Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1642
Peer-review started: December 5, 2022
First decision: January 12, 2023
Revised: January 19, 2023
Accepted: February 16, 2023
Article in press: February 16, 2023
Published online: March 6, 2023
Processing time: 87 Days and 1 Hours
Extragonadal yolk sac tumors (YSTs) are rare, with only a low reported tumor occurrence outside the gonads locally and abroad. Extragonadal YSTs are usually a diagnostic challenge, because they are infrequent, but also because a thoughtful and detailed differential diagnostic process must be performed.
Here we present a case of an abdominal wall YST in a 20-year-old woman admitted with a tumor in the lower abdomen close to the umbilicus. The tumorectomy was performed. The histological examination revealed characteristic findings such as Schiller-Duval bodies, loose reticular structures, papillary structures, and eosinophilic globules. According to the immunohistochemical staining, the tumor tissue was positive for broad-spectrum cytokeratin, Spalt-like transcription factor 4, glypican-3, CD117, and epithelial membrane antigen. Based on the clinical information, histological features, and immunohistochemical staining profile, the tumor was diagnosed as a YST present in the abdominal wall.
Based on the clinical information, histological features, and immunohistochemical staining profile described above, the tumor was diagnosed as a primary YST in the abdominal wall.
Core Tip: Extragonadal yolk sac tumors (YSTs) are usually a diagnostic challenge, because they are infrequent, but also because a thoughtful and detailed differential diagnostic process must be performed. In this case, since the tumor presented classic yolk sac tumor features, and immunohistochemistry was concordant, the diagnostic process was more straightforward, but not simplistic. And this is the first reported case of a primary YST in the abdominal wall of an adult.
- Citation: Wang Y, Yang J. Primary yolk sac tumor in the abdominal wall in a 20-year-old woman: A case report . World J Clin Cases 2023; 11(7): 1642-1649
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1642.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1642
Yolk sac tumor (YST), also known as endodermal sinus tumor or primitive endodermal tumor, is a rare and highly malignant tumor of germ cell origin. It is common in infant and adolescent children with a median age at diagnosis of 19 years, with 40% of patients diagnosed in the prepubertal period[1,2]. This tumor occurs mostly in the ovary and the testis, and it rarely occurs outside the gonads, with only 10%–15% of YST cases reported locally and abroad[3]. Extragonadal YST can occur in the mediastinum[4,5], retroperitoneum[6], cervix[7], endometrium[8], sacrococcygeal region[9], vagina[10,11], omentum[12], urachus[13], and liver[14]. Moreover, the clinical manifestations vary according to the site of tumorigenesis, which often makes the clinical diagnosis difficult. Some cases lack typical structures for proper morphological observation, while others have diverse pathological morphology.
YSTs arising in the abdominal wall are even rarer, with less than 2 cases of primary abdominal wall YST reported in the literature[15,16]. Here, we report a special case of primary YST in the abdominal wall of a 20-year-old woman, which to the best of our knowledge, is the first report of this disease in an adult.
A 20-year-old Chinese woman was admitted to the First Affiliated Hospital of Jinzhou Medical University in January 2022 with a lower abdominal mass, which had progressively enlarged since November 2021. It was not painful or itchy.
Palpation revealed that the mass was located in the hypogastric region, with a size of about 5 cm × 3 cm, The lesion was well defined, was not capsulated, normal skin without redness or swelling, poor range of motion, and no tenderness.
The patient has no past medical history.
The patient denied any family history. Healthy parents, no siblings.
On physical examination, the vital signs were as follows: Body temperature, 36.3 °C; blood pressure, 113/63 mmHg; heart rate, 86 beats per min; respiratory rate, 20 breaths per min. Palpation revealed that the mass was located in the hypogastric region, with a size of about 5 cm × 3 cm, no redness and tenderness.
The results such as routine hematological testing, blood sedimentation rate, and tumor-associated markers were normal.
Because the location of the tumor was relatively superficial, there was no imaging information at the beginning. The Enhanced Computed tomography (CT) examination revealed no abnormalities in the abdominal organs and other regions, so this case was considered a primary tumor in the abdominal wall (Supplementary Figure 1).
A primary YST in the abdominal wall.
The patient underwent subcutaneous tumor resection. The mass was found to be located in the deep layer of subcutaneous fat, and the entire mass was completely dissected and sent for biopsy. We found the cut surface of the tumor was greyish-red and greyish-yellow in colour, soft to medium in quality, with visible necrosis in some areas (about 10%) (Supplementary Figure 2). It was first considered as a possible soft tissue tumor or metastatic carcinoma.
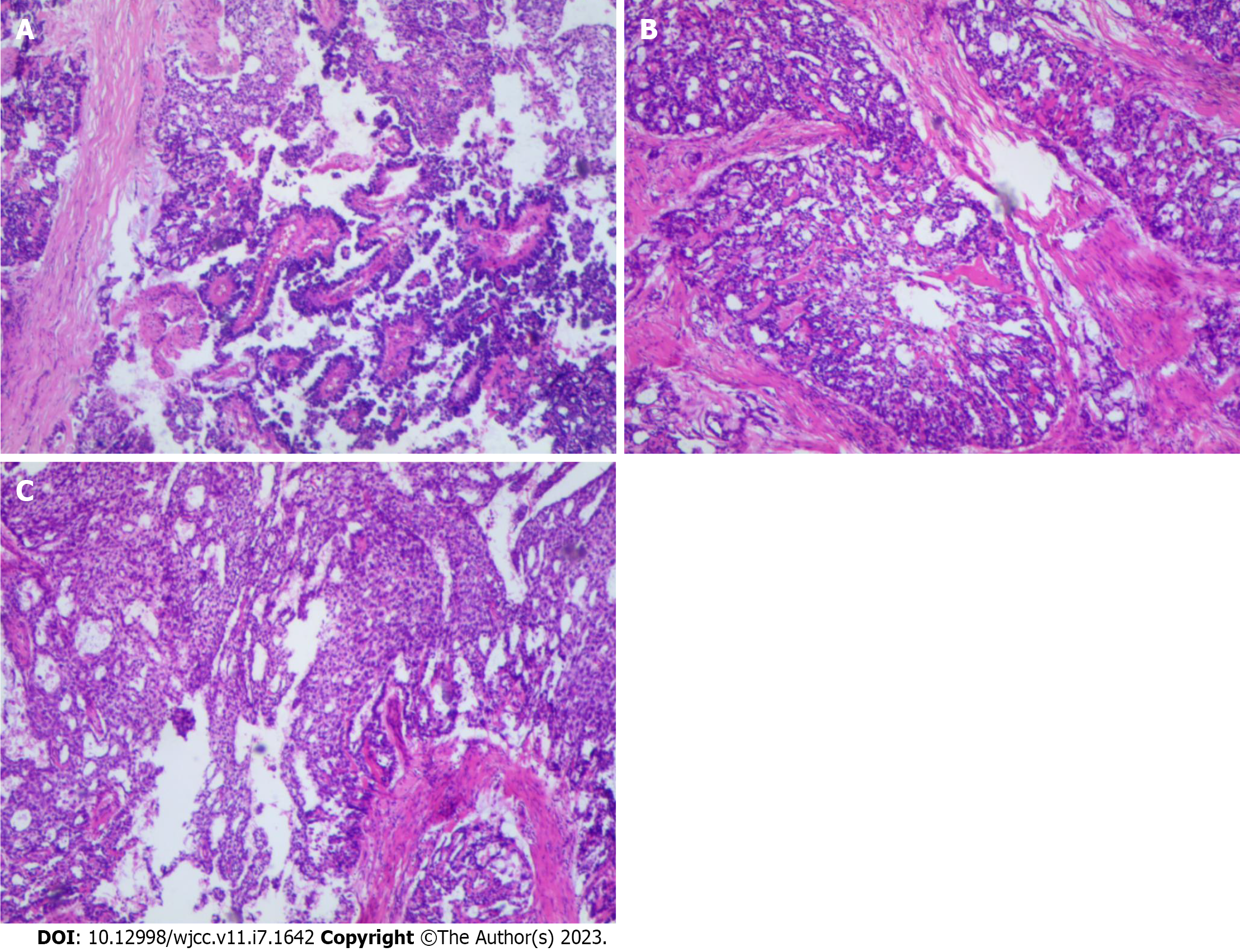
After the tumor tissue was resected, it was fixed with 10% neutral-buffered formalin and then embedded in paraffin blocks. The tissue blocks were cut into 4-μm sections. Then, the hematoxylin and eosin-stained sections were used for histological evaluation. Histological findings from the resected tumor specimen showed classic micro vesicular, solid trabecular, papillary morphology and cystically area at low power magnification (Figure 1). The surgical margins were negative, and it had visible necrosis, with no vascular invasion.
The morphology of the tumor tissues was diverse (Figure 2), and the main microscopic manifestations were as follows: (1) Characteristic Schiller-Duval bodies, which had central capillaries surrounded by a layer of cuboidal or low columnar embryonal epithelioid cells attached to the periphery; (2) Loose reticular structure, composed of a basophilic myxoid matrix, reticular microcysts, with or without a slit-like structure; the capsule wall was covered with flat, pleomorphic, or mesothelioid cells, which often had large and dark nuclei and cysts of varying sizes; (3) Papillary structure, with papillae composed of connective tissue axes and overlying epithelioid cells, with significant cellular and nuclear pleomorphism; the connective tissue had varying degrees of hyalinization; (4) Solid structure, with an area composed of aggregates of small epithelioid polygonal cells with clear cytoplasm, large nuclei, and prominent nucleoli; (5) Mucoid structure. A few cords and adenoids composed of epithelioid cells arranged in myxoid stroma were observed; and (6) Eosinophilic globules or hyaline bodies.
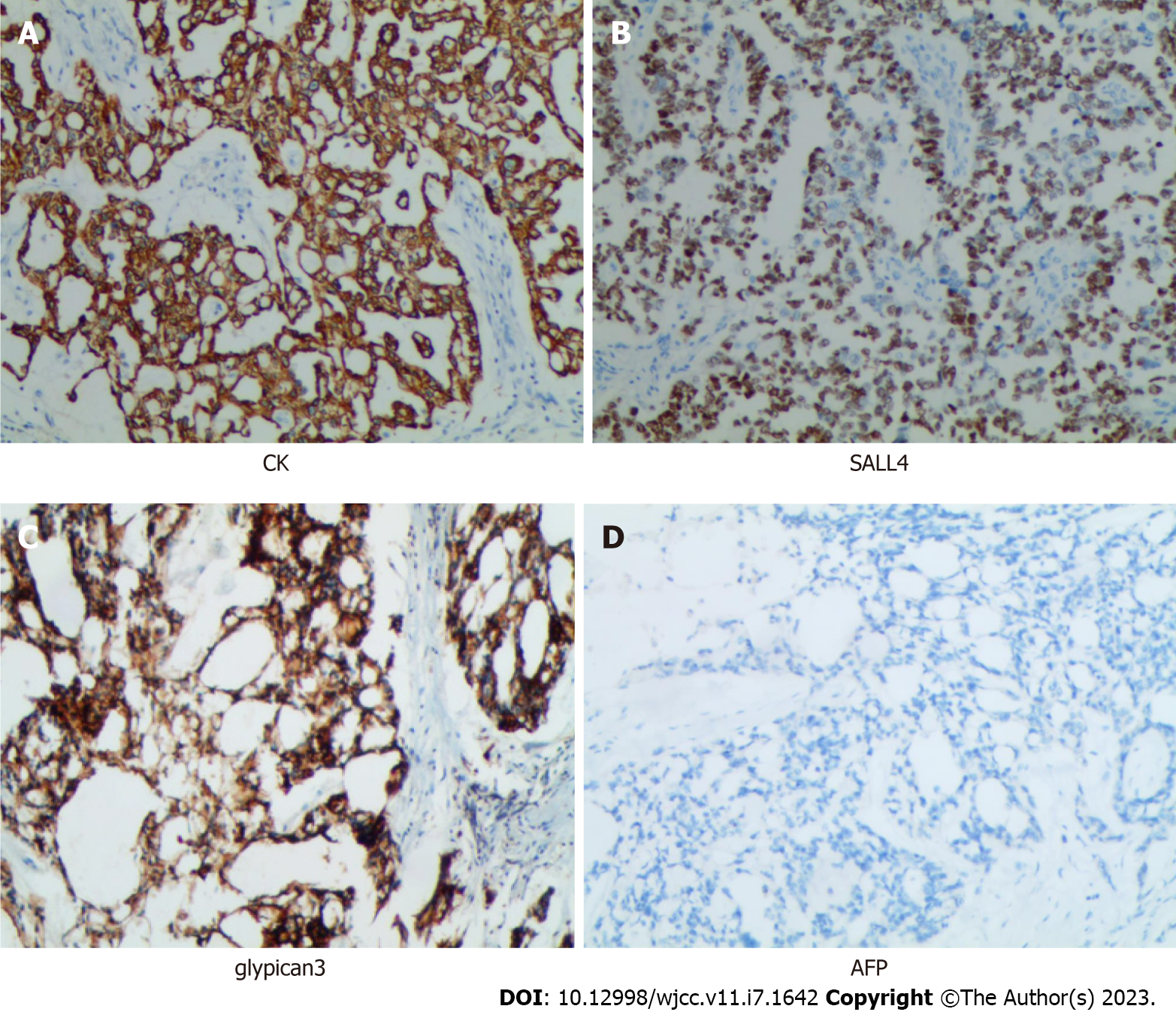
Immunohistochemistry were performed to establish a definitive diagnosis. The detection of the antibodies was accomplished using the streptavidin peroxidase method[17]. Immunostaining showed that broad-spectrum creatine kinase (CK), glypican-3 (GPC3) was strongly positive in almost all neoplastic cells. Spalt-like transcription factor 4 (SALL4) was also strongly positive in the nuclei of the neoplastic cells (Figure 3), while CD117 and epithelial membrane antigen (EMA) were positive in a little range of neoplastic cells [Because the positive range was minimal (around 5%), we have reason to suspect that they were false positives]. The percentage of cells that stained positively for Ki67 was about 40%. The expression of alpha-fetoprotein (AFP), Vimentin, CD31, CD34, D2-40, S100, Octamer-binding transcription factor 3/4 (OCT3/4), Desmin, Smooth Muscle Antibody, Paired box protein 8, Wilms Tumor gene 1, Placental alkaline phosphatase, Estrogen receptor, Progesterone receptor, and human chorionic gonadotrophin were negative in almost all neoplastic cells. Because it was shown in the literature that nearly all YST expressed AFP, we performed the second stain of AFP, and the result was also negative. In addition, we sent the samples to "Cancer Hospital, Chinese Academy of Medical Sciences" for counterstaining, AFP was still negative in expression.
The tumor was then resected without significant bleeding, and the patient recovered well without any signs of recurrence up to now. Remission was achieved after radical surgery, the patient was in good health. At the 3-, 6-, and 9-mo follow-up, the patient did not show local recurrence or distant metastasis, and the serum AFP was within normal levels. The patient will be followed up with clinical examinations and serum AFP level measurements every 3 mo for the first 2 years and every 6 mo thereafter for the next 3 years.
Based on the clinical information, histological features, and immunohistochemical staining profile described above, the tumor was diagnosed as a primary YST in the abdominal wall. YST is a common tumor in the gonads, especially in the ovary and testis, and is rarely found in regions other than the gonads, according to a few documented reports. Extragonadal YST may have derived from the malposition of germ cells during embryogenesis. During embryonic development, germ cells migrate from the yolk sac via the midline dorsal mesentery to the genital ridge. During migration, some germ cells may remain anywhere along the migration pathway and develop into germ cell tumors[18]. These tumor locations include the liver and sacrococcygeal, gastric, retroperitoneal, and other regions[19-22]. Even fewer cases have been reported of YST occurrence in the abdominal wall. To date, only 2 cases of primary abdominal wall YST have been reported in the medical literature (Table 1)[15,16]. Both reports describe tumors in female infants, while our case occurred in a 20-year-old adult. And the 2 cases all expressed AFP, while in our case AFP was negative in expression. Ultimately, we believe that this was also a special feature of our case that is different from previous YST cases.
Previous reports, including this case, have summarised the features of the primary abdominal wall YST, which often occurs in younger children. Macroscopically, these tumors are typically well-circumscribed, round, or spherical. The cut surface can appear greyish-white, greyish-red, or greyish-yellow. The histological pattern of this tumor is similar to the patterns found in the gonads. In this case, because the mass was located in the lower abdomen, the initial consideration of the clinicians was a sebaceous gland tumor, soft tissue tumor, or metastatic carcinoma. Thus, the patient in this case was not tested for AFP before the surgery. After resection, the patient’s serum AFP level was 2.33 ng/mL (normal range: 0–8.04 ng/mL).
To make a correct diagnosis, the metastatic YST from the ovary should first be excluded through detailed clinical examination and evaluation. Moreover, the enhanced CT examination revealed no abnormalities in the abdominal organs and other regions, so this case was considered a primary tumor in the abdominal wall. Other differential diagnoses include embryonal carcinoma, dysgerminoma, clear cell carcinoma, teratoma, and other types of germ cell tumors. These different types of tumors can be distinguished by their respective histological features and immunohistochemical results. Embryonal carcinoma may show a solid structure, and the CK, SALL4, and GPC3 expression can be measured using immunohistochemistry. However in embryonal carcinoma, the cells were more atypical, with large nuclei, prominent nucleoli, and no basement membrane-like material. The immunomarkers CD30 and OCT3/4 were positive in embryonal carcinoma, even though these were rarely observed in YST. Compared with YST tumor cells, dysgerminoma cells were more polygonal, the nucleoli were more obvious, and most were arranged in solid, cord-like, small tubular, and insular patterns. Meanwhile, the YST mostly showed cystic reticular structures with single solid structure. As for the immunohistochemical analysis, D2-40 and OCT4 were positive, and GPC3 and CK were negative in dysgerminoma, while D2-40 and OCT4 were negative, and CK and GPC3 were positive in YST. Clear cell carcinoma had a more regular tubular structure, often accompanied by papillary projections, cells overlying the tubules were cuboidal, clear cytoplasm, or boot stud-like cells, and lacked the typical perivascular structure of YST.
Immunohistochemically, clear cell carcinoma was diffusely strong positive for EMA and negative or partially positive for GPC3, while our results showed that very few areas of EMA are positive, while GPC3 was diffusely strong positive. Cystic mature teratomas were generally composed of three well-differentiated germ layers (endoderm, mesoderm, and ectoderm), and tumors were often cystic with a relatively intact capsule. Not consistent with the tumor we saw.
At present, there is no specific tumor marker for YST. The immunostaining of YST often displays the broad-spectrum CK, GPC3, and SALL4 positivity, which can be helpful for its diagnosis. YST has variable morphological features: microcystic/reticular, glandular, and solid structures are the more common patterns, while papillary and hepatoid structures are less common. Mixed histologic patterns were present in two-thirds of the cases, while Schiller-Duval bodies were seen in one-fifth of the cases[6]. As for the solid patterns, the neoplastic cells usually have mostly pale to clear abundant cytoplasm, with frequent intercellular basement membrane deposits, rare microcysts, nuclear pleomorphism, and hyaline globules. Thus, the present case showed a typical structure of YST.
Extragonadal YSTs are usually a diagnostic challenge, because they are infrequent, but also because a thoughtful and detailed differential diagnostic process must be performed. In this case, since the tumor presented classic YST features, and immunohistochemistry was concordant, the diagnostic process was more straightforward, but not simplistic. And this is the first reported case of a primary YST in the abdominal wall of an adult. As for the prognosis of the disease is generally poor, the authors could follow up the patient closely.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hasan A, Egypt; Pandey A, India; Tsujinaka S, Japan S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Fischerova D, Indrielle-Kelly T, Burgetova A, Bennett RJ, Gregova M, Dundr P, Nanka O, Gambino G, Frühauf F, Kocian R, Borcinova M, Cibula D. Yolk Sac Tumor of the Omentum: A Case Report and Literature Review. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Ronchi A, Cozzolino I, Montella M, Panarese I, Zito Marino F, Rossetti S, Chieffi P, Accardo M, Facchini G, Franco R. Extragonadal germ cell tumors: Not just a matter of location. A review about clinical, molecular and pathological features. Cancer Med. 2019;8:6832-6840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Shebib S, Sabbah RS, Sackey K, Akhtar M, Aur RJ. Endodermal sinus (yolk sac) tumor in infants and children. A clinical and pathologic study: an 11 year review. Am J Pediatr Hematol Oncol. 1989;11:36-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Aroshidze B, Boyapati L, Pokhrel A, Gotlieb V, Khan A, Erdinc B, Cheema MA. Yolk Sac Tumor in the Anterior Mediastinum Presenting as Acute Pericarditis. Am J Case Rep. 2022;23:e932616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Darif K, Benbrahim Z, Acharfi N, Khacha A, Maaroufi M, Amaadour L, Oualla K, Arifi S, Mellas N. A primary mediastinal germ cell tumor of yolk sac type: case report. Pan Afr Med J. 2021;38:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Ravishankar S, Malpica A, Ramalingam P, Euscher ED. Yolk Sac Tumor in Extragonadal Pelvic Sites: Still a Diagnostic Challenge. Am J Surg Pathol. 2017;41:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Torino G, Roberti A, Turrà F, Donofrio V, Bifano D, Abate M, Iorio GD. Laparoscopic Trachelectomy for Cervix Yolk Sac Tumor in 11-Month-Old Girl: The Youngest Case. J Pediatr Adolesc Gynecol. 2021;34:872-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 8. | Lu T, Qi L, Ma Y, Lu G, Zhang X, Liu P. Primary yolk sac tumor of the endometrium: a case report and review of the literatures. Arch Gynecol Obstet. 2019;300:1177-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Sharma R, Khera S, Sinha A, Yadav T. Pure yolk sac tumor of sacrococcygeal region. Autops Case Rep. 2021;11:e2021287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Elbaz M, Qadiry RE, Fouraiji K, Jalal H, Elhoudzi J. Yolk sac tumor of vagina: a rare cause of vaginal bleeding in adolescents - a case report. Pan Afr Med J. 2020;37:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Zunana C, Peña TM, Osorio N, López Martí J, Califano P. [Primary vaginal yolk sac tumor in a girl. Case report]. Arch Argent Pediatr. 2021;119:e643-e647. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Kim SW, Park JH, Lim MC, Park JY, Yoo CW, Park SY. Primary yolk sac tumor of the omentum: a case report and review of the literature. Arch Gynecol Obstet. 2009;279:189-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Suma TL, Ramanathan S, Padma M, Appaji L, Suma MN. A Case of Urachal Yolk Sac Tumor With Spontaneous Rupture in a Child. J Pediatr Hematol Oncol. 2017;39:e82-e84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Vanidassane I, Sharma V, Ramteke P, Yadav MK, Batra A. Primary Yolk Sac Tumor of the Liver in an Adult Man. ACG Case Rep J. 2019;6:e00050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Reddy M, Roy PS, Menon P, Solanki S, Gupta S, Samujh R, Trehan A. Abdominal Wall Yolk Sac Tumor in a Child. J Indian Assoc Pediatr Surg. 2022;27:94-96. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | van den Akker M, Vervloessem D, Huybrechs A, Declercq S, van der Werff Ten Bosch J. Yolk sac tumor in the abdominal wall of an 18-month-old girl: a case report. J Med Case Rep. 2017;11:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Lei L, Jiang G, Yang L, Xu H. Primary pleomorphic adenoma of the lung with positive staining of TTF-1: a case report and review of literature. Int J Clin Exp Med. 2018;11:378-383. |
| 18. | Spatz A, Bouron D, Pautier P, Castaigne D, Duvillard P. Primary yolk sac tumor of the endometrium: a case report and review of the literature. Gynecol Oncol. 1998;70:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Merchant A, Stewart RW. Sacrococcygeal yolk sac tumor presenting as subcutaneous fluid collection initially treated as abscess. South Med J. 2010;103:1068-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Jindal A, Mukund A, Bihari C. Primary yolk sac tumour of liver. Liver Int. 2021;41:2212-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Qureshi A, Al-Moundhri M, Al-Shaibi M, Al-Haddabi I, Mittal A. Primary Gastric Yolk Sac Tumour. Sultan Qaboos Univ Med J. 2018;18:e383-e385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Nair VG, Kiran HS, Shanthala PR. Pure Primary Extragonadal Retroperitoneal Yolk Sac Tumour in a Young Child: A Case Report. J Clin Diagn Res. 2017;11:ED09-ED11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |