was always found to be transmitted along with C321* and was indicated to be benign and served as an indicator of C321*[4]. Arg288 is a potential specific cleavage site of matriptase-2 and is highly conserved across species. Maxson and coworkers found that the R288A mutant specifically and completely blocked the cleavage of hemojuvelin by matriptase-2 in vitro[10]. Compared to the wild type protein, the mutant protein was expressed at a 35% lower level on the plasma membrane than the wild type. Autoproteolysis did not occur in the R288A mutant, resulting in the loss of its ability to function as a BMP coreceptor. Consequently, the mutant was unable to activate hepcidin and was insensitive to BMP6 stimulation[11]. Mutation R288W in homozygous status was previously identified in three patients from France[12,13]. All of them presented with hypogonadism, which was also observed in the present case, suggesting that hypogonadism could be a common phenotype in homozygotes with the R288 mutation. Based on the above evidence, we speculated that the R288Q mutation first identified in this case was likely disease-causing due to its loss of activity as a BMP coreceptor. In addition, mutations including I281T, I287S, Y46*, V274M, and E3D were previously reported in Chinese patients (Table 2)[4-6,8].
| Serial number | Publication date | Mutations | Gender | Age | Clinical manifestation | Treatment | Prognosis | Ref. |
| 1 | 2018 | HJV p.E3D, HFE p.H63D, TFR2 p.I238M, SUGP2 p.R639Q, DENND3 p.L708V | Male | 67 | Hepatic fibrosis | NA | NA | [4] |
| 2 | 2018 | HJV p.F103L (homozygous) | Female | 36 | Abnormal liver function test, amenorrhea | NA | NA | [4] |
| 3 | 2018 | HJV p.C321X/Q6H/I281T | Male | 26 | Diabetes, cardiopathy | NA | NA | [4] |
| 4 | 2018 | HJV p.Q6H/C321X/V274M | Male | 57 | Abnormal liver function test | phlebotomy | NA | [4] |
| 5 | 2018 | HJV p.Q6H/C321X/H104R, TFR2 p.A75V | Male | 18 | Abnormal liver function test | NA | NA | [4] |
| 6 | 2018 | HJV p.E3D, BMP4 p.R269Q | Female | 53 | Jaundice, abnormal liver function test | NA | NA | [4] |
| 7 | 2018 | HJV p.E3D, HFE p.H63D, TMPRSS6 p.T331M | Male | 33 | Abnormal liver function test | NA | NA | [4] |
| 8 | 2004 | HJV p.C321X/Q6H/I281T | Female | 19 | Hepatic fibrosis, diabetes, dilated cardiomyopathy, hypogonadotropic hypogonadism skin pigmentation | Phlebotomy | Normal cardiac function, and normal serum ferritin after 2 years of follow-up | [5] |
| 9 | 2014 | HJV p.C321X/Q6H (heterozygous) | Male | 37 | Liver cirrhosis diabetes, dilated cardiomyopathy, hypogonadism skin pigmentation | Phlebotomy | Died of sudden cardiac arrhythmia 1 mo later | [6] |
| 10 | 2014 | HJVp.C321X/Q6H (homozygous) | Male | 29 | Liver fibrosis, diabetes, dilated cardiomyopathy, hypogonadism skin pigmentation | NA | NA | [7] |
| 11 | 2017 | HJV p.Y46x (homozygous) | Male | 13 | Liver cirrhosis | NA | NA | [8] |
| 12 | 2017 | HJV p.I287S (homozygous) | Male | 37 | Diabetes | NA | NA | [8] |
Notably, the heterozygous status of the HJV mutation can lead to hemochromatosis, which has been mainly observed in Chinese individuals[4-6], suggesting that genetic screening and management of relatives should be emphasized after diagnosis. In the present pedigree, all of the heterozygotes, either the father and son of the patient with monoallelic R288Q or his mother with monoallelic (Q6H; C321*), had normal ferritin levels and transferrin saturation rates, which will be carefully followed up in the future.
Multiorgan dysfunction caused by excess iron accumulation is the major consequence of hemochromatosis. In addition to hepatic accumulation of excess iron causing liver fibrosis or cirrhosis, progressive iron deposition selectively in pancreatic β-cells or the anterior part of the pituitary gland results in diabetes or hypogonadism, which are the two most common extrahepatic complications of hemochromatosis. For JH patients, the total iron burden was significantly greater than that for HFE-HH patients. Hypogonadism or associated reproductive problems are often the first manifestation of patients in their 20s. De Gobbi et al[14] reported that 96.1% of the JH patients presented with hypogonadism, which was significantly higher than that in HFE C282Y homozygotes (18.4%) or hemochromatosis type 3 patients (27.3%)[14]. Moreover, one-third of JH patients could suffer from cardiomyopathy, which is also the major cause of their death. Notably, it was also shown that the prevalence of liver complications in JH patients was similar to, or even lower than that in HFE-HH patients. It seems that the earlier onset and greater prevalence of hypogonadism or cardiopathy in JH patients than in those with liver cirrhosis indicate that the pituitary hypophysis and heart of JH patients are relatively more vulnerable to iron toxicity than the liver is at a young age. Alternatively, it is possible that the rapid iron accumulation that occurs is less dangerous to the liver, which is a natural iron store, or that cirrhosis requires more time to develop.
HJV-HH has been speculated to constitute of the majority of JH. A systematic review of JH indicated that the age at diagnosis among the biallelic mutation probands was earlier than that of the monoallelic HJV mutation cases, and more individuals with early-onset (onset age ≤ 30) were identified[15]. For HJV-HH, hypogonadism was the most frequently reported phenotype in Caucasians with biallelic HJV mutations, whereas glucose intolerance was also common but less frequent. In the Chinese population, patients with HJV-HH could have a different spectrum of clinical symptoms (Table 2). Among the eight previously reported biallelic probands of Chinese ancestry, three had cardiomyopathy, three had hypogonadism, four had glucose intolerance or diabetes, and two had hepatic fibrosis[4,5,7,8]. In our HJV-HH patient, diabetes was the initial clinically significant manifestation at the age of 26, followed by the presentation of hypogonadotropic hypogonadism six years later. At the same time, although his ferritin level was extremely high, only mild to moderate hepatic fibrosis was observed, which might have resulted from his genotype and his lack of alcohol intake, as excess alcohol intake was proven to be a major risk factor for the development of liver disease in hemochromatosis patients[16]. Fortunately, this patient has not yet shown any abnormalities in the cardiac function tests. In fact, there is a lack of awareness of hemochromatosis in China because of its rarity, especially for those with mild hepatic outcomes, such as in HJV-HH. Patients are not always promptly and accurately diagnosed until liver cirrhosis is observed. Our findings indicated that if diabetes is comorbid with unexplained hypogonadotropic hypogonadism and/or skin hyperpigmentation, regardless of the liver-related symptoms, hemochromatosis should be considered and screened for.
In addition to hypogonadism, other pituitary deficiencies may occur. In this case, the patient showed a typical presentation of impairment of two pituitary axes, both the hypothalamic-pituitary-gonadal axis and hypothalamic-pituitary-thyroid axis, which was not observed in other reported cases of Chinese ancestry, suggesting that a systemic evaluation of endocrine functions is necessary.
Therapeutic phlebotomy is the simplest, least expensive, and most effective way to remove accumulated iron in non-anemic patients with iron overload. The amount and frequency of phlebotomy depend on the individual's clinical characteristics and tolerance. Usually, one unit of blood (400-500 mL), containing approximately 200-250 mg of iron can be removed once or twice per week as tolerated. Treatment with JH typically requires initial aggressive phlebotomy to reduce accumulated iron stores. Furthermore, based on phlebotomy requirements to maintain iron balance, it was calculated that iron absorption in JH is 3-4 times greater than that in HFE-associated HH[17]. Experience in the phlebotomy management of JH caused by HJV gene deficiency in Chinese patients is still lacking. Among the previously reported Chinese cases, only four reported uses of this therapy, and only two of them had outcome data[4-6]. One patient died after diagnosis[6]. The other patient achieved therapeutic targets through 53 wk of weekly 500 mL phlebotomy[5]. Here, we reported a detailed and successful treatment procedure involving 500- or 1000-ml phlebotomy per week for at least 72 wk, along with a satisfactory outcome, which surely provided valuable experiences of phlebotomy for a long duration, especially for the Chinese population. This study suggested for the first time that more active and aggressive phlebotomy can be efficient and well tolerated in Chinese patients with heavy iron overload.
It is well recognized that a significant reduction in hepatic iron stores can be achieved by phlebotomy in HH. In HJV-HH, there’re several studies based on Caucasians reported that the hepatic iron overload could be significantly alleviated after phlebotomy through CT or MRI images. Moreover, phlebotomy could well prevent hepatic iron deposition in HJV-HH patients diagnosed early by family screening[18]. However, it has also been reported that hepatic fibrosis in patients cannot be reversed even though the hepatic iron has been fully depleted[19]. In Chinese patients, hepatic function can be normalized by phlebotomy, but direct evidence of hepatic iron depletion has not been reported. In the present case, for the first time, we reported that hepatic CT images after phlebotomy revealed a complete depletion of hepatic iron at week 72, when the iron in circulation was largely depleted by adequate phlebotomy. This finding provides the first-hand evidence of hepatic iron depletion after phlebotomy in HJV-HH in a Chinese population, which strongly supports that hepatic iron stores induced by HJV gene deficiency can be efficiently depleted by phlebotomy.
Unfortunately, recovery of the affected endocrine glands is rare in HJV-HH. Most of the HJV-HH patients should be rely on hormone replacement therapies. Iron toxicity to endocrine cells makes recovery difficult. In this case, although iron depletion was successfully achieved, the dose of insulin in this patient did not significantly decrease. For secondary hypogonadism in HH, one study suggested that the combination of intensive phlebotomy and hormonal replacement therapy completely reversed hypogonadotropic hypogonadism in some patients under 40 years of age at diagnosis[20,21]. Thus, as the present patient is relatively young, there could be a chance to restore the gonadal axis in the future.
In summary, our study reports a young Chinese male with novel compound heterozygous mutations in the HJV gene with coexistence of diabetes, hypogonadotropic hypogonadism, hypophyseal hypothyroidism, skin hyperpigmentation, and hepatomegaly. This study emphasizes the importance of screening for hemochromatosis in patients with diabetes or unexplained hypogonadotropic hypogonadism, along with skin hyperpigmentation. In addition, if tolerated, more aggressive, long-term phlebotomy therapy may improve the prognosis of patients with severe JH.
We sincerely thank the patient and his family for their willingness to allow their experience to be shared.
| 1. | Wallace DF, Subramaniam VN. The global prevalence of HFE and non-HFE hemochromatosis estimated from analysis of next-generation sequencing data. Genet Med. 2016;18:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Malyszko J. Hemojuvelin: the hepcidin story continues. Kidney Blood Press Res. 2009;32:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Deugnier YM, Loréal O, Turlin B, Guyader D, Jouanolle H, Moirand R, Jacquelinet C, Brissot P. Liver pathology in genetic hemochromatosis: a review of 135 homozygous cases and their bioclinical correlations. Gastroenterology. 1992;102:2050-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 221] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Lv T, Zhang W, Xu A, Li Y, Zhou D, Zhang B, Li X, Zhao X, Wang Y, Wang X, Duan W, Wang Q, Xu H, Zheng J, Zhao R, Zhu L, Dong Y, Lu L, Chen Y, Long J, Zheng S, Wang W, You H, Jia J, Ou X, Huang J. Non-HFE mutations in haemochromatosis in China: combination of heterozygous mutations involving HJV signal peptide variants. J Med Genet. 2018;55:650-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Huang FW, Rubio-Aliaga I, Kushner JP, Andrews NC, Fleming MD. Identification of a novel mutation (C321X) in HJV. Blood. 2004;104:2176-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Li S, Xue J, Chen B, Wang Q, Shi M, Xie X, Zhang L. Two middle-age-onset hemochromatosis patients with heterozygous mutations in the hemojuvelin gene in a Chinese family. Int J Hematol. 2014;99:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Yuanfeng L, Hongxing Z, Haitao Z, Xiaobo P, Lili B, Fuchu H, Zewu Q, Gangqiao Z. [Mutation analysis of the pathogenic gene in a Chinese family with hereditary hemochromatosis]. Yi Chuan. 2014;36:1152-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Wang Y, Du Y, Liu G, Guo S, Hou B, Jiang X, Han B, Chang Y, Nie G. Identification of novel mutations in HFE, HFE2, TfR2, and SLC40A1 genes in Chinese patients affected by hereditary hemochromatosis. Int J Hematol. 2017;105:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Silvestri L, Pagani A, Fazi C, Gerardi G, Levi S, Arosio P, Camaschella C. Defective targeting of hemojuvelin to plasma membrane is a common pathogenetic mechanism in juvenile hemochromatosis. Blood. 2007;109:4503-4510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Maxson JE, Chen J, Enns CA, Zhang AS. Matriptase-2- and proprotein convertase-cleaved forms of hemojuvelin have different roles in the down-regulation of hepcidin expression. J Biol Chem. 2010;285:39021-39028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Rausa M, Ghitti M, Pagani A, Nai A, Campanella A, Musco G, Camaschella C, Silvestri L. Identification of TMPRSS6 cleavage sites of hemojuvelin. J Cell Mol Med. 2015;19:879-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Filali M, Le Jeunne C, Durand E, Grinda JM, Roetto A, Daraio F, Bruneval P, Jeunemaitre X, Gimenez-Roqueplo AP. Juvenile hemochromatosis HJV-related revealed by cardiogenic shock. Blood Cells Mol Dis. 2004;33:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Lanzara C, Roetto A, Daraio F, Rivard S, Ficarella R, Simard H, Cox TM, Cazzola M, Piperno A, Gimenez-Roqueplo AP, Grammatico P, Volinia S, Gasparini P, Camaschella C. Spectrum of hemojuvelin gene mutations in 1q-linked juvenile hemochromatosis. Blood. 2004;103:4317-4321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | De Gobbi M, Roetto A, Piperno A, Mariani R, Alberti F, Papanikolaou G, Politou M, Lockitch G, Girelli D, Fargion S, Cox TM, Gasparini P, Cazzola M, Camaschella C. Natural history of juvenile haemochromatosis. Br J Haematol. 2002;117:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Kong X, Xie L, Zhu H, Song L, Xing X, Yang W, Chen X. Genotypic and phenotypic spectra of hemojuvelin mutations in primary hemochromatosis patients: a systematic review. Orphanet J Rare Dis. 2019;14:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Wood MJ, Powell LW, Dixon JL, Ramm GA. Clinical cofactors and hepatic fibrosis in hereditary hemochromatosis: the role of diabetes mellitus. Hepatology. 2012;56:904-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Cazzola M, Cerani P, Rovati A, Iannone A, Claudiani G, Bergamaschi G. Juvenile genetic hemochromatosis is clinically and genetically distinct from the classical HLA-related disorder. Blood. 1998;92:2979-2981. [PubMed] |
| 18. | Barton JC, Rao SV, Pereira NM, Gelbart T, Beutler E, Rivers CA, Acton RT. Juvenile hemochromatosis in the southeastern United States: a report of seven cases in two kinships. Blood Cells Mol Dis. 2002;29:104-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Daraio F, Ryan E, Gleeson F, Roetto A, Crowe J, Camaschella C. Juvenile hemochromatosis due to G320V/Q116X compound heterozygosity of hemojuvelin in an Irish patient. Blood Cells Mol Dis. 2005;35:174-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Gama R, Smith MJ, Wright J, Marks V. Hypopituitarism in primary haemochromatosis; recovery after iron depletion. Postgrad Med J. 1995;71:297-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Hamer OW, Gnad M, Schölmerich J, Palitzsch KD. Successful treatment of erectile dysfunction and infertility by venesection in a patient with primary haemochromatosis. Eur J Gastroenterol Hepatol. 2001;13:985-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |