Published online May 16, 2024. doi: 10.12998/wjcc.v12.i14.2451
Revised: March 13, 2024
Accepted: April 3, 2024
Published online: May 16, 2024
Processing time: 74 Days and 22 Hours
Awake fiberoptic nasotracheal intubation (AFNI) is the preferred airway ma
A 63-year-old female with a history of right maxillary osteosarcoma underwent craniotomy for a suspected malignant brain lesion. The patient’s medical history included prior surgery, chemotherapy, and radiation therapy, resulting in signi
The SPG block represents a promising analgesic approach in AFNI, offering po
Core Tip: This is the first clinical case report of the application of a sphenopalatine ganglion (SPG) block for awake fiberoptic nasotracheal intubation (AFNI). The SPG block provided sufficient analgesia during AFNI. This case report suggests that an alternative analgesic modality for AFNI is the most reasonable option for airway management.
- Citation: Kang H, Park S, Jin Y. Ultrasound-guided sphenopalatine ganglion block for effective analgesia during awake fiberoptic nasotracheal intubation: A case report. World J Clin Cases 2024; 12(14): 2451-2456
- URL: https://www.wjgnet.com/2307-8960/full/v12/i14/2451.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i14.2451
Patients with malignant oral tumors who undergo surgical intervention and chemoradiotherapy often experience pro
AFNI is considered a safe and effective alternative approach for patients with significant airway compromise owing to oral surgery[2]. This method enables the preservation of spontaneous breathing, thereby minimizing potential complications such as critical desaturation due to difficulties with mask ventilation[3]. However, AFNI causes considerable physical and psychological distress. In addition, reflexive movements triggered by stimulation can occur, necessitating an appropriate response. Nerve blocks aimed at alleviating pain in the vocal cords and lower airways have been developed and widely used in clinical settings[4].
Blocks of the superior and recurrent laryngeal nerves have been used to alleviate discomfort in the laryngeal region during intubation[4]. Transcricothyroid membrane blocks have been used to induce anesthesia in the subglottic region. However, challenges have arisen during AFNI, particularly regarding the associated pain and discomfort in the upper airway and nasopharyngeal regions. This case report proposes that using an ultrasound-guided sphenopalatine ganglion (SPG) block when performing AFNI is effective in alleviating pain and discomfort, particularly in the upper airway and nasopharyngeal regions.
A 63-year-old female (height, 153 cm; weight, 42 kg) was scheduled for craniotomy for a suspected malignant brain lesion.
The patient was diagnosed with right maxillary osteosarcoma 28 years ago and underwent radical orbitomaxillectomy, chemotherapy, and radiotherapy. However, disease recurrence prompted further surgical interventions, including man
The patient’s past medical history revealed only hypertension.
She denied any other medical history or family history of medical issues.
The patient presented with persistent complications secondary to her prior treatments, including significant jaw im
The patient’s arterial blood gas analysis showed mild respiratory acidosis with CO2 retention, as follows: pH, 7.37; PaCO2, 47.3 mmHg; PaO2, 87.0 mmHg; HCO3 concentration, 25.7 mmol/L; base excess, 1.5 mmol/L; and oxygen saturation, 96.7%. Considering the patient’s general condition, pulmonary function tests were not performed. The results of all other laboratory tests, including blood and urine analyses, were within acceptable ranges.
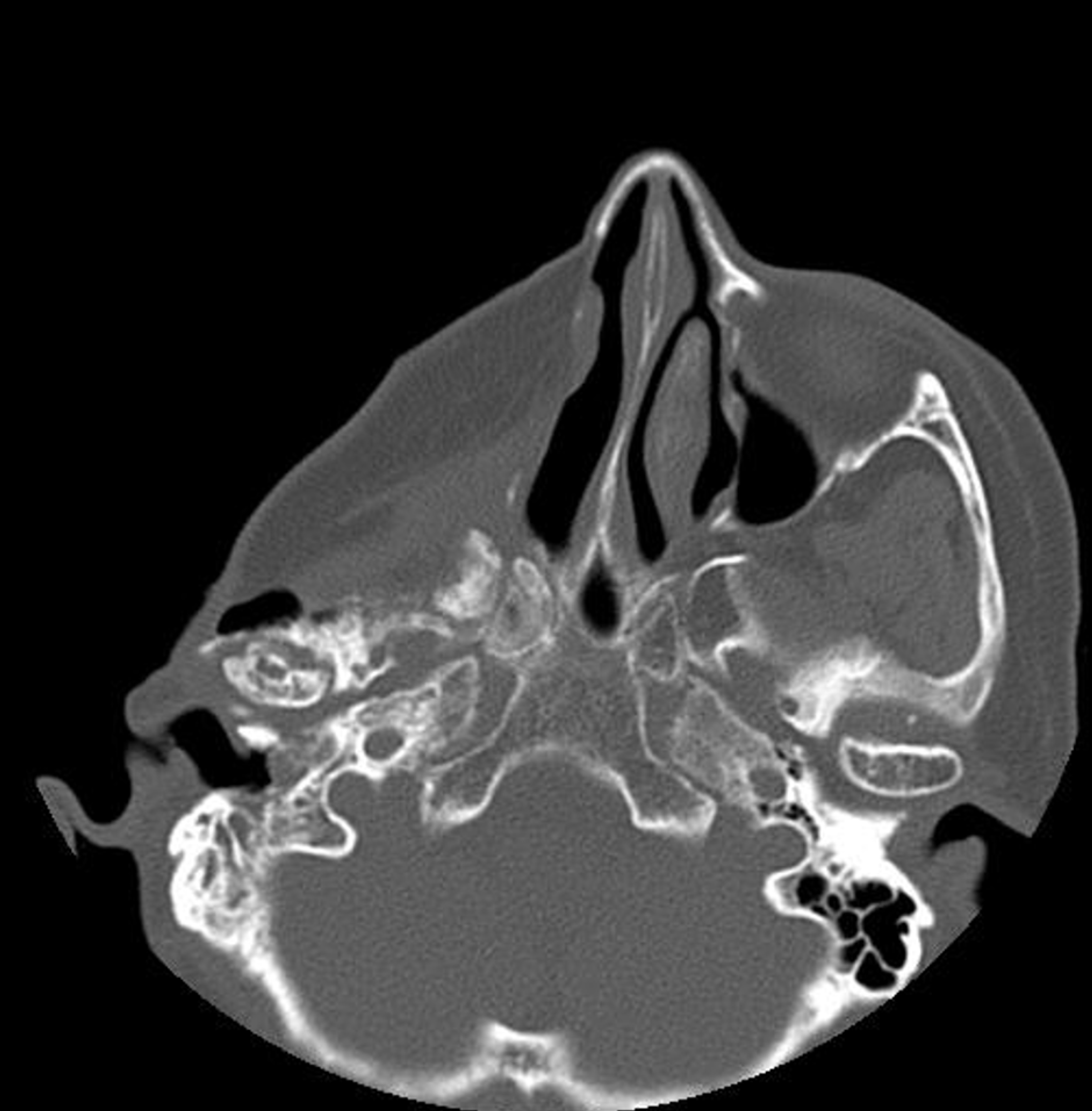
Preoperative brain computed tomography (CT) revealed osteosarcoma recurrence along the inner cortex of the right frontotemporal craniotomy site, accompanied by chronic otomastoiditis on the right side. In addition, CT findings in
Due to restricted mouth opening and limited neck extension, difficulty in airway management or ventilation was anti
Informed consent regarding the potential risks of anesthesia was obtained from the patient and her family. The patient was admitted to our hospital without premedication. Upon entering the operating room, routine monitoring measures, such as pulse oximetry, electrocardiography, noninvasive blood pressure measurements, bispectral index, and capno
The patient’s vital signs measured immediately after admission were as follows: Blood pressure, 126/73 mmHg; heart rate, 73 beats/min; and oxygen saturation, 97% on room air. To prevent hypoxemia, oxygen was administered for several minutes through a high-flow nasal cannula (Optiflow, Fisher & Paykel Healthcare). An intravenous injection of 0.2 mg of glycopyrrolate was administered, as a form of premedication, to decrease saliva production. Additionally, 2 mg of mi
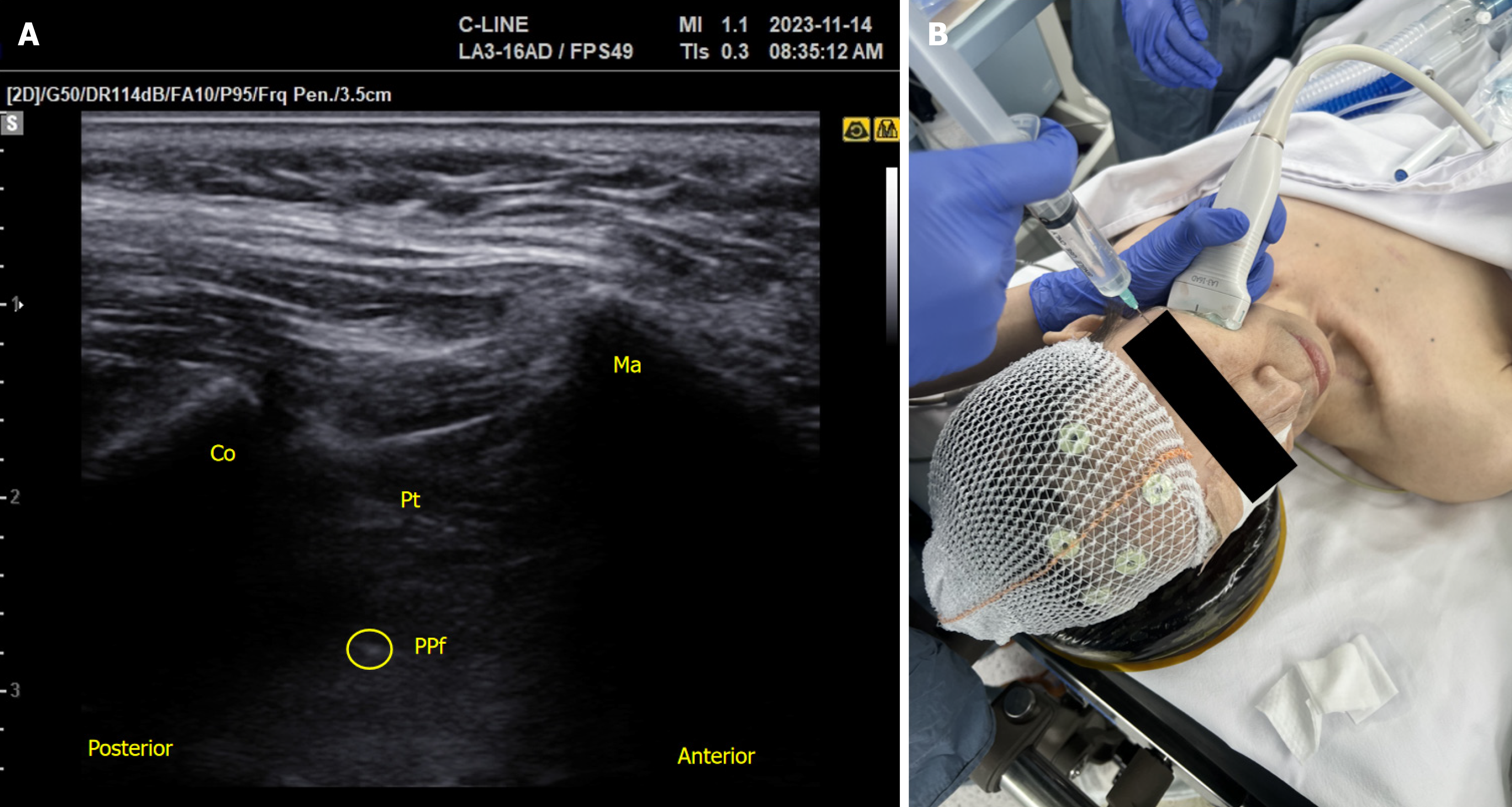
Using an out-of-plane approach with a 25-gauge, 2-inch needle, a left SPG block was performed under real-time ul
Through the cricothyroid membrane puncture, we administered a 5 mL bolus of 2% lidocaine into the trachea. Oral gargling was performed with 10 mL of 4% lidocaine. Nasal intubation was performed using a 6.5 mm nasal endotracheal tube with a preloaded flexible bronchoscope. The nasotracheal tube was seamlessly inserted into the left nostril without the patient coughing or gagging. The patient did not complain of pain or discomfort as the nasotracheal tube passed through the left nostril. We verified the accurate positioning of the nasotracheal tube by observing the carina and its tips and subsequently withdrawing the bronchoscope.
Once the presence of an end-tidal carbon dioxide waveform was confirmed, anesthesia was induced using propofol and remifentanil, followed by the intravenous administration of 40 mg of rocuronium. Propofol and remifentanil-based total intravenous anesthesia with target-controlled infusion was administered to maintaingeneral anesthesia. The patient received mechanical ventilation with a fresh gas flow of 3 L/min consisting of 50% oxygen mixed with ambient air. Arterial cannulation of the right radial artery and central venouscannulation of the right internal jugular vein were performed.
The surgery was extended over a 6 h duration while maintaining stable vital signs. Although spontaneous breathing resumed postoperatively, nasotracheal intubation was maintained for half a day as a precaution against the anticipated airway edema. On the following day, the patient was extubated. She did not complain of nasopharyngeal pain or epis
SPG block is commonly used to alleviate pain in the nasopharyngeal region. Its effectiveness has been demonstrated in the treatment of post-dural puncture headaches, migraines, and various facial pain syndromes, as well as in the setting of endoscopic sinus surgery[5,7-9]. The application of this procedure during AFNI proved beneficial, such that the effective reduction of patient discomfort and pain in the nasal cavity and pharynx facilitated tube insertion without compromising airway integrity.
SPG, also referred to as the pterygopalatine ganglion, is located within the cranial division of the autonomic nervous system and has distinctive features[7]. It interconnects with three main neural pathways, the somatosensory, sympathetic, and parasympathetic systems, making it well-suited for addressing various painful conditions affecting the facial and cranial regions[7].
Anatomically, the maxillary division of the trigeminal nerve courses through the foramen rotundum and proceeds anteriorly through the PPF[10]. The efferent branches of the SPG form the nasopalatine nerve, the posterior, superior, and inferior lateral nasal branches, and the pharyngeal nerves; additionally, the SPG is directly connected to the greater and lesser palatine nerves. Sensory nerve fibers arising from the maxillary nerve traverse the SPG, facilitating the sensory innervation of the nasal mucosa, palate, and pharyngeal areas. SPG blocks modulate the transmission of pain signals by suppressing the activity of these sensory nerves.
The sympathetic pathway of the SPG originates from the superior cervical ganglion, following a trajectory through the internal carotid plexus as the deep petrosal nerve. This nerve merges with the greater petrosal nerve, forming the pterygoid canal nerve, also known as the vidian nerve[10]. Vasoconstrictive innervation of the nasal cavity, upper pharynx, and palate is supplied by the postganglionic sympathetic fibers that pass through the SPG[10]. The parasympathetic pathway originates from the superior salivatory nucleus (SSN) within the brainstem and extends towards the SPG. Efferent fibers from the SSN traverse via the nervus intermedius, combining to form the greater petrosal nerve, which contributes to the parasympathetic innervation of the SPG[8,10]. This pathway stimulates the secretory function of the nasal cavity, pharyngeal mucosa, and lacrimal and palatine glands[8]. Therefore, through its effects on the sympa
SPG block is commonly performed using transnasal and percutaneous approaches. In contrast to the transnasal approach, the percutaneous approach offers the advantage of delivering medication directly to the SPG without encountering barriers such as the nasal mucosa, sphenopalatine foramen, and fat tissue before reaching the PPF[7,11]. The transnasal method results in inconsistent coverage of the contents within the PPF, leading to fewer enduring outcomes[11].
A superior laryngeal nerve block was not performed in this case, which could be considered a limitation. The superior laryngeal nerve innervates the cricothyroid muscle, whereas the recurrent laryngeal nerve innervates the remaining muscles[12]. Although we can reduce reflective contractions by performing a translaryngeal block targeting the recurrent laryngeal nerve, this may be inadequate as the cricothyroid muscle of the larynx would remain unaffected. Overall, this was not deemed essential for awake intubation, and no disruptive reflexes were observed in our patient during the procedure.
In conclusion, when performing AFNI, the implementation of a nerve block strategy such as the SPG block improves patient cooperation and minimizes physical and mental pain without compromising airway integrity. Further clinical studies are needed to investigate the comparative outcomes and establish optimized protocols.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grawish ME, Egypt; Yuan HJ, China S-Editor: Zheng XM L-Editor: A P-Editor: Xu ZH
| 1. | Nikhar SA, Sharma A, Ramdaspally M, Gopinath R. Airway Management of Patients Undergoing Oral Cancer Surgery: A Retrospective Analysis of 156 Patients. Turk J Anaesthesiol Reanim. 2017;45:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | El-Boghdadly K, Onwochei DN, Cuddihy J, Ahmad I. A prospective cohort study of awake fibreoptic intubation practice at a tertiary centre. Anaesthesia. 2017;72:694-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Cabrini L, Baiardo Redaelli M, Ball L, Filippini M, Fominskiy E, Pintaudi M, Putzu A, Votta CD, Sorbello M, Antonelli M, Landoni G, Pelosi P, Zangrillo A. Awake Fiberoptic Intubation Protocols in the Operating Room for Anticipated Difficult Airway: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Anesth Analg. 2019;128:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Pani N, Kumar Rath S. Regional & topical anaesthesia of upper airways. Indian J Anaesth. 2009;53:641-648. [PubMed] |
| 5. | Smith CR, Dickinson KJ, Carrazana G, Beyer A, Spana JC, Teixeira FJP, Zamajtuk K, Maciel CB, Busl KM. Ultrasound-Guided Suprazygomatic Nerve Blocks to the Pterygopalatine Fossa: A Safe Procedure. Pain Med. 2022;23:1366-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Cometa MA, Zasimovich Y, Smith CR. Sphenopalatine ganglion block: do not give up on it just yet! Br J Anaesth. 2021;126:e198-e200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Piagkou M, Demesticha T, Troupis T, Vlasis K, Skandalakis P, Makri A, Mazarakis A, Lappas D, Piagkos G, Johnson EO. The pterygopalatine ganglion and its role in various pain syndromes: from anatomy to clinical practice. Pain Pract. 2012;12:399-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Binfalah M, Alghawi E, Shosha E, Alhilly A, Bakhiet M. Sphenopalatine Ganglion Block for the Treatment of Acute Migraine Headache. Pain Res Treat. 2018;2018:2516953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Wang P. The efficacy of sphenopalatine ganglion block for pain management after endoscopic sinus surgery: a meta-analysis of randomized controlled studies. Eur Arch Otorhinolaryngol. 2021;278:2681-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Robbins MS, Robertson CE, Kaplan E, Ailani J, Charleston L 4th, Kuruvilla D, Blumenfeld A, Berliner R, Rosen NL, Duarte R, Vidwan J, Halker RB, Gill N, Ashkenazi A. The Sphenopalatine Ganglion: Anatomy, Pathophysiology, and Therapeutic Targeting in Headache. Headache. 2016;56:240-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Anthony Cometa M, Zasimovich Y, Smith CR. Percutaneous sphenopalatine ganglion block: an alternative to the transnasal approach. Int J Obstet Anesth. 2021;45:163-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Wada N, Furutani A, Tokumine J, Nakazawa H, Shimazu K, Yorozu T. Ultrasound-Guided Glossopharyngeal Nerve Block for an Awake Intubation in a Patient Predicted to Have a Difficult Airway: A Case Report. A A Pract. 2023;17:e01682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |