Published online May 26, 2024. doi: 10.12998/wjcc.v12.i15.2542
Peer-review started: January 25, 2024
First decision: February 8, 2024
Revised: February 24, 2024
Accepted: March 28, 2024
Article in press: March 28, 2024
Published online: May 26, 2024
Processing time: 110 Days and 3.5 Hours
The number of patients undergoing solid organ transplantation has increased annually. However, infections in solid organ transplant recipients can have a severe effect on patient survival owing to the continued use of immunosuppressants. Carrimycin is a novel macrolide antibiotic produced by genetically engineered streptomyces spiramyceticus harboring a 4’’-O-isovaleryltransferase gene (ist) from streptomyces thermotoleran. Carrimycin has good antibacterial and antiviral effects. However, no relevant studies have been conducted on the efficacy and safety of carrimycin in patients with severe pneumonia (SP) after solid organ transplantation.
To explore the efficacy and safety of carrimycin in patients with SP after solid organ transplantation to provide a medication reference for clinical treatment.
In March 2022, ten patients with SP following solid-organ transplantation were treated at our hospital between January 2021 and March 2022. When the condition was critical and difficult to control with other drugs, carrimycin was admi
All ten patients were included in the analysis. Regarding etiological agent detection, there were three cases of fungal pneumonia, two cases of bacterial pneumonia, two cases of Pneumocystis pneumonia, and three cases of mixed infections. After treatment with carrimycin, the disease in seven patients significantly improved, the course of the disease was significantly shortened, fever was quickly controlled, chest computed tomography was significantly improved, and oxygenation was significantly improved. Finally, the patients were discharged after curing. One patient died of acute respiratory distress syndrome, and two patients discontinued treatment.
Carrimycin is a safe and effective treatment modality for SP following solid organ transplantation. Carrimycin may have antibacterial and antiviral effects in patients with SP following solid organ transplantation.
Core Tip: In this study, the clinical features and treatment projects of 10 cases of severe pneumonia (SP) after solid organ transplantation were retrospectively analyzed. It was conformed that carrimycin is a safe and effective modality in the treatment of SP after solid organ transplantation.
- Citation: Cui XQ, Zhang LW, Zhao P, Feng JJ. Efficacy and safety of carrimycin in ten patients with severe pneumonia following solid organ transplantation. World J Clin Cases 2024; 12(15): 2542-2550
- URL: https://www.wjgnet.com/2307-8960/full/v12/i15/2542.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i15.2542
The number of patients undergoing solid-organ transplantation (SOT) is increasing. Post-operative complications can severely affect patient survival[1]. Among these, pneumonia is a frequent infectious complication of SOT. It is considered the chief cause of morbidity and mortality in renal allograft recipients due to the continued use of immunosuppressants[2,3]. Pneumonia can lead to acute respiratory distress syndrome (ARDS)[4], with an occurrence rate ranging from 0.2% to 4.3%[5,6] and a mortality rate of 22.5%–67%[7-11]. The mortality rate has been reported to be nearly 100% in patients on ventilation[12]. However, the curative effect of conventional drugs is often poor and seriously puzzling for clinicians.
Carrimycin is a novel macrolide antibiotic produced by genetically engineered streptomyces spiramyceticus harboring a 4’’-O-isovaleryltransferase gene (ist) from streptomyces thermotolerans. Carrimycin is a broad-spectrum antibacterial agent and is effective against Gram-positive cocci, β-lactamase-producing bacteria, Mycoplasma, and Chlamydia, as well as some Gram-negative bacilli and fungi. Additionally, it has been reported that carrimycin can be used to treat Coronavirus disease 2019 (COVID-19) with good therapeutic effect[13]. Previous studies have verified the antitumor effects of carrimycin in vitro and in vivo[14].
However, no relevant studies have been conducted on the efficacy of carrimycin in patients with post-SOT infections. In this study, we investigated the efficacy and safety of carrimycin in SOT recipients in vitro and in vivo for the first time between January 2021 and March 2022; 10 patients with severe pneumonia (SP) following SOT were treated at our hospital. When the condition was critical and difficult to control with other drugs, carrimycin was administered. Through a retrospective analysis and summary of the clinical data of these patients, this study preliminarily discusses the efficacy and safety of carrimycin for treating SP following SOT to provide a medication reference for clinical treatment.
Among the ten patients, there were six males and four females, aged 27-63 years, all of whom underwent transplantation for the first time, including nine renal transplant recipients and one liver transplant recipient (Table 1).
| Recipient | Gender | Age | Type of transplanted organ | Transplant time |
| 1 | Male | 46 | Renal transplantation | 27 d |
| 2 | Male | 63 | Renal transplantation | 5 months |
| 3 | Female | 34 | Renal transplantation | 13 months |
| 4 | Male | 55 | Liver transplantation | 7 d |
| 5 | Male | 56 | Renal transplantation | 4 months |
| 6 | Female | 27 | Renal transplantation | 5 months |
| 7 | Female | 55 | Renal transplantation | 3 months |
| 8 | Male | 60 | Renal transplantation | 16 yr |
| 9 | Male | 27 | Renal transplantation | 4 months |
| 10 | Female | 40 | Renal transplantation | 7 yr |
In this study, the diagnostic criteria of SP following SOT were based on the American Thoracic Society criteria and slightly adjusted in combination with the characteristics of SOT[15]: (1) Long-term application of immunosuppressants after SOT; (2) clinical symptoms of persistent fever, cough and progressive dyspnea; (3) an aspiratory rate > 30 per min; (4) an arterial oxygen partial pressure/oxygen inspiratory fraction (PaO2/FiO2) ratio < 250; and (5) chest film pneumonia showed and rapidly expanded by more than 50% in a short time. Diagnostic criteria of ARDS[16]: (1) Timing: Acute onset; (2) oxygenation: PaO2/FiO2 ≤ 200 mmHg regardless of positive end-expiratory pressure; (3) chest radiograph: Bilateral infiltrates seen on frontal chest radiograph; and (4) airway pressure: ≤ 18 mm Hg when measured or no clinical evidence of left atrial hypertension.
Eight patients (excluding cases 4 and 8), were treated with basiliximab or anti-thymocyte globulin for immunity induction therapy. Methylprednisolone (1000 mg) was administered intravenously during surgery, and methylprednisolone (500 mg/d) was administered intravenously for two consecutive d after transplantation. Methylprednisolone tablets were administered orally at 0.8 mg/kg·d on the fourth day after transplantation, decreasing by 4 mg per d to 16 mg/d and then by 4 mg per wk to 4 mg/d. After transplantation, except in case 8, cyclosporine + mycophenolate mofetil + methylprednisolone was administered; in the other 9 cases, tacrolimus + mycophenolate mofetil + methylprednisolone was administered. The blood tacrolimus and cyclosporine concentrations were maintained at 5-10 ng/mL, and the concentration of cyclosporine was maintained at 100-150 ng/mL. The dosage of mycophenolate mofetil used was 1000-1500 mg/d.
According to Rubinl's "infection schedule" stage[17], the time distribution of SP cases in this group is 2 cases (20%, 2/10) in the first month after surgery, 5 cases (50%, 5/10) in 2–6 months, and 3 cases (30%, 3/10) after 6 months. Clinical features: (1) Most patients have a regular temperature, but some present low or high fever. Fever was evident in the morning. The onset of fever is often delayed by 1–2 h daily. After sweating, the fever resolved. A few patients showed massive sweating, but their body temperatures were normal. When they developed a high fever, it was mostly persistent; and (2) there may be no obvious dyspnea symptoms in the early stage, followed by chest tightness, suffocation, accelerated heart rate, and other symptoms that worsen after activity, with or without coughing. No sputum was detected in the early or middle stages, and blood gas analysis indicated severe hypoxemia; (3) this disease progresses rapidly. After the symptoms of dyspnea and hypoxia, the disease progresses rapidly. Although noninvasive or invasive ventilation is used, some patients die from respiratory failure; and (4) it is often accompanied by multiple-organ dysfunction syndrome (MODS).
According to our center's experience, we summarized the "five early" scheme for treating pulmonary infection after SOT. (1) Early discontinuation of immunosuppressants. Immunosuppressants were discontinued immediately after the patient experienced symptoms of hypoxia. During this period, rejection generally does not occur due to patients' excessive immunosuppressive state; (2) early use of glucocorticoids. Glucocorticoids have anti-inflammatory and anti-rejection effects. The dose and frequency of methylprednisolone administration were determined based on the patient's body temperature. The dose was 40–80 mg every 12 h in the early stages. After the body temperature normalized for 3 d, methylprednisolone was gradually reduced to 40 mg qd and then changed to oral methylprednisolone tablets for maintenance; (3) early use of human immunoglobulin. The dosage of human immunoglobulin is 0.5 g/(kg/d), which is usually treated for 3-5 d. The course of treatment and dosage should be adjusted according to the patient’s situation; (4) early combined use of multiple drugs. It can be seen from the ten patients in this study that before the pathogen is determined, the treatment needs to include gram-positive bacteria, gram-negative bacteria, fungi, viruses, and Pneumocystis yersiniae. After identifying the pathogen, select the appropriate anti-infective drugs according to the pathogen (Table 2); and (5) early use of noninvasive ventilation. For patients with hypoxemia on blood gas analysis, the early use of non-invasive ventilation is conducive to reducing respiratory muscle fatigue, improving ventilation function, reducing lung injury, and reducing oxygen consumption. Delayed treatment may aggravate the patient's disease and lead to the use of invasive ventilation, resulting in complications such as ventilator-associated pneumonia. In addition, attention should be paid to increasing the patients' nutrition and treating hypoproteinemia, which is conducive to maintaining blood pressure stability and improving immunity. For those who had used the above measures and were still worsening, carrimycin was used for treatment. The dose of carrimycin was 400 mg orally on the first day and 200 mg orally every d for seven d.
| Recipient | Treatment protocols |
| 1 | Carrimycin + Vancocin + Levofloxacin |
| 2 | Carrimycin + Amphotericin B |
| 3 | Carrimycin + Caspofungin + Trimethoprim-sulfamethoxazole |
| 4 | Carrimycin + Meropenem + Linezolid + Caspofungin |
| 5 | Carrimycin + Ganciclovir + Moxifloxacin + Trimethoprim-sulfamethoxazole + Peramivir |
| 6 | Carrimycin + Caspofungin + Piperacillin sodium and tazobactam sodium + Imipenem + Trimethoprim-sulfamethoxazole |
| 7 | Carrimycin + Meropenem + Azithromycin + Caspofungin |
| 8 | Carrimycin + Moxifloxacin + Ganciclovir + Peramivir + Imipenem + Piperacillin sodium and tazobactam sodium + Tigecycline + Linezolid + Fluconazole |
| 9 | Carrimycin + Caspofungin + Trimethoprim-sulfamethoxazole + Imipenem + Moxifloxacin |
| 10 | Carrimycin + Imipenem + Ganciclovir + Voriconazole |
There were three cases of fungal pneumonia, two cases of bacterial pneumonia, two cases of pneumocystis pneumonia, and three cases with mixed infections (Table 3).
| Recipients | Pathogens |
| 1 | Legionnella + Corynebacterium striatum |
| 2 | Mucor |
| 3 | Pneumocystis jirovecii |
| 4 | Baumanii + Staphylococcus epidermidis |
| 5 | Pneumocystis jirovecii + Cytomegalovirus |
| 6 | Pneumocystis jirovecii |
| 7 | Haemophilus parainfluenzae + Mycoplasma |
| 8 | Acinetobacter + Coronavirus |
| 9 | Acinetobacter |
| 10 | Aspergillus |
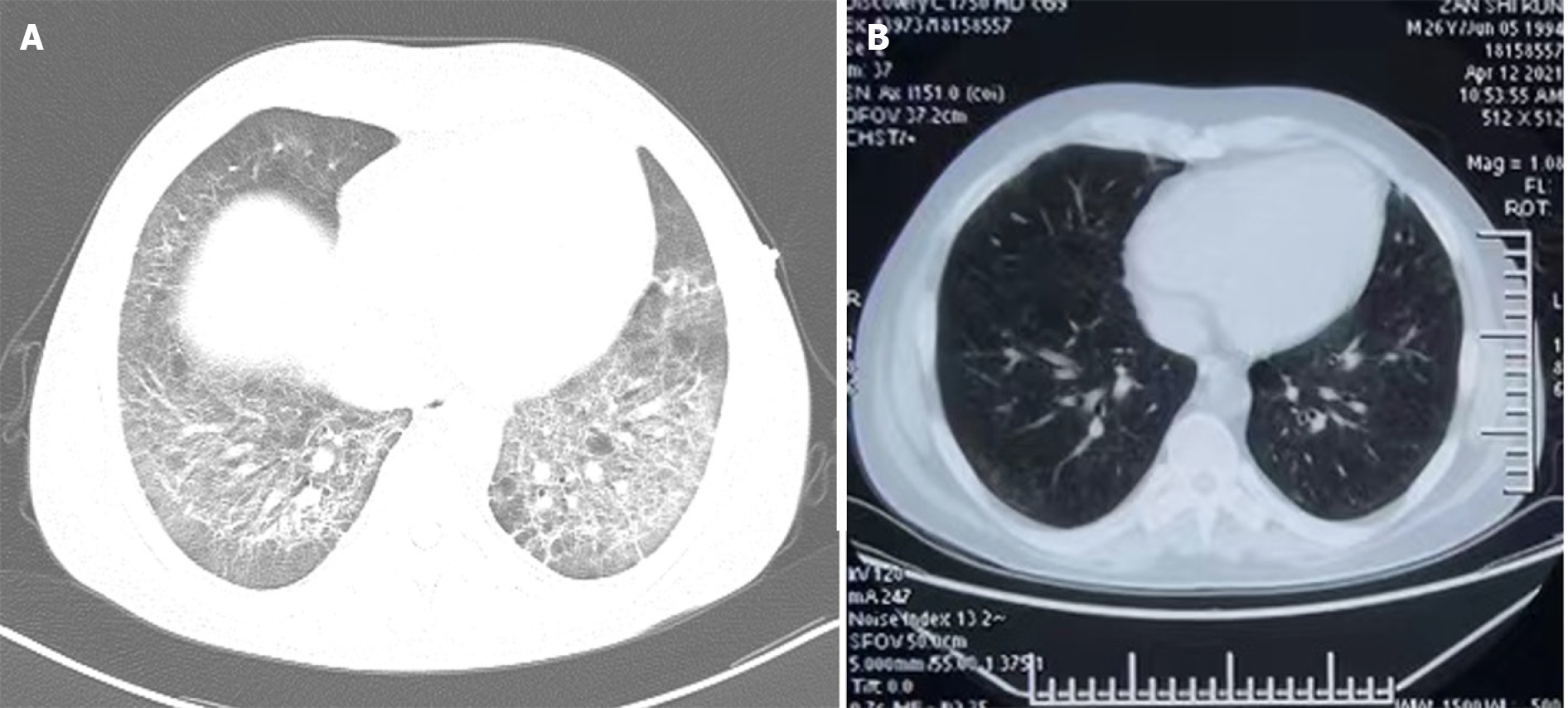
After treatment with carrimycin, the disease in seven patients significantly improved, the course of disease was significantly shortened, fever was quickly controlled, chest computed tomography was significantly improved (Figure 1), and oxygenation was significantly improved. Finally, all anti-infective drugs were gradually discontinued, and the patients were discharged after treatment. One patient died from ARDS, and two patients discontinued treatment (Table 4).
| Recipient | Outcome of transplants | Clinical outcomes |
| 1 | Remove | Abandoning treatment |
| 2 | Normal | Cure |
| 3 | Normal | Death |
| 4 | Normal | Cure |
| 5 | Normal | Cure |
| 6 | Normal | Abandoning treatment |
| 7 | Normal | Cure |
| 8 | Normal | Cure |
| 9 | Normal | Cure |
| 10 | Normal | Cure |
After transplantation, patients require immunosuppressants for anti-rejection therapy. Immunosuppressants include cyclosporine, tacrolimus, prednisone, and mycophenolate mofetil, which can prevent rejection by inhibiting cellular and humoral immunity; however, long-term use of immunosuppressants weakens the immunity of patients. Compared to the general population, infections in SOT recipients are far more complicated and prone to opportunistic infections, mixed infections, rare bacterial infections, and severe infections, making diagnosis more difficult. Delayed diagnosis leads to delayed treatment and poor prognosis[18,19].
Pneumonia is a major threat in renal allograft recipients[20]. According to various studies, pneumonia rates range from 4.5% to 20%[21-28], and pneumonia accounts for 8%–70.3% of all infectious complications[29-32] following kidney transplantation. SP can lead to cellular immune disorders and pro- and anti-inflammatory cytokine imbalances[33]. SP is characterized by occult onset, rapid progress, difficulty in identifying pathogens, and poor treatment effects, and can develop into respiratory failure and even systemic failure in a short time. SP easily develops into ARDS, which is the most direct cause of death in patients with pulmonary infection after renal transplantation[34]. Bacteria are the most common pathogens causing infections after SOT, particularly in the early post-transplantation period[35,36]. In addition to bacterial infections, many other pathogens, such as viruses, fungi, Mycoplasma, and Pneumocystis jirovecii, can also cause pneumonia. With the application of next generation sequencing technology, bronchoalveolar lavage, and other technologies, the technology for identifying pathogens is improving; however, some patients are still unable to identify pathogens in a short time, resulting in disease progression and even death.
In terms of treatment, we generally use high-efficiency broad-spectrum antibiotics after admission, combined with antiviral, anti-Pneumocystis yersinia, and antifungal drugs. However, multiple previous hospitalizations, invasive interventions, and prior antibiotic use make SOT recipients susceptible to colonization by nosocomial and antibiotic-resistant pathogens, including Methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and resistant gram-negative bacilli[37-39]. Patients with SP often exhibit anorexia, and the body is in a highly decomposed state, resulting in malnutrition and a further reduction in immune function. Therefore, conventional drugs typically have poor efficacy. When patients are infected, many inflammatory mediators and cytokines released during the inflammatory reaction cause acute lung injury, which can cause ARDS and even lead to systemic inflammatory response syndrome and MODS[40]. Although various treatment measures have been adopted, the condition of some patients cannot be effectively controlled. Therefore, there is an urgent need to identify drugs with good antibacterial activity and low biotoxicity.
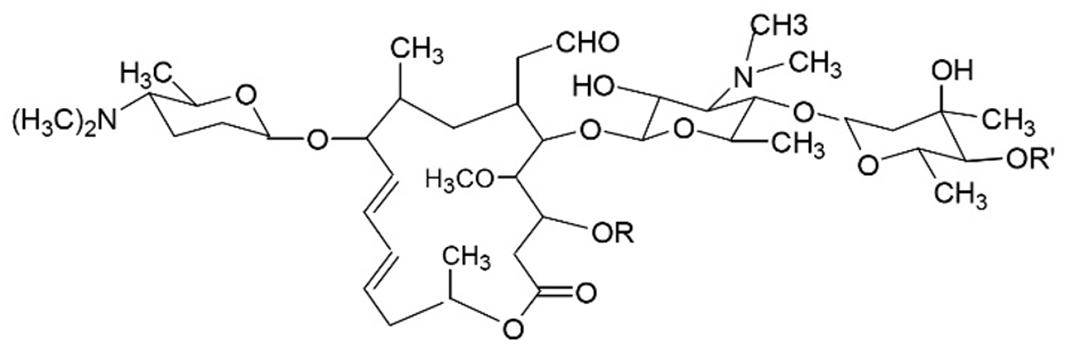
Carrimycin is a new class I drug developed by the Institute of Pharmaceutical Biotechnology of the Chinese Academy of Medical Sciences. Shenyang Tonglian Group Co., Ltd. Carrimycin was approved for listing in June 2019. It is a new drug with independent intellectual property rights in China. Carrimycin is a genetically engineered drug developed using synthetic biological technology and belongs to the macrolide class of antibiotics. The main components of carrimycin were isovalerylspiramycin III, II, and I (Figure 2), with minor components consisting of butyrylspiramycin III, propionylspiramycin III, acetylspiramycin III, butyrylspiramycin II, propionylspiramycin II, acetylspiramycin II[41-44]. Compared with acylspiramycin, the longer alkyl chains at position 4’’ render carrimycin increased antibacterial potency, especially in vivo, because of its higher lipophilicity[45,46].
The China Food and Drug Administration approved carrimycin for the treatment of acute tracheal bronchitis caused by Haemophilus influenzae, Streptococcus pneumoniae and acute sinusitis caused by Streptococcus pneumoniae, Haemophilus influenzae, Streptococcus pyogenes, Moraxella catarrhalis, and Staphylococcus. Carrimycin can inhibit bacterial protein synthesis by blocking the activity of peptidyl transferase in the 50s ribosome, which can directly inhibit bacterial growth. Carrimycin can combine with peroxide-scavenging enzymes to induce peroxide and destroy biological macromolecules such as DNA to achieve sterilization (Table 5). In vivo, carrimycin can promote neutrophils to migrate to inflammatory sites to destroy and phagocytize bacteria and damaged cells and enhance the phagocytosis of macrophages to regulate immunity and enhance resistance. In addition, capreomycin also plays a certain role in inhibiting inflammation and improving inflammation and edema of important organs such as the heart and lungs. A pharmacokinetic study of carrimycin confirmed that its half-life was longer than that of spiramycin, and some metabolites of carrimycin maintained antibacterial activity. The tissue permeability of carrimycin is enhanced, and it has a marked post-antibiotic effect[46]. Carrimycin has broad prospects in clinical applications, but there has been no research on the application of carrimycin in SP following SOT.
| Pathogens | Antimicrobial spectrum |
| Gram-positive bacterium | Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus digestae, Other Streptococcus, Staphylococcus aureus, Legionella pneumophila |
| Gram-negative bacterium | Haemophilus influenzae, Moraxella catarrh, Neisseria gonorrhoeae |
| Anaerobe | Digestococcus, Bacteroid, Clostridium, Prevotella, Propionibacterium |
| Atypical pathogens | Mycoplasma pneumoniae, Ureaplasma urealyticum, Chlamydia trachomatis, Chlamydia pneumonia |
Based on our previous observations, we found that the use of carrimycin in patients with low immune function often achieved unexpected results. The specific mechanism is unclear, which may be related to clindamycin improving T lymphocyte function and repairing immune damage. Therefore, we administered carrimycin to patients receiving long-term oral immunosuppressants after organ transplantation. The ten patients in this study were treated with carrimycin following poor response to other drugs. Carrimycin treatment was found to be effective. It is speculated that this is related to the direct bactericidal and bacteriostatic effects of carrimycin, which may also be related to the effects of carrimycin in improving immune function and repairing tissue damage. Our center will further study the specific mechanisms of action of carrimycin in improving the immune function of patients.
In addition, among the ten patients treated with carrimycin, case 5 was complicated by cytomegalovirus infection, and case 8 was complicated by coronavirus infection. After treatment with carrimycin, these patients showed positive effects, suggesting that carrimycin may have antiviral effects. Our results are consistent with those of previous studies, which have found that the main active component of carrimycin strongly binds the angiotensin-converting enzyme type 2 receptor protein and can also bind to the 3-chymotrypsin-like protease binding site; therefore, it has great potential value and unique therapeutic advantages in anti-Severe acute respiratory syndrome coronavirus 2 infection[13]. However, further studies are required to confirm the antiviral effects of carrimycin.
This study has some limitations. As our study had only a comparatively small number of samples from a single center, more studies need to verify the general applicability of our research conclusions. In addition, more research is needed on the timing, course of treatment, and antibacterial spectrum of carrimycin for treating pulmonary infections in SOT recipients. Our center will continue to collect clinical data on carrimycin in treating SP in SOT recipients to make a more reliable evidence-based evaluation of its efficacy and safety and provide new medication options for treating SP in SOT recipients.
This study found that carrimycin is safe and effective for treating SP in SOT recipients. Because carrimycin is a newly developed drug in China, many current experimental studies are based on basic and animal trials. The efficacy and safety of carrimycin in clinical treatment require further confirmation of clinical data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koerkamp BG, Netherlands S-Editor: Liu H L-Editor: A P-Editor: Yu HG
| 1. | Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:961-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 2. | Dulek DE, Mueller NJ; AST Infectious Diseases Community of Practice. Pneumonia in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Wilmes D, Coche E, Rodriguez-Villalobos H, Kanaan N. Bacterial pneumonia in kidney transplant recipients. Respir Med. 2018;137:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Dong B, Wang Y, Wang G, Wang W, Zhou H, Fu Y. A retrospective study of cytomegalovirus pneumonia in renal transplant patients. Exp Ther Med. 2014;7:1111-1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Shih CJ, Tarng DC, Yang WC, Yang CY. Immunosuppressant dose reduction and long-term rejection risk in renal transplant recipients with severe bacterial pneumonia. Singapore Med J. 2014;55:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Shorr AF, Abbott KC, Agadoa LY. Acute respiratory distress syndrome after kidney transplantation: epidemiology, risk factors, and outcomes. Crit Care Med. 2003;31:1325-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Canet E, Osman D, Lambert J, Guitton C, Heng AE, Argaud L, Klouche K, Mourad G, Legendre C, Timsit JF, Rondeau E, Hourmant M, Durrbach A, Glotz D, Souweine B, Schlemmer B, Azoulay E. Acute respiratory failure in kidney transplant recipients: a multicenter study. Crit Care. 2011;15:R91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Candan S, Pirat A, Varol G, Torgay A, Zeyneloglu P, Arslan G. Respiratory problems in renal transplant recipients admitted to intensive care during long-term follow-up. Transplant Proc. 2006;38:1354-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Kirilov D, Cohen J, Shapiro M, Grozovski E, Singer P. The course and outcome of renal transplant recipients admitted to a general intensive care unit. Transplant Proc. 2003;35:606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Sun Q, Liu ZH, Chen J, Ji S, Tang Z, Cheng Z, Ji D, Li LS. An aggressive systematic strategy for acute respiratory distress syndrome caused by severe pneumonia after renal transplantation. Transpl Int. 2006;19:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Tu GW, Ju MJ, Zheng YJ, Zhu DM, Xu M, Rong RM, Zhu TY, Luo Z. An interdisciplinary approach for renal transplant recipients with severe pneumonia: a single ICU experience. Intensive Care Med. 2014;40:914-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Capulong MG, Mendoza M, Chavez J. Cytomegalovirus pneumonia in renal transplant patients. Transplant Proc. 1998;30:3151-3153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Yan H, Sun J, Wang K, Wang H, Wu S, Bao L, He W, Wang D, Zhu A, Zhang T, Gao R, Dong B, Li J, Yang L, Zhong M, Lv Q, Qin F, Zhuang Z, Huang X, Yang X, Li Y, Che Y, Jiang J. Repurposing carrimycin as an antiviral agent against human coronaviruses, including the currently pandemic SARS-CoV-2. Acta Pharm Sin B. 2021;11:2850-2858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Liang SY, Zhao TC, Zhou ZH, Ju WT, Liu Y, Tan YR, Zhu DW, Zhang ZY, Zhong LP. Anti-tumor effect of carrimycin on oral squamous cell carcinoma cells in vitro and in vivo. Transl Oncol. 2021;14:101074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3978] [Cited by in RCA: 4253] [Article Influence: 236.3] [Reference Citation Analysis (0)] |
| 16. | Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 481] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 17. | Rubin RH, Ikonen T, Gummert JF, Morris RE. The therapeutic prescription for the organ transplant recipient: the linkage of immunosuppression and antimicrobial strategies. Transpl Infect Dis. 1999;1:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Xu Y, Jin T. Manipulating cell motility by Legionella: Speeding up or slowing down? J Transl Int Med. 2023;11:24-25. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Xu Z, Mo X, Kong Y, Wen Q, Han T, Lyu M, Xu L, Chang Y, Zhang X, Huang X, Wang Y. Mini-dose methotrexate combined with methylprednisolone as a first-line treatment for acute graft-versus-host disease: A phase 2 trial. J Transl Int Med. 2023;11:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 20. | Jackson KR, Motter JD, Bae S, Kernodle A, Long JJ, Werbel W, Avery R, Durand C, Massie AB, Desai N, Garonzik-Wang J, Segev DL. Characterizing the landscape and impact of infections following kidney transplantation. Am J Transplant. 2021;21:198-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Yıldırım F, Karaman İ, Kaya A. Current situation in ARDS in the light of recent studies: Classification, epidemiology and pharmacotherapeutics. Tuberk Toraks. 2021;69:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 22. | Shah A, Shah M. Advancement of deep learning in pneumonia/Covid-19 classification and localization: A systematic review with qualitative and quantitative analysis. Chronic Dis Transl Med. 2022;8:154-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Pourmand G, Salem S, Mehrsai A, Taherimahmoudi M, Ebrahimi R, Pourmand MR. Infectious complications after kidney transplantation: a single-center experience. Transpl Infect Dis. 2007;9:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | García-Prado ME, Cordero E, Cabello V, Pereira P, Torrubia FJ, Ruíz M, Cisneros JM. [Infectious complications in 159 consecutive kidney transplant recipients]. Enferm Infecc Microbiol Clin. 2009;27:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Veroux M, Giuffrida G, Corona D, Gagliano M, Scriffignano V, Vizcarra D, Tallarita T, Zerbo D, Virgilio C, Sciacca A, Cappello D, Stefani S, Veroux P. Infective complications in renal allograft recipients: epidemiology and outcome. Transplant Proc. 2008;40:1873-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Charfeddine K, Zaghden S, Kharrat M, Kamoun K, Jarraya F, Hachicha J. Infectious complications in kidney transplant recipients: a single-center experience. Transplant Proc. 2005;37:2823-2825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Kalra V, Agarwal SK, Khilnani GC, Kapil A, Dar L, Singh UB, Mirdha BR, Xess I, Gupta S, Bhowmik D, Tiwari SC, Dash SC. Spectrum of pulmonary infections in renal transplant recipients in the tropics: a single center study. Int Urol Nephrol. 2005;37:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Sileri P, Pursell KJ, Coady NT, Giacomoni A, Berliti S, Tzoracoleftherakis E, Testa G, Benedetti E. A standardized protocol for the treatment of severe pneumonia in kidney transplant recipients. Clin Transplant. 2002;16:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | de Carvalho MA, Freitas FG, Silva Junior HT, Bafi AT, Machado FR, Pestana JO. Mortality predictors in renal transplant recipients with severe sepsis and septic shock. PLoS One. 2014;9:e111610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Bige N, Zafrani L, Lambert J, Peraldi MN, Snanoudj R, Reuter D, Legendre C, Chevret S, Lemiale V, Schlemmer B, Azoulay E, Canet E. Severe infections requiring intensive care unit admission in kidney transplant recipients: impact on graft outcome. Transpl Infect Dis. 2014;16:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Alangaden GJ, Thyagarajan R, Gruber SA, Morawski K, Garnick J, El-Amm JM, West MS, Sillix DH, Chandrasekar PH, Haririan A. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant. 2006;20:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 279] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 32. | Mouloudi E, Massa E, Georgiadou E, Iosifidis E, Katsika E, Rembelakos G, Gakis D, Imvrios G, Papanikolaou V, Papadopoulos S, Gritsi-Gerogianni N. Infections related to renal transplantation requiring intensive care admission: a 20-year study. Transplant Proc. 2012;44:2721-2723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Dukhinova M, Kokinos E, Kuchur P, Komissarov A, Shtro A. Macrophage-derived cytokines in pneumonia: Linking cellular immunology and genetics. Cytokine Growth Factor Rev. 2021;59:46-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Lee I, Barton TD. Viral respiratory tract infections in transplant patients: epidemiology, recognition and management. Drugs. 2007;67:1411-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Garzoni C; AST Infectious Diseases Community of Practice. Multiply resistant gram-positive bacteria methicillin-resistant, vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus (MRSA, VISA, VRSA) in solid organ transplant recipients. Am J Transplant. 2009;9 Suppl 4:S41-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Shafiekhani M, Mirjalili M, Vazin A. Prevalence, Risk Factors And Treatment Of The Most Common Gram-Negative Bacterial Infections In Liver Transplant Recipients: A Review. Infect Drug Resist. 2019;12:3485-3495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | van Delden C, Blumberg EA; AST Infectious Diseases Community of Practice. Multidrug resistant gram-negative bacteria in solid organ transplant recipients. Am J Transplant. 2009;9 Suppl 4:S27-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Cervera C, van Delden C, Gavaldà J, Welte T, Akova M, Carratalà J; ESCMID Study Group for Infections in Compromised Hosts. Multidrug-resistant bacteria in solid organ transplant recipients. Clin Microbiol Infect. 2014;20 Suppl 7:49-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 39. | Shafiekhani M, Karimzadeh I, Nikeghbalian S, Firoozifar M, Pouladfar G, Vazin A. Comparison of Ceftizoxime Plus Ampicillin-Sulbactam versus Gentamicin Plus Ampicillin-Sulbactam in the Prevention of Post-Transplant Early Bacterial Infections in Liver Transplant Recipients: A Randomized Controlled Trial. Infect Drug Resist. 2020;13:89-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Cao H, Huang J, Chang J, Zhu Y, Liang J, Sun C, Lin J. Predictors of progression in idiopathic inflammatory myopathies with interstitial lung disease. J Transl Int Med. 2023;11:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 41. | Lu Z, Zhang X, Dai J, Wang Y, He W. Engineering of leucine-responsive regulatory protein improves spiramycin and bitespiramycin biosynthesis. Microb Cell Fact. 2019;18:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Sano M, Sunazuka T, Tanaka H, Yamashita K, Okachi R, Omura S. Chemical modification of spiramycins. VI. Synthesis and antibacterial activities of 3,3''-di-O-acyl-4''-O-sulfonyl and 3,3''-di-O-acyl-4''-O-alkyl derivatives of spiramycin I. J Antibiot (Tokyo). 1985;38:1350-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Epp JK, Huber ML, Turner JR, Goodson T, Schoner BE. Production of a hybrid macrolide antibiotic in Streptomyces ambofaciens and Streptomyces lividans by introduction of a cloned carbomycin biosynthetic gene from Streptomyces thermotolerans. Gene. 1989;85:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Guangdong S, Jianlu D, Yiguang W. Construction and physiological studies on a stable bioengineered strain of shengjimycin. J Antibiot (Tokyo). 2001;54:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Shi XG, Sun YM, Zhang YF, Zhong DF. Tissue distribution of bitespiramycin and spiramycin in rats. Acta Pharmacol Sin. 2004;25:1396-1401. [PubMed] |
| 46. | Shi XG, Fawcett JP, Chen XY, Zhong DF. Structural identification of bitespiramycin metabolites in rat: a single oral dose study. Xenobiotica. 2005;35:343-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |