Published online Mar 16, 2023. doi: 10.12998/wjcc.v11.i8.1814
Peer-review started: November 17, 2022
First decision: November 25, 2022
Revised: December 1, 2022
Accepted: January 20, 2023
Article in press: January 20, 2023
Published online: March 16, 2023
Processing time: 109 Days and 21.3 Hours
This article examines primary hepatic angiosarcoma (PHA) and fat-poor angiomyolipoma (AML), two uncommon vascular cancers. Clinical decisions in these situations are frequently aided by pathology reports and imaging techniques. Uncommon malignant tumors of the vascular endothelium include PHA. Another diagnosis that should not be overlooked when employing contrast-enhanced MR and contrast-enhanced computed tomography (CT) imaging techniques is fat-poor AML, one of the uncommon vascular tumors of the liver. In both conditions, biopsy is the primary means of diagnosis.
In our article, besides the diagnosis of PHA, fat-poor AML, one of the other rare vascular tumors of the liver, is mentioned. In the case, a 50-year-old female patient with VHL Syndrome was admitted to our hospital with nonspecific lesions such as right upper quadrant pain, weight loss, and nausea. Abdominal ultrasonography (US) revealed a hypoechoic heterogeneous lesion with occasional faint contours. In computed tomography, it was observed as a hyperdense nodular lesion in segment 4. Magnetic resonance imaging (MRI) revealed that the lesion did not contain fat. In connection with the known history of VHL Syndrome, we first evaluated the possibility of AML. Thereupon, a histopathological sample was taken and the diagnosis was made as fat-poor AML with 5% fat content.
In conclusion, PHA in our case report and fat-poor AML in our clinic are two uncommon liver vascular malignancies with comparable incidences. Important imaging techniques like contrast-enhanced US (CEUS), CECT, and CEMRI give us substantial advantages in both cases. However, a biopsy is used to provide the final diagnosis.
Core Tip: In this review, two rare vascular tumors, namely primary hepatic angiosarcoma (PHA) and fat-poor angiomyolipoma (AML), were mentioned. Special mention is made of the diagnosis of PHA by contrast-enhanced ultrasound (CEUS) in these case reports. Meanwhile we introduced a new ultrasound technology and CEUS has many specific manifestations in the diagnosis and differential diagnosis of PHA and has great clinical value in diagnosing PHA. Although imaging methods have an important place in the diagnosis of fat-poor AML, one of the points especially mentioned in the study is that the definitive diagnosis of both tumors will be made with a pathology report after biopsy.
- Citation: Gulmez AO, Aydin S, Kantarci M. A complementary comment on primary hepatic angiosarcoma: A case report. World J Clin Cases 2023; 11(8): 1814-1822
- URL: https://www.wjgnet.com/2307-8960/full/v11/i8/1814.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i8.1814
This article reviews two uncommon vascular cancers, primary hepatic angiosarcoma (PHA) and fat-poor angiomyolipoma (AML). However, in this study, besides the diagnosis of PHA, fat-poor AML, one of the other rare vascular tumors of the liver, is mentioned. In these cases, pathology reports and imaging techniques often assist clinical decisions. Another diagnosis that should not be overlooked when using contrast-enhanced MR and contrast-enhanced computed tomography (CT) imaging techniques is fat-poor AML, one of the rare vascular tumors of the liver. In both cases, biopsy is the primary diagnostic tool. We will mention two rare diagnoses that doctors and healthcare professionals involved in the diagnosis should be careful about.
We would like to talk about the case report of our patient who was diagnosed with fat-poor AML, which is one of the rare liver tumors like PHA. Our patient is a 50-year-old female patient who was previously diagnosed with VHL syndrome. Nonspecific symptoms such as right upper quadrant pain, weight loss, and nausea are the primary complaints at admission to our clinic.
If we look at the patient’s current disease, nonspecific symptoms such as right upper quadrant pain, weight loss and nausea make us think that he already has a disease related to the gastroenterological system.
When we look at the patient’s past disease history, VHL syndrome was diagnosed and it is known that he has AMLs in both kidneys.
When the family history was taken apart from the patient’s history, no condition that could be associated with the current disease status was found in the family members.
If we look at the physical examination findings, no significant finding was observed except for the right upper quadrant tenderness.
Laboratory examinations, especially liver function tests, were within normal limits.
At the beginning of the introduction, we would like to talk about the case presentation of our patient, who was diagnosed with fat-poor AML, which is one of the rare liver tumors such as PHA. Our patient is a 50-year-old female patient who was previously diagnosed with VHL syndrome. The main complaints of admission to our clinic are nonspecific symptoms such as right upper quadrant pain, weight loss and nausea. When evaluated together with the patient’s current disease and past disease history, he was diagnosed with VHL syndrome and had AMLs in both kidneys. Apart from the patient’s personal health history, when the family history was taken, no related condition was found in the family members to be associated with the current disease state. When evaluated by physical examination, no significant finding was observed except right upper quadrant tenderness. In laboratory examinations, other tests, especially liver function tests, were within normal ranges.
If we look at the imaging methods, a hypoechoic heterogeneous lesion with faint contours was detected in the abdominal ultrasonography (US). The mass lesion was 15 mm × 16 mm in size and was located in segment 4. On computed tomography (CT), it was observed as a hyperdense nodular (approximately 16 mm in diameter) lesion in the venous phase. In magnetic resonance imaging (MRI), in-phase and out-of-phase images obtained with the ‘Dual echo’ method; When both sequences were compared, it was understood that the lesion did not contain fat. Iohexol (Opaxol 350 mg/100 mL) was used in CT and gadoxic acid disodium (Primovist 0.25 mmol/mL) was used as hepatospecific agent in MR. In addition, the lesion showed slight diffusion restriction on diffusion-weighted images. It was hypointense compared to normal liver parenchyma on pre-contrast T1-weighted images. It showed strong peripheral enhancement in the arterial phase. Hepatobiliary phase images showed hypointense associated with normal parenchyma.
Radiographic findings showed a benign, highly vascular tumor devoid of hepatocytes. In connection with the known history of VHL Syndrome, we first evaluated the possibility of AML; however, the lack of lesion fat made it difficult to establish the diagnosis. Thereupon, histopathological sampling was taken for diagnosis and the final diagnosis came as fat-poor AML with 5% fat content.
If we look at the treatment point, medical, surgical or embolic ablative treatment methods were not required at this stage because of the fact that the patient did not grow more than 0.5 cm per year in the follow-ups and the dimensions did not exceed 3 cm at the initial diagnosis stage. It was decided that the patient should come to the controls at regular intervals.
As a result, histopathological sampling is required for definitive diagnosis together with physical examination, laboratory and imaging methods. The patient was followed up with laboratory and imaging methods at regular intervals.
Although more detailed information is given in the continuation of the article about hepatic AMLs, which we mentioned over the case, it is important to remember that it should be kept in mind as it is a rare tumor.
PHA in our case report and fat-poor AML in our clinic are two rare vascular tumors of the liver with a similar incidence. In both, important imaging modalities such as CEUS, CECT and CEMRI provide us with significant gains. However, the definitive diagnosis is made by biopsy.
We read with great interest the case report of Wang et al[1] on the diagnosis and treatment of PHA in the November 2022 issue of the World Journal of Clinical Cases. They described a situation when the patient complained of abdominal soreness. After a comprehensive investigation, including contrast-enhanced ultrasound (CEUS), CECT, and CEMRI, the findings were combined with the biopsy result and the diagnosis of PHA was made. We appreciate the dedication of the authors to raise awareness of the diagnosis and treatment of PHA.
PHA is a rare malignant tumor. It arises from spindle pleomorphic cells that line or grow within the lumen of sinusoids and pre-existing vascular spaces such as terminal hepatic venules in the liver. Worldwide, only about 200 cases are detected annually. However, it is the most common primary malignant mesenchymal tumor of the liver in adults, accounting for 2% of all primary hepatic malignancies. It accounts for less than 5% of all angiosarcoma[2,3]. A quarter of PHA is thought to be bound to various substances such as vinyl chloride[1]. The cause of the remaining three quarters is unknown. Patients most commonly present with vague symptoms such as right upper quadrant pain, weight loss, fatigue, and abdominal mass[4].
If we look at the differential diagnosis point, as stated in many studies, it is difficult to distinguish PHA from hemangioma, hepatocellular carcinoma (HCC), cholangiocarcinoma, metastases and hepatic abscess[1,5]. Although CECT and CEMRI guide us, CEUS, which was especially emphasized in the study, has started to take its place in daily practice as an important and simultaneous imaging method[1]. In the arterial and portal phases of the CEUS, nodular peripheral enhancement is seen, and in the late phase nodules, low contrast enhancement is shown together with non-contrast areas[1,6,7].
Although radiological imaging has an important place in the diagnosis of PHA, the actual diagnosis is finalized with the result of pathological biopsy[8]. In immunohistochemical stains, CD31, CD34, and factor VIII-associated antigen are often used in combination for the diagnosis of angiosarcomas, as 40% of tumors lose expression of one or more markers. The combination of CD31 and factor VIII-related antigen is defined as the most sensitive one by expressing one of the two markers in 90% of cases[8,9]. Although the treatment point was also mentioned in our study, when combined with some other studies, surgical resection seems to be the key to improving the prognosis in the best way[10,11].
In addition to the article, we would like to talk about fat-poor AML, which is one of the rare vascular tumors of the liver like PHA. AMLs are benign mesenchymal tumors that usually involve the kidneys and rarely the liver[12]. Renal AMLs are also seen as a subcomponent of some syndromes. Von Hippel-Lindau syndrome (VHL syndrome) is one of these syndromes. VHL syndrome is caused by germline mutations of the VHL tumor suppressor gene located on chromosome 3p25. VHL syndrome is an inherited cancer syndrome characterized by the development of vascular tumors of the nervous system and retina, pheochromocytomas, pancreatic islet cell tumors, endolymphatic sac tumors, AMLs, especially cysts in the kidney, as well as the development of benign cysts affecting various organs[13].
Before proceeding to the case report of our patient with a prediagnosis of VHL, we would like to give some more general information about VHL syndrome. VHL syndrome, as we mentioned in the above paragraph, is an autosomal dominant inherited tumor disease that occurs due to germline mutations in the VHL gene located on the short arm of chromosome 3. Patients with VHL can develop multiple benign and malignant tumor structures that can affect various organ systems at various levels. To give examples, retinal hemangioblastomas (HBs), central nervous system (CNS) HBs, endolymphatic sac tumors, pancreatic neuroendocrine tumors, pancreatic cystadenomas, pancreatic cysts, clear cell renal cell carcinomas, renal cysts, pheochromocytomas, paragangliomas, and epididia and large ligament cystadenomas can be given as examples of many findings. One of the most important points in making a clinically meaningful diagnosis and initiating treatment is to know that VHL syndrome can be divided into groups according to the forms that we may encounter in daily practice. Each phenotype is associated with a particular genotype. It is basically divided into 2 types, type 1 and type 2. Type 2 is divided into 3 types as type 2A, type 2B and type 2C. In Type 1, there is a minimal likelihood of developing a medullary adrenal gland tumor, but a high likelihood of developing clear cell kidney cancer, hemangioblastomas, and different pancreatic diseasesAlthough there is a high risk of pheochromocytoma in all kinds 2A, 2b, and 2C, the fact that type 2C alone has a very high risk is significant. Additionally crucial to the distinction of 2A and 2B types is clear cell renal cancer. Type 2B is more likely to experience it than type 2A, where its occurrence is lower. We have summarized the types of VHL syndrome in general[14].
VHL syndrome is a syndrome that requires lifelong prophylactic surveillance. The surveillance data we have are based on best medical judgment. However, there is no evidence of any effect[15]. VHL syndrome is a multisystem-related familial cancer syndrome with a prevalence ranging from 1 in 31000 to 1 in 85000[16,17]. It is autosomal dominant in inheritance type and the estimated incidence of its newly developed mutation is 1%-23%[18,19]. After the diagnosis of VHL syndrome, various surveillance begins in patients; because this syndrome affects many organs at the same time. We will now touch on these through examples. Imaging of the central nervous system begins at age 15; but if the diagnosis is made earlier, a basic examination can be done once in the age range of 5-14 years. Eye examination starts directly upon diagnosis and is repeated every 12 mo. Imaging of the abdominal region begins at age 15; but if the diagnosis is made earlier, a basic examination can be done once in the age range of 5-14 years. Neurological examination starts directly upon diagnosis and is repeated every 12 mo. A 24-h urine test for catecholamine levels basically begins at age 15; but if the diagnosis is made earlier, a basic examination can be done once in the age range of 5-14 years. Audiometric examination begins at age 15. Since the risk of ocular and neurological findings and poor prognosis is higher, examinations are performed at more frequent intervals as the diagnosis is made[14].
We want to get started with the imaging features. Here we will relate it to the finding that it is associated with organs. Starting with the kidney first, it is seen in more than two-thirds of patients with histological subtype VHL syndrome with multicentric renal cysts and clear cell RCCs in the kidney[14]. Although there is a high risk of pheochromocytoma in all kinds 2A, 2B, and 2C, the fact that type 2C alone has a very high risk is significant. Additionally crucial to the distinction of 2A and 2B types is clear cell renal cancer. Type 2B is more likely to experience it than type 2A, where its occurrence is lower[14,20,21]. Especially CT and MRI are two important imaging modalities that are frequently used in the evaluation of kidney lesions suspected to be BCC and in the staging of such lesions. In CT, increases below 10 HU are considered within the normal range and are not classified as increases[22]. Another crucial fact to keep in mind is that even straightforward cystic lesions may become more prevalent in more than one in twenty MRI findings[23]. The main purpose of imaging and the treatment applied with it is to detect lesions before new lesions appear. If we look at the imaging features of the pancreas, pancreatic cyst may develop in 42% of patients with VHL syndrome, while serous cystadenoma and neuroendocrine tumor influencing the pancreas can be observed in approximately one in 10 patients, respectively[24]. Such pancreatic cysts are usually multicenter and can be seen as hypotenuated lesions without contrast enhancement. On MRI, serous cystadenomas are typically hyperintense on T2-weighted images and hypointense on T1-weighted images; however, if there is intracystic hemorrhage, an increase in signal is observed that can be hyperintense in both. When a fibrotic central scar is present, a hypointense signal is produced with delayed contrast enhancement on T1- and T2-weighted images. Although pathognomonic, a central scar is seen only in 20%-30% of cases. In the absence of scar, the combination of microcystic appearance and vascular contrast enhancement may support the diagnosis. Serous cystadenomas are not associated with the pancreatic duct. Pancreatic neuroendocrine tumors can be seen in 9%-17% of patients with VHL syndrome. Compared with sporadic pancreatic neuroendocrine tumors, those associated with VHL syndrome appear earlier (mean, 35 years vs 58 years). The neuroendocrine tumors seen in VHL are typically multifocal and most commonly located in the pancreatic head section and the uncinate process. On non-contrast CT imaging, they often appear hypotenuated. On imaging, it usually exhibits the same contrast enhancement as the rest of the body. Pancreatic neuroendocrine tumors in people with VHL syndrome are typically identified solely by imaging[14,24]. These individuals frequently experience the triad of headache, perspiration, and tachycardia linked to arterial hypertension. In cases of VHL disease, approximately one in every 4 patients has an adrenal medullary tumor, while paragangliomas are seen in about one in every 6 patients[24,25]. Like its clinical presentations, imaging findings are diverse. These individuals frequently experience the triad of headache, perspiration, and tachycardia linked to arterial hypertension. Despite the fact that there is a strong augmentation visible after contrast application, this shows that they are hypervascular, especially in their solid components[14,21]. It’s crucial to keep in mind that absolute or relative resolution may be seen on CT in benign lesions like adenoma or malignant tumors[26,27]. The bulb sign, or high signal intensity on T2-weighted MRI scans of a pheochromocytoma, is a crucial component for diagnosis and is present in 11%-65% of patients[28]. Usually isointense; but if there is bleeding it may also present with a hyperintense appearance. As a result of different degrees of pathological degeneration, pheochromocytomas may present in many different forms as imaging. Radiologists refer to them as "chameleon tumors" because of this. Functional investigations are frequently necessary to be included in the diagnosis for pheochromocytomas and paragangliomas, as well as to detect non-adrenal or metastatic illness, due to the wide range of imaging symptoms[29]. In conclusion, it is crucial for the initial diagnosis as well as the detection and monitoring of lesions in accordance with the advised abdominal imaging follow-up protocols[15,30,31]. Radiologists, with multidisciplinary approaches and medical equipment more treatment modalities for patients with VHL syndrome They seek to improve their quality of life and aim to reduce the mortality and morbidity caused by the disease.
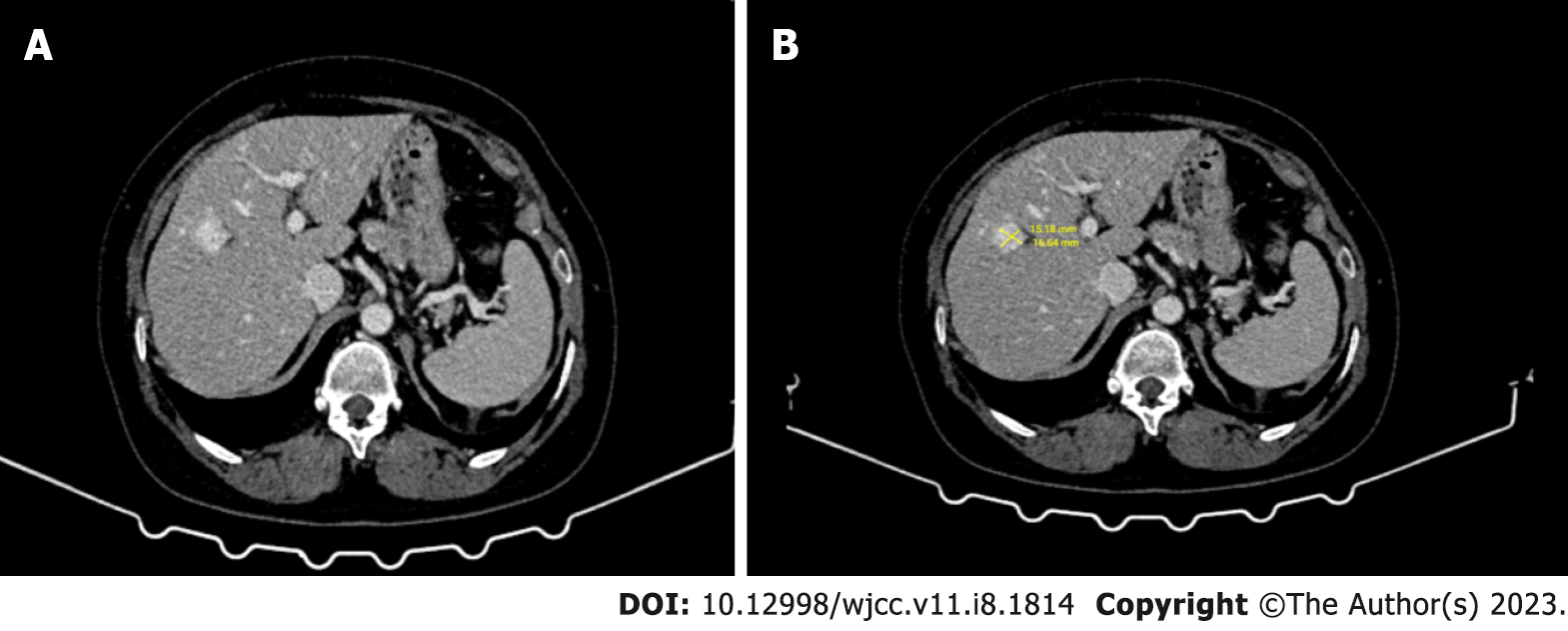
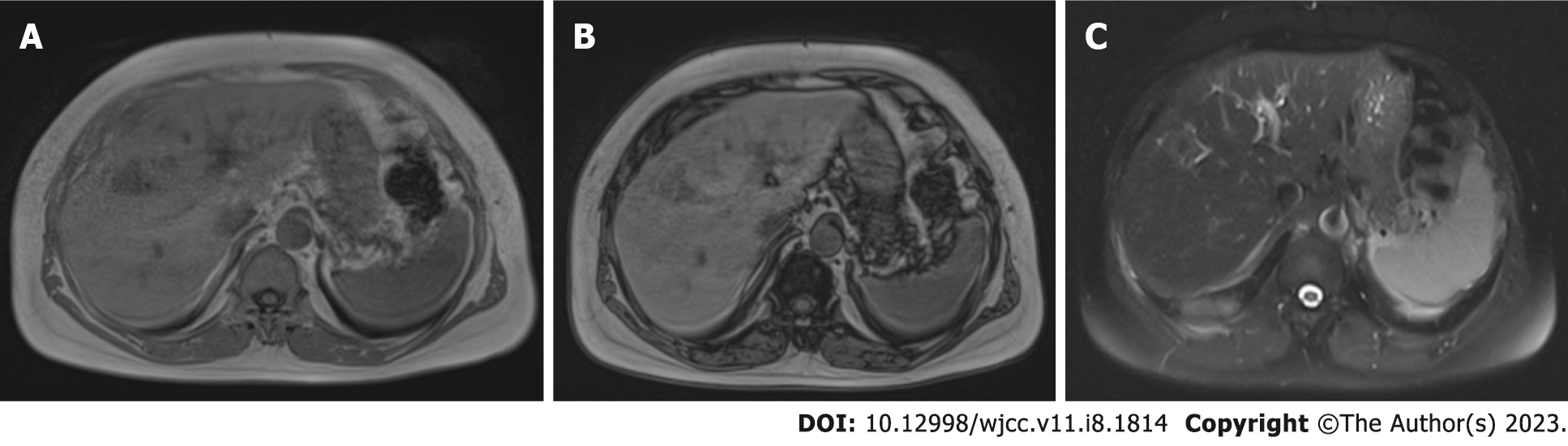
A 50-year-old female patient with VHL Syndrome was admitted to our hospital with nonspecific lesions such as right upper quadrant pain, weight loss and nausea. In laboratory tests, liver function test results were measured in normal values. Abdominal ultrasonography (US) revealed a hypoechoic heterogeneous lesion with faint contours in places; The tumor was 15 mm × 16 mm in size and was located in the 4th (Figure 1B) segment of the liver. On computed tomography (CT), it was observed as a nodular (diameter 16 mm) lesion with hyperdense appearance in the venous phase (Figure 1). In MRI, in phase and out phase images obtained by “Dual echo” method; When both sequences are compared, it is understood that the lesion does not contain any fat (Figure 2).
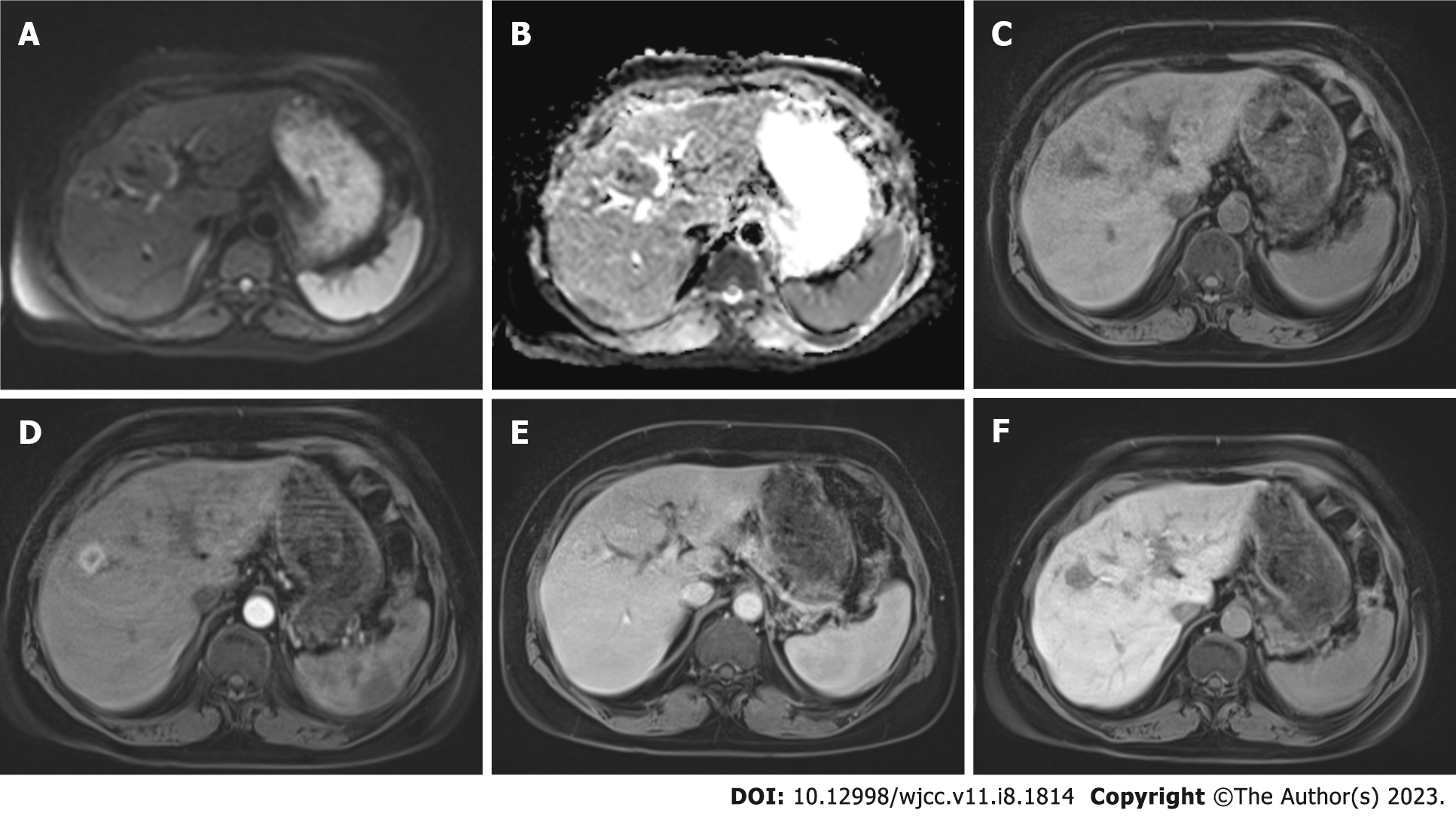
In this case from our hospital, iohexol (Opaxol 350 mg/100 mL) was used in CT and gadoxtic acid disodium (Primovist 0.25 mmol/mL) was used as hepatospecific agent in MR. In addition, the lesion revealed mild diffusion restriction on diffusion-weighted images, it was hypointense in comparison with normal liver parenchyma on T1 weighted image precontrast phase, it showed strong peripheral enhancement in the arterial phase, and continued to enhance in the venous and late venous phases. In the hepatobiliary phase images it was seen hypointense related to the normal parenchyma (Figure 3). The radiographic findings indicated a benign, highly vascular tumour devoid of hepatocytes. In conjunction with the known history of VHL Syndrome, we primarily considered the possibility of AML; nevertheless, the lesion’s lack of fat made it difficult to determine the diagnosis. Thereupon, histopathological sampling was taken and the diagnosis came as fat-poor AML with 5% fat content.
We would like to give some more general information about the hepatic AMLs we have mentioned over the case. Ishak[32] first described hepatic AMLs in 1976; they are tumors made up of altered fat, epithelioid and spindle smooth muscle cells, and thick-walled blood vessels. There is a clear female predominance in hepatic AMLs, which can affect patients of all ages. Patients with hepatic AML are typically asymptomatic; the tumor is frequently discovered by chance during physicals or tests for other illnesses. Patients with big AMLs experience symptoms brought on by the tumor’s compression[33,34].
The prevalence of this condition has increased as a result of recent developments in imaging techniques and a deeper comprehension of hepatic AMLs. Approximately 200 cases of hepatic AML have so far been recorded[16]. Additionally, it has been discovered that the relative proportions of the tumor components affect the imaging characteristics of hepatic AMLs[14-34]. Due to their rarity and varying imaging characteristics, hepatic AMLs are challenging to correctly diagnose preoperatively. In regions where HCC is prevalent, it is crucial to differentiate between hepatic AMLs and HCCs. Hepatic AMLs typically have a characteristic appearance on imaging tests due to their fat content, allowing for the preoperative separation of hepatic AMLs from HCCs[17-35].
In conclusion, the PHA in our case report and the fat-poor AML we presented from our clinic are two rare vascular tumors of the liver with similar incidences. In both, important imaging methods such as CEUS, CECT, and CEMRI provide us with significant gains. However, the definitive diagnosis is found by biopsy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moshref RH, Saudi Arabia; Reis F, Brazil S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Wang J, Sun LT. Primary hepatic angiosarcoma: A case report. World J Clin Cases. 2022;10:11590-11596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou C, Smith KD, Phung TL, Pollock RE, Benjamin R, Hunt KK, Lazar AJ, Lev D. Angiosarcoma: clinical and molecular insights. Ann Surg. 2010;251:1098-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Fayette J, Martin E, Piperno-Neumann S, Le Cesne A, Robert C, Bonvalot S, Ranchère D, Pouillart P, Coindre JM, Blay JY. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007;18:2030-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 4. | Chaudhary P, Bhadana U, Singh RA, Ahuja A. Primary hepatic angiosarcoma. Eur J Surg Oncol. 2015;41:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Zhu YP, Chen YM, Matro E, Chen RB, Jiang ZN, Mou YP, Hu HJ, Huang CJ, Wang GY. Primary hepatic angiosarcoma: A report of two cases and literature review. World J Gastroenterol. 2015;21:6088-6096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Zhou Y, Hou P, Wang F, Li B, Gao J. Primary hepatic malignant vascular tumors: a follow-up study of imaging characteristics and clinicopathological features. Cancer Imaging. 2020;20:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Trojan J, Hammerstingl R, Engels K, Schneider AR, Zeuzem S, Dietrich CF. Contrast-enhanced ultrasound in the diagnosis of malignant mesenchymal liver tumors. J Clin Ultrasound. 2010;38:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Zheng YW, Zhang XW, Zhang JL, Hui ZZ, Du WJ, Li RM, Ren XB. Primary hepatic angiosarcoma and potential treatment options. J Gastroenterol Hepatol. 2014;29:906-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Rao P, Lahat G, Arnold C, Gavino AC, Lahat S, Hornick JL, Lev D, Lazar AJ. Angiosarcoma: a tissue microarray study with diagnostic implications. Am J Dermatopathol. 2013;35:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Duan XF, Li Q. Primary hepatic angiosarcoma: a retrospective analysis of 6 cases. J Dig Dis. 2012;13:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Weitz J, Klimstra DS, Cymes K, Jarnagin WR, D'Angelica M, La Quaglia MP, Fong Y, Brennan MF, Blumgart LH, Dematteo RP. Management of primary liver sarcomas. Cancer. 2007;109:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Banshodani M, Ishiyama K, Amano H, Tashiro H, Arihiro K, Itamoto T, Ohdan H. Hepatic Angiomyolipoma with Minimal Intratumoral Fat Content. Case Rep Gastroenterol. 2009;3:324-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Maher ER, Kaelin WG. Von Hippel-Lindau disease. Medicine. 1997;76:381-391. [RCA] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 349] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Ganeshan D, Menias CO, Pickhardt PJ, Sandrasegaran K, Lubner MG, Ramalingam P, Bhalla S. Tumors in von Hippel-Lindau Syndrome: From Head to Toe-Comprehensive State-of-the-Art Review. Radiographics. 2018;38:849-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Poulsen ML, Budtz-Jørgensen E, Bisgaard ML. Surveillance in von Hippel-Lindau disease (vHL). Clin Genet. 2010;77:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Maher ER, Iselius L, Yates JR, Littler M, Benjamin C, Harris R, Sampson J, Williams A, Ferguson-Smith MA, Morton N. Von Hippel-Lindau disease: a genetic study. J Med Genet. 1991;28:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 358] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Neumann HP, Berger DP, Sigmund G, Blum U, Schmidt D, Parmer RJ, Volk B, Kirste G. Pheochromocytomas, multiple endocrine neoplasia type 2, and von Hippel-Lindau disease. N Engl J Med. 1993;329:1531-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 251] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Choyke PL, Glenn GM, Walther MM, Patronas NJ, Linehan WM, Zbar B. von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology. 1995;194:629-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 305] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Sgambati MT, Stolle C, Choyke PL, Walther MM, Zbar B, Linehan WM, Glenn GM. Mosaicism in von Hippel-Lindau disease: lessons from kindreds with germline mutations identified in offspring with mosaic parents. Am J Hum Genet. 2000;66:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Fernandes DA, Mourão JLV, Duarte JÁ, Dalaqua M, Reis F, Caserta NMG. Imaging manifestations of von Hippel-Lindau disease: an illustrated guide focusing on abdominal manifestations. Radiol Bras. 2022;55:317-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Schwingel R, Duarte SB, Oshima MM, Mesquita JV, Reis F. Which is your diagnosis? Radiol Bras. 2015;48:XI-XIII. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Tappouni R, Kissane J, Sarwani N, Lehman EB. Pseudoenhancement of renal cysts: influence of lesion size, lesion location, slice thickness, and number of MDCT detectors. AJR Am J Roentgenol. 2012;198:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Gläsker S, Vergauwen E, Koch CA, Kutikov A, Vortmeyer AO. Von Hippel-Lindau Disease: Current Challenges and Future Prospects. Onco Targets Ther. 2020;13:5669-5690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 24. | Choi YA, Kim CK, Park BK, Kim B. Evaluation of adrenal metastases from renal cell carcinoma and hepatocellular carcinoma: use of delayed contrast-enhanced CT. Radiology. 2013;266:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Aufforth RD, Ramakant P, Sadowski SM, Mehta A, Trebska-McGowan K, Nilubol N, Pacak K, Kebebew E. Pheochromocytoma Screening Initiation and Frequency in von Hippel-Lindau Syndrome. J Clin Endocrinol Metab. 2015;100:4498-4504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Blake MA, Kalra MK, Maher MM, Sahani DV, Sweeney AT, Mueller PR, Hahn PF, Boland GW. Pheochromocytoma: an imaging chameleon. Radiographics. 2004;24 Suppl 1:S87-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | Schieda N, Alrashed A, Flood TA, Samji K, Shabana W, McInnes MD. Comparison of Quantitative MRI and CT Washout Analysis for Differentiation of Adrenal Pheochromocytoma From Adrenal Adenoma. AJR Am J Roentgenol. 2016;206:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Jacques AE, Sahdev A, Sandrasagara M, Goldstein R, Berney D, Rockall AG, Chew S, Reznek RH. Adrenal phaeochromocytoma: correlation of MRI appearances with histology and function. Eur Radiol. 2008;18:2885-2892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Prasad V, Tiling N, Denecke T, Brenner W, Plöckinger U. Potential role of (68)Ga-DOTATOC PET/CT in screening for pancreatic neuroendocrine tumour in patients with von Hippel-Lindau disease. Eur J Nucl Med Mol Imaging. 2016;43:2014-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Mourão JLV, Borella LFM, Duarte JÁ, Dalaqua M, Fernandes DA, Reis F. Imaging manifestations of von Hippel-Lindau disease: an illustrated guide focusing on the central nervous system. Radiol Bras. 2022;55:188-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | VHL Alliance. The VHL handbook: What you need to know about VHL. Boston, MA: VHL Alliance. Independently published, 2020. [DOI] [Full Text] |
| 32. | Ishak KG. Mesenchymal Tumors of the Liver. New York: John Wiley Medical, 1976: 247-307. |
| 33. | Ren N, Qin LX, Tang ZY, Wu ZQ, Fan J. Diagnosis and treatment of hepatic angiomyolipoma in 26 cases. World J Gastroenterol. 2003;9:1856-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Jiang TA, Zhao QY, Chen MY, Wang LJ, Ao JY. Diagnostic analysis of hepatic angiomyolipoma. Hepatobiliary Pancreat Dis Int. 2005;4:152-155. [PubMed] |
| 35. | Högemann D, Flemming P, Kreipe H, Galanski M. Correlation of MRI and CT findings with histopathology in hepatic angiomyolipoma. Eur Radiol. 2001;11:1389-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |