Published online Apr 16, 2024. doi: 10.12998/wjcc.v12.i11.1900
Peer-review started: January 15, 2024
First decision: February 23, 2024
Revised: March 4, 2024
Accepted: March 20, 2024
Article in press: March 20, 2024
Published online: April 16, 2024
Processing time: 86 Days and 19.3 Hours
The coexistence of venous thromboembolism (VTE) within patients with cancer, known as cancer-associated thrombosis (CAT), stands as a prominent cause of mortality in this population. Over recent years, the incidence of VTE has demonstrated a steady increase across diverse tumor types, influenced by several factors such as patient management, tumor-specific risks, and treatment-related aspects. Furthermore, mutations in specific genes have been identified as potential contributors to increased CAT occurrence in particular cancer subtypes. We conducted an extensive review encompassing pivotal historical and ongoing studies on CAT. This review elucidates the risks, mechanisms, reliable markers, and risk assessment methodologies that can significantly guide effective interventions in clinical practice.
Core Tip: Treatment-related risks involve therapies such as chemotherapy, endocrine therapy, angiogenesis inhibitors, immunotherapy, protein kinase inhibitors, blood transfusions, and cell line stimulants, all contributing to venous thromboembolism. This review summarizes the pathogenesis of cancer-associated thrombosis and treatment approaches for this condition. This review elucidates the risks, mechanisms, reliable markers, and risk assessment methodologies that can significantly guide effective interventions in clinical practice.
- Citation: Wang TF, Chen Q, Deng J, Li SL, Xu Y, Ma SX. Research progress on venous thrombosis development in patients with malignant tumors. World J Clin Cases 2024; 12(11): 1900-1908
- URL: https://www.wjgnet.com/2307-8960/full/v12/i11/1900.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i11.1900
With demographic shifts and an aging population, the global incidence of tumors has steadily risen, establishing malignant tumors as a leading cause of disease mortality in the 21st century[1-3]. Cancer-associated thrombosis (CAT) stands out as a common complication of malignant tumors, affecting up to approximately 20% of individuals, according to relevant studies[4,5]. The risk of CAT is multifaceted, lacking a singular predictive risk factor or biomarker for its occurrence[6,7]. The correlation between venous thrombosis and malignancy was initially suggested by Baptiste Builaud, and later confirmed 44 years afterward by the French physician Armand Trousseau[8,9]. Among CAT patients, the risk of tumor recurrence and bleeding post-anticoagulation is notably higher than patients without tumor. Specifically, the risk of venous thromboembolism (VTE) recurrence is three times higher than that in the general population without tumor, while the risk of bleeding escalates three to six times higher than in the population without tumor[10].
The risk of recurrent VTE (rVTE) in patients with tumor significantly amplifies within one month of experiencing VTE[11]. Numerous factors may influence the risk of CAT, including patient-related, tumor-related, and treatment-related risk factors[12]. Patient factors include age, gender, smoking, alcohol consumption, obesity, and nutritional requirements[13]. Tumor-related risks are closely associated with the type and stage of malignant tumors, with brain tumors[14], pancreatic cancer[15], and gastric carcinoma[16]posing the highest CAT risk, followed by lung[17], liver[18], ovarian cancers[19], and certain hematologic tumors such as multiple myeloma[20] and acute leukemia[20].
Treatment-related risks involve therapies such as chemotherapy, endocrine therapy, angiogenesis inhibitors, immunotherapy, protein kinase inhibitors, blood transfusions, and cell line stimulants, all contributing to VTE. Chemotherapy, notably, elevates thrombotic events nearly sevenfold compared with patients with cancer who do not undergo chemotherapy[21,22]. Additionally, other treatments (e.g., surgeries) and factors related to treatment (e.g., hospitalization and central venous catheters) heighten VTE risk in patients with cancer.
The paradigm of cancer treatment has undergone a significant shift in the past decade with the emergence of precision medicine and the development of various targeted therapies[23]. As medical care quality has improved, cancer patient survival rates have seen a proportional increase, leading to the emergence of new CAT risk groups. The GARFIELDVTE study revealed that 10.1% of patients had an active tumor upon VTE diagnosis, and patients with active tumors exhibited higher rates of mortality, rVTE, and major bleeding than patients without tumor[24]. A recent study involving 150000 cancer diagnoses during 2006-2007 identified approximately 7200 cases of VTE, showcasing substantial variations in CAT prevalence based on patient characteristics, follow-up duration, and detection/reporting methods for venous thrombosis[25]. A registry study in Denmark assessed the survival duration of patients with general tumors vs CAT tumors, demonstrating that CAT patients had a notably lower 1-year survival rate, at least 24% lower than those with general tumors, and a 11% higher rate of distant metastases than patients without CAT tumors[26]. Intriguingly, this study also illustrated that patients with bilateral deep vein thrombosis (DVT) had lower survival rates than those with unilateral DVT, with a 2-year survival rate of approximately 70% for unilateral proximal DVT, 64% for bilateral proximal DVT, and 66% for bilateral distal proximal DVT[27].
Although certain factors such as patient age, gender, history of VTE, and various metastatic diseases have been recognized as predictive factors in some studies, the comprehensive understanding of risk factors contributing to CAT within this population remains incomplete.
The primary clinical presentations of malignancy-associated thromboembolism encompass DVT[28], pulmonary embolism (PE)[29], wandering thrombophlebitis, arterial thromboembolism, nonbacterial thrombotic endocarditis, portal vein thrombosis, and disseminated intravascular coagulation[30]. However, the etiology of CAT varies among cases and primarily involves stagnant blood flow due to the hypercoagulable state of blood, vessel wall injury, and tumor compression[31]. The presence of additional risk factors contributes significantly to VTE development, primarily including patient-related factors, tumor-related factors, and treatment-related factors. Patient-specific factors encompass age, gender, obesity, and history of VTE, where a prior history of VTE can independently elevate the risk for recurrent CAT, notably more prevalent in patients with malignant tumors compared to common VTE cases[32]. For instance, in the general population, the incidence of VTE escalates notably with advancing age. One study findings suggest that patient age may universally influence VTE occurrence and could impact the location of thrombus presentation[33]. Correspondingly, aging emerges as a substantial risk factor for VTE in cancer patients. In retrospective cohort analyses, cancer patients aged ≥ 65 years were notably more prone to VTE development compared to younger patients. In a case control study, Matern et al[34] conducted a multifactorial analysis of data related to patients with cervical cancer, and the results showed that age is an independent risk factor for CAT formation, and that attention should be paid to screening for DVT in patients of advanced age[34]. Furthermore, systemic infections also pose a risk for CAT development[35]. In a controlled study, hospitalized patients with malignant tumors who acquired infections demonstrated a 3- to 5-times higher risk of developing CAT than non-infected patients. Infections such as respiratory, skin, intra-abdominal infections, and bacteremia all contributed to this heightened risk[35]. The second risk factor, specifically linked to malignancy-related VTE, includes the anatomical location of the tumor, tumor stage, and tissue origin. Some studies highlight that the incidence of VTE is significantly higher in patients with advanced tumors developing distant metastases than in patients whose lesions do not progress to distant metastases[36]. The third risk factor emanates from the therapeutic dimension of tumor treatment, encompassing systemic chemotherapy, hormonal therapy, anti-angiogenic therapy, major surgery, postoperative bed rest, and other factors capable of influencing CAT development. Among the predictive factors for VTE in hospitalized patients with malignancies are blood transfusions and central venous cannulation The development of blood clots from central venous catheters may be related to venous stasis and endothelial injury after the procedure. The formation of blood clots from central venous catheters may relate to venous blood stasis and endothelial injury post-procedure[37].
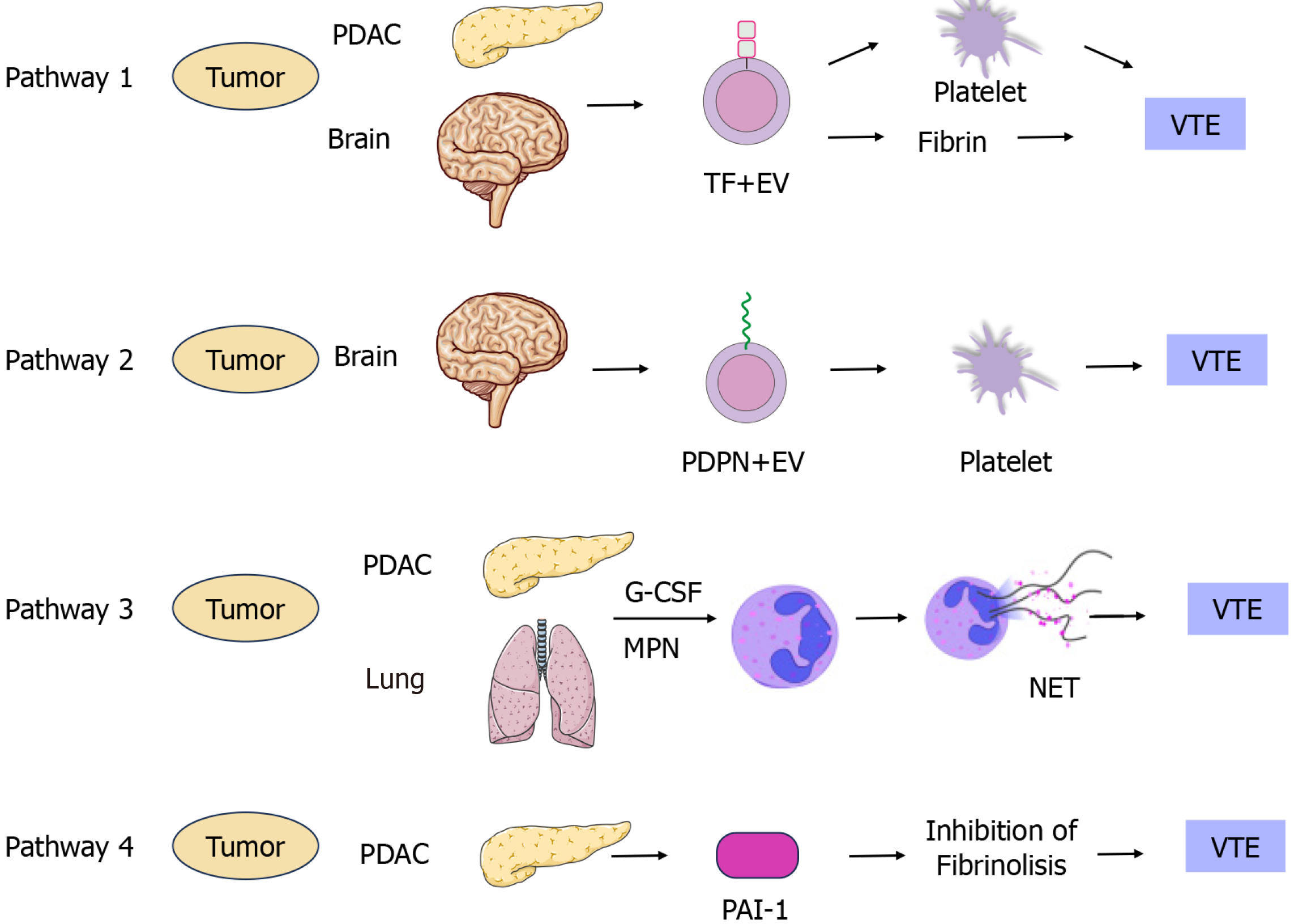
Malignant tumors disrupt the body's coagulation, anticoagulation, and fibrinolytic systems through various mechanisms, inducing hypercoagulability and pre-thrombotic alterations. This disruption promotes the growth and metastasis of the tumor, forming a vicious circle. Numerous reports delve into the mechanisms underlying CAT occurrence, and this review consolidates four potential aspects involved in CAT formation: Tissue factor (TF)[38], podoplanin (PDPN)[39], neutrophil extracellular traps (NETs)[40], and plasminogen activator inhibitor-1 (PAI-1)[41] (Figure 1).
TF: Endothelial cells, monocytes, and tumor cells express TF. TF is now widely acknowledged as a major contributor to cancer-associated coagulation disorders and CAT. TF directly triggers the conversion of coagulation factor VII to coagulation factor VIIa, playing a pivotal role in activating the exogenous coagulation pathways. TF, a transmembrane protein, exhibits heightened expression on the plasma membrane of cancer cells or microvesicles derived from circulating cancer cells[42]. In cancer patients, TF's expression and activity are significantly elevated compared to normal tissues, often correlating with thromboembolic complications and a poorer prognosis. Several ongoing clinical studies indicate a correlation between CAT incidence and TF in pancreatic cancer, glioma, and other tumors[14]. Activated TF is frequently released from tumor cells in the form of extracellular vesicles (EV), specifically termed EVTF[43]. Patients with tumors have higher levels of EVTF activity than healthy individuals, and intriguingly, patients with tumors of different histological origins have different levels of EVTF activity, with patients with tumors originating from adenocarcinomas also having higher levels of EVTF activity than those with other histological types of tumors. Elevated EVTF activity levels are associated with an increased CAT risk in patients with multiple tumors. A recent study on the relationship between EVTF activity and VTE in patients with glioblastoma showed no direct association between EVTF activity and VTE in patients with glioblastoma during a 2-year follow-up. Notably, patients with glioblastoma and wild-type IDH1/2 displayed higher levels of TF expression and a greater CAT incidence than the mutant type[44]. However, further investigation is needed to determine whether tumor-derived TF + EV contributes to VTE in patients with glioblastoma[45]. Nick et al[45] collected autopsy specimens from 180 patients, including 66 patients without tumor and 114 patients with tumor. Among the patients with tumor, 30 (26.3%) showed CAT formation. Upon analyzing TF expression in this group, their results revealed that 23 (76.7%) patients exhibited higher TF levels[45]. Collectively, these TF-expressing tumor cells likely contribute to CAT formation through various pathways in this patient cohort.
PDPN: PDPN represents a class of cell surface glycoproteins that play a pivotal role in tumor development. Overexpressed in various tumors such as hepatocellular carcinoma, lung cancer, and breast cancer[46,47], PDPN induces platelet aggregation through specific binding to platelet receptors. Additionally, PDPN participates in the proliferation, differentiation, epithelial mesenchymal transition and maintenance of tumor stem cell-like properties of malignant tumor cells.
Several studies have investigated PDPN's regulatory mechanisms. Hantusch et al[48] initially analyzed the base-rich region upstream of PDPN's promoters and identified multiple transcription factors promoting its transcription, including SP1, AP4, NF-1, among others. Moreover, in lymphatic endothelial cells, the transcription factor PROX-1 was recognized as a potential regulator for transcription factor for the transcriptional regulation of PDPN. Interestingly, analysis confirmed by chromatin immunoprecipitation confirmed the recruitment of SP1/SP3 to the upstream promoter region of PDPN, suggesting the presence of additional transcription factor complexes in this region. Peterziel et al[49] demonstrated a negative correlation between PDPN expression levels in primary human glioblastoma and glioma cells at the cellular level. At the same time, they experimentally observed increased PDPN expression in the ventricles of the brain in phosphatase and tensin homolog (PTEN) knockout mice and confirmed using western blot, that the PI3K/AKT/AP-1 signaling axis activation and PTEN loss of function led to PDPN expression in glioblastoma.
The tumor microenvironment (TME) comprises extracellular matrix (ECM), cytokines, and numerous stromal cells. Oncogenic stromal cells significantly contribute to TME construction, involving ECM production, activation of cancer-associated fibroblasts (CAFs), immune suppression, and angiogenesis promotion[50]. PDPN-positive CAFs actively participate in tumor malignancy by modifying the TME. Furthermore, PDPN acts as a co-inhibitory receptor expressed on T cells[50]. Understanding this mechanism elucidates PDPN's role in immunosuppression, offering new directions for cancer immunotherapy.
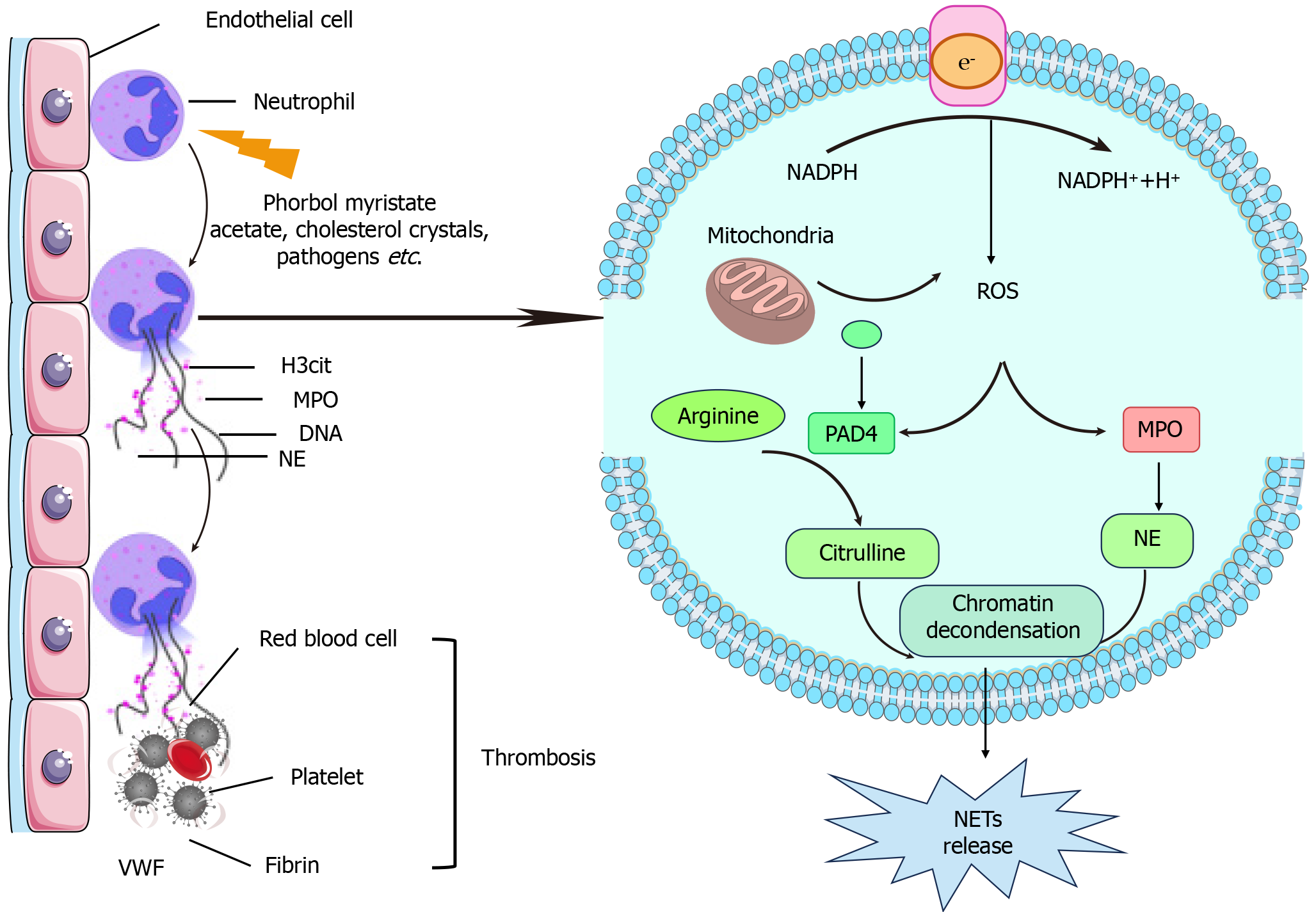
NETs: In 2004, Brinkmann et al[51] identified a network of DNA-histone complexes and proteins released by activated neutrophils, naming it NETs[51]. NETs formation represents a specific cellular process leading toward death, involving the release of granule proteins and chromatin depolymerization[52]. The mechanisms underlying NETs formation primarily stem from two aspects. First, the cleavage of NETs is induced by fopperol acetate myristate or cholesterol crystals, leading to histone arginine citrullination. Subsequently, neutrophils undergo rapid actin cleavage, detachment of cytosolic membranes, and reestablishment of microtubules and the cytoskeleton, followed by rupture of the cytoplasm and nucleus to release the chromatin. Finally, NET is released after cytoplasmic membrane rupture and release of the cytoplasmic contents. Another mechanism is the formation of nonlysing NETs, mediated by the activation of Toll-like receptors by certain bacteria or by the activation of a few complement-mediated reactions, all of which occurs independent of the oxidative activity of nicotinamide adenine dinucleotide phosphate [53] (Figure 2).
Previous studies have highlighted NETs' role as a defense mechanism for host cells and their involvement in non-infectious diseases such as rheumatoid arthritis, systemic lupus erythematosus, diabetes mellitus, atherosclerosis, and periodontitis[54]. Notably, several investigations have linked NETs to tumor cells, indicating their involvement in the tumor immune microenvironment, proliferation, metastasis, and CAT[55]. NETs further facilitate tumor cell metastasis by degrading extracellular stromal components.
Interestingly, NETs are closely associated with tumor progression and metastasis, with significantly higher expression levels detected in the plasma of patients with pancreatic, bladder, and lung cancers compared to healthy individuals[56]. In colorectal cancer patients, heightened in vitro stimuli correlate with increased NETs expression, linking to poor patient prognosis. Park et al[57] demonstrated the highest expression of NETs in metastatic triple-negative breast cancer patients through immunofluorescent staining.
PAI-1: PAI-1 acts as a serine protease (serpin) inhibitor and the principal regulator of the plasminogen activation system[58]. Studies conducted since the 1990s have consistently found high levels of PAI-1 protein in human primary malignant tumor extracts, serving as a significant biochemical marker for poor prognosis across various human cancer types. Recent research highlighted PAI-1's influence on the transition of tumor cells from G1 to S phase by regulating cell cycle proteins D1/CDK3/4 and, consequently, the transition of tumor cells from G1 to S phase[59]. However, conflicting findings exist; in breast tumor cells, PAI-1 exhibited an inhibitory effect on proliferation. The exact molecular mechanisms confirming PAI-1's direct regulation of malignant tumor cell cycles lack validation, although its role in inhibiting apoptosis is extensively reported and characterized. PAI-1's anti-apoptotic effect involves inhibiting cell adhesion to waveform proteins, prompting tumor cell separation and migration, thereby exerting its anti-apoptotic effect[60].
Extensive literature underscores PAI-1's pro-cancer role in malignant tumors. Surprisingly, despite numerous studies, there remains insufficient evidence supporting the therapeutic efficacy of targeting PAI-1 on tumor cells[60]. Notably, recent investigations over the past 3 years have seen the development of several small molecule inhibitors of PAI-1, tested in animal models. While these inhibitors have shown promise in promoting thrombus recanalization in some models, their significant impact on tumor cell growth and metastasis in animal tumor models remains limited. Placencio et al[61] reported that PAI-039, also known as tiplaxtinin, an inhibitor of PAI-1, demonstrated antitumor activity in T47 bladder cancer and HeLa cell tumors in mice. However, another PAI-1 inhibitor, TM554, displayed activity in certain preclinical cancer models but lacked antithrombotic activity in other models.
Conventional anticoagulation for CAT: The current treatment program for CAT is based on DVT treatment. All patients who are considered for VTE should commence anticoagulation therapy alongside diagnostic assessments. Guidelines advocate for low-molecular-weight heparin (LMWH) as the preferred choice for both initial and prolonged anticoagulation in CAT patients. Several guidelines support LMWH as the primary option for initial and ongoing anticoagulation in CAT patients. According to a randomized controlled study comparing low molecular heparin to oral anticoagulants in preventing rVTE in cancer patients, a 6-month LMWH treatment notably reduced the risk of rVTE from 17% to 9% compared with conventional treatment (LMWH bridged to warfarin)[62].
While many guidelines support LMWH therapy for CAT, some analyses propose VKA bridging after 6 months of LMWH might be effective in patients with tumor. A study of 1502 patients with tumor treated with LMWH for 6 months demonstrated similar rates of rVTE [hazard ratio (HR) = 0.67, 95%CI: 0.44-1.02] and major bleeding (HR = 1.05, 95%CI: 0.79-1.55) for those continuing LMWH vs those transitioned to VKAs. Optimal anticoagulation duration remains inconclusive; guidelines suggest its continuation during active tumor presence or ongoing antitumor therapy[63].
ASCO guidelines recommend starting pharmacological prophylaxis preoperatively, ITAC recommends starting 2 to 12 h preoperatively[64], and ASH recommends starting postoperatively; for patients with malignancies treated with outpatient chemotherapy, risk stratification using the Khorana Risk Assessment Model recommends rivaroxaban as a primary prophylaxis for thrombosis[65]. These guidelines are applicable to all patients with malignancies, but how to more accurately individualise the regimen for gynecological patients with malignancies in different risk strata is a major challenge in prophylactic anticoagulation for a wide range of malignancies, including gynecological oncology patients.
Recent advancements in novel oral anticoagulants (NOACs) mark a significant breakthrough in CAT prophylaxis and treatment, presenting an alternative to heparin and vitamin K antagonists (VKAs)[66]. However, efficacy and safety data for patients with tumor using NOACs are limited. Despite the advantages of NOACs over other anticoagulants, such as ease of administration (oral and fixed-dose regimens), no need for frequent testing, half-life similar to that of heparin, predictable anticoagulant efficacy, and minimal adverse effects, their safety and effectiveness in patients with tumor require further exploration[66].
Subgroup and meta-analyses of six phase III clinical trials investigating long-term oral anticoagulant therapy using NOACs in patients with CAT who have a prior history of tumor or are currently in an active tumor stage (approximately 5% of the total population) revealed that NOACs exhibit comparable safety and efficacy in both patients with and without tumor[67]. In the Zhang et al study[68], a randomized subgroup meta-analysis examining the treatment of active CAT with rivaroxaban (15 mg/dose, twice daily), compared to the Select-D study-a randomized, unblinded trial contrasting rivaroxaban (15 mg/dose, twice daily for 21 d, followed by 20 mg/dose once daily) with dalteparin (200 IU/kg for the initial month, then 150 IU/kg/d)-explored the efficacy of prolonged anticoagulant therapy. This evaluation assessed the incidence of hemorrhagic events and clinically relevant non-major hemorrhagic events in patients over 6 months, revealing that the rVTE at 6 months stood at 4% in the rivaroxaban group and 11% in the dalteparin group. Moreover, major and non-major clinically relevant bleeding rates were 17% and 6% in the rivaroxaban group, respectively. A meta-analysis indicated a decrease in rVTE following LMWH treatment in contrast to patients treated with VKAs [relative risk (RR) = 0.52, 95%CI: 0.36-0.74]. However, direct oral anticoagulants (DOACs) did not exhibit a significant reduction in rVTE (RR = 0.66, 95%CI: 0.39-1.11). Neither LMWH nor DOACs were linked to the development of major bleeding events[69]. Contrary to these findings, the International Society on Thrombosis and Haemostasis Guidance Statement suggests that NOACs might not be suitable for use in all patients with CAT due to the elevated risk of gastrointestinal bleeding. The statement emphasizes the necessity for more comprehensive and rigorous examination of the efficacy and safety of these medications through randomized, controlled trials.
In situations where anticoagulation is contraindicated for CAT, caution should be exercised when placing an IVCF in patients with CAT because of the high risk of thrombotic recurrence risks in this population[70]. IVTE treatment strategies encompass mechanical thrombus removal, catheter-directed thrombolysis, angioplasty, and other endoluminal therapies[71].
In cases where CAT leads to severe functional impairment or significantly affects the quality of life, such as when a tumor compresses nearby major blood vessels or metastasizes in lymph nodes, more aggressive treatment approaches may be considered. These may involve venous stenting, either with or without Contact thrombolysis with preserved catheter. The aim is to prevent further deterioration in patients' quality of life[72] due to tumor-related issues. For acute malignant superior vena cava obstruction syndrome, the primary treatment is endovascular stenting of the superior vena cava, either solely or in conjunction with radiotherapy and/or chemotherapy[72].
Although endovascular interventions prove effective and safe in alleviating symptoms and enhancing the quality of life, there's a scarcity of comprehensive data from international researchers on endoluminal treatment for tumor-related VTE. Existing data are mainly obtained from of case reports and studies with small sample sizes. This limited information might be attributed to the shorter life expectancy of most patients with tumor, where preventing potentially fatal PE becomes a therapeutic priority. Additionally, patients with advanced tumors often lack sufficient survival time to develop post-thrombotic syndrome post-thrombotic syndrome or chronic thromboembolic pulmonary hypertension[73]. Moreover, individuals with tumors have a heightened risk of rVTE and are more susceptible to in-stent reocclusion post-thromboplasty than patients without tumor. Hence, the risk-benefit analysis of angioplasty in patients with CAT should be thoroughly evaluated.
The risk associated with CAT varies based on the malignancy type, stage of development, and the patient's susceptibility to both thrombosis and anticancer therapies. However, CAT significantly impacts patient survival, mortality rates, and the overall quality of life in individuals with tumors. Consequently, enhancing risk assessment models to predict thrombosis risk and comprehending the pathogenesis of CAT are crucial. These steps aid in identifying high-risk CAT patients and devising suitable preventive measures.
Therapeutic approaches for CAT remain uniquely challenging, demanding tailored anticoagulation durations aligned with tumor activity and ongoing anticancer treatments. In the era of personalized medicine, frequent individualization of drugs, doses, and durations is imperative. While endoluminal therapy gains attention in CAT research, various aspects of its clinical application require further exploration.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Di Donato V, Italy S-Editor: Liu H L-Editor: A P-Editor: Guo X
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11936] [Article Influence: 2984.0] [Reference Citation Analysis (4)] |
| 2. | de Visser KE, Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1574] [Reference Citation Analysis (0)] |
| 3. | Shah SC, Itzkowitz SH. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology. 2022;162:715-730.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 445] [Article Influence: 148.3] [Reference Citation Analysis (0)] |
| 4. | Sanfilippo KM, Moik F, Candeloro M, Ay C, Di Nisio M, Lee AYY. Unanswered questions in cancer-associated thrombosis. Br J Haematol. 2022;198:812-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Cohen O, Caiano LM, Tufano A, Ageno W. Cancer-Associated Splanchnic Vein Thrombosis. Semin Thromb Hemost. 2021;47:931-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Schaefer JK, Elshoury A, Nachar VR, Streiff MB, Lim MY. How to Choose An Appropriate Anticoagulant for Cancer-Associated Thrombosis. J Natl Compr Canc Netw. 2021;19:1203-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Falanga A, Marchetti M. Cancer-associated thrombosis: enhanced awareness and pathophysiologic complexity. J Thromb Haemost. 2023;21:1397-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 8. | Kirwan CC, Blower EL. Contemporary breast cancer treatment-associated thrombosis. Thromb Res. 2022;213 Suppl 1:S8-S15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 9. | Mahajan A, Brunson A, Adesina O, Keegan THM, Wun T. The incidence of cancer-associated thrombosis is increasing over time. Blood Adv. 2022;6:307-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 10. | Bolek H, Ürün Y. Cancer-associated thrombosis and drug-drug interactions of antithrombotic and antineoplastic agents. Cancer. 2023;129:3216-3229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 11. | Jin S, Qin D, Liang BS, Zhang LC, Wei XX, Wang YJ, Zhuang B, Zhang T, Yang ZP, Cao YW, Jin SL, Yang P, Jiang B, Rao BQ, Shi HP, Lu Q. Machine learning predicts cancer-associated deep vein thrombosis using clinically available variables. Int J Med Inform. 2022;161:104733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Streiff MB, Holmstrom B, Angelini D, Ashrani A, Elshoury A, Fanikos J, Fertrin KY, Fogerty AE, Gao S, Goldhaber SZ, Gundabolu K, Ibrahim I, Kraut E, Leavitt AD, Lee A, Lee JT, Lim M, Mann J, Martin K, McMahon B, Moriarty J, Morton C, Ortel TL, Paschal R, Schaefer J, Shattil S, Siddiqi T, Sudheendra D, Williams E, Hollinger L, Nguyen MQ. Cancer-Associated Venous Thromboembolic Disease, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:1181-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 13. | Lyman GH, Kuderer NM. Clinical practice guidelines for the treatment and prevention of cancer-associated thrombosis. Thromb Res. 2020;191 Suppl 1:S79-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Khorana AA, Mackman N, Falanga A, Pabinger I, Noble S, Ageno W, Moik F, Lee AYY. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 222] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 15. | Sano M, Takahashi R, Ijichi H, Ishigaki K, Yamada T, Miyabayashi K, Kimura G, Mizuno S, Kato H, Fujiwara H, Nakatsuka T, Tanaka Y, Kim J, Masugi Y, Morishita Y, Tanaka M, Ushiku T, Nakai Y, Tateishi K, Ishii Y, Isayama H, Moses HL, Koike K. Blocking VCAM-1 inhibits pancreatic tumour progression and cancer-associated thrombosis/thromboembolism. Gut. 2021;70:1713-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Li JC, Zou XM, Yang SF, Jin JQ, Zhu L, Li CJ, Yang H, Zhang AG, Zhao TQ, Chen CY. Neutrophil extracellular traps participate in the development of cancer-associated thrombosis in patients with gastric cancer. World J Gastroenterol. 2022;28:3132-3149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Shirai T, Tsukiji N, Sasaki T, Oishi S, Yokomori R, Takano K, Suzuki-Inoue K. Cancer-associated fibroblasts promote venous thrombosis through podoplanin/CLEC-2 interaction in podoplanin-negative lung cancer mouse model. J Thromb Haemost. 2023;21:3153-3165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 18. | Yamamura K, Beppu T, Kinoshita K, Oda E, Sato N, Yuki H, Motohara T, Miyamoto H, Kawaguchi H, Komohara Y, Akahoshi S. Hepatocellular Carcinoma With Extensive Cancer-associated Thrombosis Successfully Treated With Liver Resection and Direct Oral Anticoagulant: A Case Report. Anticancer Res. 2020;40:6465-6471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Sasano T, Gonzalez-Delgado R, Muñoz NM, Carlos-Alcade W, Cho MS, Sheth RA, Sood AK, Afshar-Kharghan V. Podoplanin promotes tumor growth, platelet aggregation, and venous thrombosis in murine models of ovarian cancer. J Thromb Haemost. 2022;20:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Li A, Wu Q, Luo S, Warnick GS, Zakai NA, Libby EN, Gage BF, Garcia DA, Lyman GH, Sanfilippo KM. Derivation and Validation of a Risk Assessment Model for Immunomodulatory Drug-Associated Thrombosis Among Patients With Multiple Myeloma. J Natl Compr Canc Netw. 2019;17:840-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 21. | Comerford C, Glavey S, Quinn J, O'Sullivan JM. The role of VWF/FVIII in thrombosis and cancer progression in multiple myeloma and other hematological malignancies. J Thromb Haemost. 2022;20:1766-1777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood. 2017;130:1499-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 279] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 23. | Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 843] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 24. | Goto S, Haas S, Ageno W, Goldhaber SZ, Turpie AGG, Weitz JI, Angchaisuksiri P, Nielsen JD, Kayani G, Farjat A, Schellong S, Bounameaux H, Mantovani LG, Prandoni P, Kakkar AK; GARFIELD-VTE Investigators. Assessment of Outcomes Among Patients With Venous Thromboembolism With and Without Chronic Kidney Disease. JAMA Netw Open. 2020;3:e2022886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Yeini E, Satchi-Fainaro R. The role of P-selectin in cancer-associated thrombosis and beyond. Thromb Res. 2022;213 Suppl 1:S22-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 26. | Barg AA, Kenet G. Cancer-associated thrombosis in pediatric patients. Thromb Res. 2020;191 Suppl 1:S22-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 28. | Dickson K, Koom-Dadzie K, Brito-Dellan N, Escalante C. Risks, diagnosis, and management of recurrent cancer-associated thrombosis (CAT): a narrative review. Support Care Cancer. 2022;30:8539-8545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 29. | Weitz JI, Haas S, Ageno W, Goldhaber SZ, Turpie AGG, Goto S, Angchaisuksiri P, Nielsen JD, Kayani G, Farjat AE, Schellong S, Bounameaux H, Mantovani LG, Prandoni P, Kakkar AK; GARFIELD-VTE investigators. Cancer associated thrombosis in everyday practice: perspectives from GARFIELD-VTE. J Thromb Thrombolysis. 2020;50:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Poenou G, Dumitru Dumitru T, Lafaie L, Mismetti V, Ayoub E, Duvillard C, Accassat S, Mismetti P, Heestermans M, Bertoletti L. Pulmonary Embolism in the Cancer Associated Thrombosis Landscape. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 31. | Dolgin E. Cancer's new normal. Nat Cancer. 2021;2:1248-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Moik F, Pabinger I, Ay C. How I treat cancer-associated thrombosis. ESMO Open. 2020;5:e000610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Hisada Y, Mackman N. Mechanisms of cancer-associated thrombosis. Res Pract Thromb Haemost. 2023;7:100123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 34. | Matern T, Kang E, Lim PC. Factors in the feasibility and safety of outpatient robotic-assisted hysterectomy for endometrial or cervical carcinoma. Gynecol Oncol. 2020;157:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Khan AM, Chiasakul T, Redd R, Patell R, McCarthy EP, Neuberg D, Zwicker JI. Survival outcomes with warfarin compared with direct oral anticoagulants in cancer-associated venous thromboembolism in the United States: A population-based cohort study. PLoS Med. 2022;19:e1004012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 36. | Fernandes CJ, Morinaga LTK, Alves JL Jr, Castro MA, Calderaro D, Jardim CVP, Souza R. Cancer-associated thrombosis: the when, how and why. Eur Respir Rev. 2019;28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 37. | Gervaso L, Dave H, Khorana AA. Venous and Arterial Thromboembolism in Patients With Cancer: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2021;3:173-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 38. | Koizume S, Miyagi Y. Tissue factor in cancer-associated thromboembolism: possible mechanisms and clinical applications. Br J Cancer. 2022;127:2099-2107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 39. | Suzuki-Inoue K. Platelets and cancer-associated thrombosis: focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood. 2019;134:1912-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 40. | Herre M, Cedervall J, Mackman N, Olsson AK. Neutrophil extracellular traps in the pathology of cancer and other inflammatory diseases. Physiol Rev. 2023;103:277-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 104] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 41. | Ilich A, Kumar V, Henderson M, Mallick R, Wells P, Carrier M, Key NS. Biomarkers in cancer patients at risk for venous thromboembolism: data from the AVERT study. Thromb Res. 2020;191 Suppl 1:S31-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, Meyer G, Segers A, Shi M, Wang TF, Yeo E, Zhang G, Zwicker JI, Weitz JI, Büller HR; Hokusai VTE Cancer Investigators. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2018;378:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 1091] [Article Influence: 155.9] [Reference Citation Analysis (0)] |
| 43. | Moik F, Chan WE, Wiedemann S, Hoeller C, Tuchmann F, Aretin MB, Fuereder T, Zöchbauer-Müller S, Preusser M, Pabinger I, Ay C. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood. 2021;137:1669-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 44. | Unruh D, Schwarze SR, Khoury L, Thomas C, Wu M, Chen L, Chen R, Liu Y, Schwartz MA, Amidei C, Kumthekar P, Benjamin CG, Song K, Dawson C, Rispoli JM, Fatterpekar G, Golfinos JG, Kondziolka D, Karajannis M, Pacione D, Zagzag D, McIntyre T, Snuderl M, Horbinski C. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol. 2016;132:917-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 45. | van Es N, Hisada Y, Di Nisio M, Cesarman G, Kleinjan A, Mahé I, Otten HM, Kamphuisen PW, Berckmans RJ, Büller HR, Mackman N, Nieuwland R. Extracellular vesicles exposing tissue factor for the prediction of venous thromboembolism in patients with cancer: A prospective cohort study. Thromb Res. 2018;166:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Bieniasz-Krzywiec P, Martín-Pérez R, Ehling M, García-Caballero M, Pinioti S, Pretto S, Kroes R, Aldeni C, Di Matteo M, Prenen H, Tribulatti MV, Campetella O, Smeets A, Noel A, Floris G, Van Ginderachter JA, Mazzone M. Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell Metab. 2019;30:917-936.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 47. | Bai J, Liu T, Tu B, Yuan M, Shu Z, Fan M, Huo S, Guo Y, Wang L, Wang H, Zhao Y. Autophagy loss impedes cancer-associated fibroblast activation via downregulating proline biosynthesis. Autophagy. 2023;19:632-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 48. | Hantusch A, Rehm M, Brunner T. Counting on Death - Quantitative aspects of Bcl-2 family regulation. FEBS J. 2018;285:4124-4138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Peterziel H, Müller J, Danner A, Barbus S, Liu HK, Radlwimmer B, Pietsch T, Lichter P, Schütz G, Hess J, Angel P. Expression of podoplanin in human astrocytic brain tumors is controlled by the PI3K-AKT-AP-1 signaling pathway and promoter methylation. Neuro Oncol. 2012;14:426-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Du R, Zhang X, Lu X, Ma X, Guo X, Shi C, Ren X, He Y, Gao Y, Liu Y. PDPN positive CAFs contribute to HER2 positive breast cancer resistance to trastuzumab by inhibiting antibody-dependent NK cell-mediated cytotoxicity. Drug Resist Updat. 2023;68:100947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 51. | Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5773] [Cited by in RCA: 7245] [Article Influence: 345.0] [Reference Citation Analysis (0)] |
| 52. | Zhang H, Wang Y, Qu M, Li W, Wu D, Cata JP, Miao C. Neutrophil, neutrophil extracellular traps and endothelial cell dysfunction in sepsis. Clin Transl Med. 2023;13:e1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 150] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 53. | Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1809] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 54. | Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei R, Lin ZF, Wang XY, Wang CQ, Lu M, Jia HL, Chen JH, Zhang JB, Qin LX. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. 2020;13:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 244] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 55. | Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis. Front Immunol. 2020;11:1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 341] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 56. | Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, Huang D, Li J, Li H, Chen F, Liu J, Xing Y, Chen X, Su S, Song E. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 632] [Article Influence: 126.4] [Reference Citation Analysis (0)] |
| 57. | Park SY, Shrestha S, Youn YJ, Kim JK, Kim SY, Kim HJ, Park SH, Ahn WG, Kim S, Lee MG, Jung KS, Park YB, Mo EK, Ko Y, Lee SY, Koh Y, Park MJ, Song DK, Hong CW. Autophagy Primes Neutrophils for Neutrophil Extracellular Trap Formation during Sepsis. Am J Respir Crit Care Med. 2017;196:577-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 58. | Morrow GB, Mutch NJ. Past, Present, and Future Perspectives of Plasminogen Activator Inhibitor 1 (PAI-1). Semin Thromb Hemost. 2023;49:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 59. | Mali RS, Cheng M, Chintala SK. Plasminogen activators promote excitotoxicity-induced retinal damage. FASEB J. 2005;19:1280-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Rossi Sebastiano M, Pozzato C, Saliakoura M, Yang Z, Peng RW, Galiè M, Oberson K, Simon HU, Karamitopoulou E, Konstantinidou G. ACSL3-PAI-1 signaling axis mediates tumor-stroma cross-talk promoting pancreatic cancer progression. Sci Adv. 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 61. | Placencio VR, DeClerck YA. Plasminogen Activator Inhibitor-1 in Cancer: Rationale and Insight for Future Therapeutic Testing. Cancer Res. 2015;75:2969-2974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 62. | Gong M, Fu G, Liu Z, Zhao B, Kong J, He X, Gu J. Angiojet pharmacomechanical thrombectomy versus anticoagulant therapy alone in massive cancer-associated thrombosis: a single centre retrospective cohort study. J Thromb Thrombolysis. 2023;55:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 63. | Gulati S, Eckman MH. Anticoagulant Therapy for Cancer-Associated Thrombosis: A Cost-Effectiveness Analysis. Ann Intern Med. 2023;176:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 64. | Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Francis CW, Gates LE, Kakkar AK, Levine MN, Liebman HA, Tempero MA, Lyman GH, Falanga A. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 951] [Article Influence: 158.5] [Reference Citation Analysis (0)] |
| 65. | Shimura T, Nakazawa S, Kobayashi S, Yokota H, Otsuka T, Nakamura T. Clinicopathological studies of diffuse axonal injury--five autopsy cases. No Shinkei Geka. 1988;16:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Chen DY, Tseng CN, Hsieh MJ, Lan WC, Chuang CK, Pang ST, Chen SW, Chen TH, Chang SH, Hsieh IC, Chu PH, Wen MS, Chen JS, Chang JW, See LC, Huang WK. Comparison Between Non-vitamin K Antagonist Oral Anticoagulants and Low-Molecular-Weight Heparin in Asian Individuals With Cancer-Associated Venous Thromboembolism. JAMA Netw Open. 2021;4:e2036304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 67. | van der Hulle T, den Exter PL, Kooiman J, van der Hoeven JJ, Huisman MV, Klok FA. Meta-analysis of the efficacy and safety of new oral anticoagulants in patients with cancer-associated acute venous thromboembolism. J Thromb Haemost. 2014;12:1116-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 68. | Zhang J, Xu J, Zhang W, Jiang M, Liu J, Xu L, Liu G, Zhao Z. Quality Appraisal of Guidelines on Cancer-Associated Thrombosis Using AGREE II Instrument and Analysis of Current Status of New Oral Anticoagulants. Clin Appl Thromb Hemost. 2019;25:1076029619846562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Mulder FI, Bosch FTM, Young AM, Marshall A, McBane RD, Zemla TJ, Carrier M, Kamphuisen PW, Bossuyt PMM, Büller HR, Weitz JI, Middeldorp S, van Es N. Direct oral anticoagulants for cancer-associated venous thromboembolism: a systematic review and meta-analysis. Blood. 2020;136:1433-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 70. | Kim AS, Khorana AA, McCrae KR. Mechanisms and biomarkers of cancer-associated thrombosis. Transl Res. 2020;225:33-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 71. | Mukai M, Oka T. Mechanism and management of cancer-associated thrombosis. J Cardiol. 2018;72:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 72. | Grover SP, Hisada YM, Kasthuri RS, Reeves BN, Mackman N. Cancer Therapy-Associated Thrombosis. Arterioscler Thromb Vasc Biol. 2021;41:1291-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 73. | Manz XD, Szulcek R, Pan X, Symersky P, Dickhoff C, Majolée J, Kremer V, Michielon E, Jordanova ES, Radonic T, Bijnsdorp IV, Piersma SR, Pham TV, Jimenez CR, Vonk Noordegraaf A, de Man FS, Boon RA, Voorberg J, Hordijk PL, Aman J, Bogaard HJ. Epigenetic Modification of the von Willebrand Factor Promoter Drives Platelet Aggregation on the Pulmonary Endothelium in Chronic Thromboembolic Pulmonary Hypertension. Am J Respir Crit Care Med. 2022;205:806-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |