Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1385
Peer-review started: November 10, 2022
First decision: November 22, 2022
Revised: November 30, 2022
Accepted: January 31, 2023
Article in press: January 31, 2023
Published online: February 26, 2023
Processing time: 106 Days and 5.6 Hours
Direct infiltration of the pancreas by acute myeloid leukemia (AML) with acute pancreatitis (AP) as an initial symptom is extremely rare. Only once in the literature, the leukemia cells in AML have been implicated as the cause of AP. Pancreatitis caused by a rare predisposing factor is often misdiagnosed as idiopathic pancreatitis or pancreatitis of other common causes. Severe AP (SAP) progresses rapidly with a high fatality rate. Therefore, it is important to identify the predisposing factors in the early stage of SAP, evaluate the condition, determine prognosis, formulate treatment plans, and prevent a recurrence. Here, we describe a case of SAP due to AML.
A 61-year-old man presented to the hospital with fever and persistent abdominal pain. Blood analysis presented significantly elevated serum amylase and severe thrombocytopenia. Computed tomography examination of the abdomen revealed peripancreatic inflammatory effusion. The patient had no common etiologies and risk factors for AP, but the concurrent severe thrombocytopenia could not be explained by pancreatitis. Finally, the bone marrow aspirate and biopsy inspection revealed the underlying reason for pancreatitis, AML (M2 type based on the French-American-British classifications system).
Direct infiltration of the pancrease by acute leukemia, particularly AML cells, is an infrequent cause of AP. Therefore, although AP is a rare extramedullary infiltration characteristic for AML patients, it should be considered when determining the etiology of AP.
Core Tip: Although acute pancreatitis (AP) is a rare extramedullary infiltration characteristic for acute myeloid leukemia patients, it should be considered when determining the etiology of AP. Early diagnosis and etiological management can help avoid ineffective treatments and improve the outcomes. To better diagnose and treat such patients, we review the literature available on leukemia complicated by AP and analyze its mechanisms and clinical symptoms.
- Citation: Yang WX, An K, Liu GF, Zhou HY, Gao JC. Acute pancreatitis as initial presentation of acute myeloid leukemia-M2 subtype: A case report. World J Clin Cases 2023; 11(6): 1385-1392
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1385.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1385
Acute pancreatitis (AP) is caused by the premature activation of pancreatic enzymes, which leads to inflammatory disorders of the pancreatic system and pancreatic cell auto-digestion[1]. AP patients often present to the emergency department with the complaint of persistent abdominal pain. The common etiologies of AP include gallstones, alcohol abuse, medication, and metabolic disorders such as hyperlipidemia, hypercalcemia, and endoscopic retrograde cholangiopancreatography. Severe AP (SAP) progresses rapidly with a high fatality rate. Therefore, physicians must identify the inducing factors early to evaluate the condition and devise treatment plans.
Acute myeloid leukemia (AML) is the most common form of acute leukemia in adults. It is characterized by abnormal proliferation of undifferentiated hematopoietic stem cells in bone marrow with damage to the normal blood cells. Its clinical features and prognosis show significant variation. Primarily due to poor prognosis and high mortality, it reduces the quality of life of patients. According to a recent study on AML in the United States, the M2 subtype was the most common (25%) of AML based on the French-American-British (FAB) classification, and the five-year survival rate for patients with AML is 28.3%[2]. The percentage of deaths increases with age.
Current literature concentrates pancreatitis associated with acute leukemia more on the use of chemotherapeutic drugs, and mainly in children with acute lymphoblastic leukemia (ALL). Direct infiltration of the pancreas by acute leukemia, particularly AML cells, is an infrequent cause of AP. Therefore, a better understanding of the extramedullary infiltration characteristic for AML is urgently needed. And when determining the etiology of AP, the possibility of acute leukemia should be considered.
Herein, we present a case of pancreas infiltration in a 61-year-old male AML patient, and through a literature review of previous cases, we analyze and summarize the features and potential mechanism for the extramedullary infiltration of AML.
A 61-year-old Chinese man was admitted to the emergency department with acute pain in the left upper abdomen with progressive worsening for 3 h.
Symptoms started 3 h before presentation with persistent epigastric pain initially, and then it gradually developed to diffuse abdominal tenderness with nausea, emesis, and lumbar-back radiating pain.
This patient had no history of chronic diseases, such as hypertension, hyperuricemia, hyperlipidemia, and coronary heart disease.
The patient was a non-smoker and there was no history of alcohol consumption. The patient denied receiving chemotherapy or undergoing recent trauma. His family history was also not significant.
Vital monitoring at admission showed a pulse rate of 111 bpm, blood pressure of 161/111 mmHg, body temperature of 38.4 °C, and respiratory rate of 21 breaths/min.
There was no jaundice detected on the skin and sclera. During chest auscultation, decreased breath sounds were heard in bilateral lungs. Abdominal examination disclosed diffuse abdomen tenderness, abdominal muscle tension, and slightly decreased bowel sounds. Cullen's, Gray-Turner's, and Murphy signs were absent.
Initial laboratory testing indicated that the white blood cell (WBC) count was 7.63 × 109/L (reference range: 3.5-9.5 × 109/L), with a monocyte percentage of 18.10%, low platelet count 12 × 109/L (reference range: 150-400 × 109/L), hemoglobin 12.2 g/dL (reference range: 12-16 g/dL), C-reactive protein 0.13 mg/dL (reference range: 0-0.33 mg/dL), elevated level of serum amylase (4288 U/L, reference range: 35-135 U/L), and normal bilirubin, triglycerides, and serum calcium. The laboratory examination revealed severe thrombocytopenia, mild anemia, increased monocyte count, and significantly increased serum amylase.
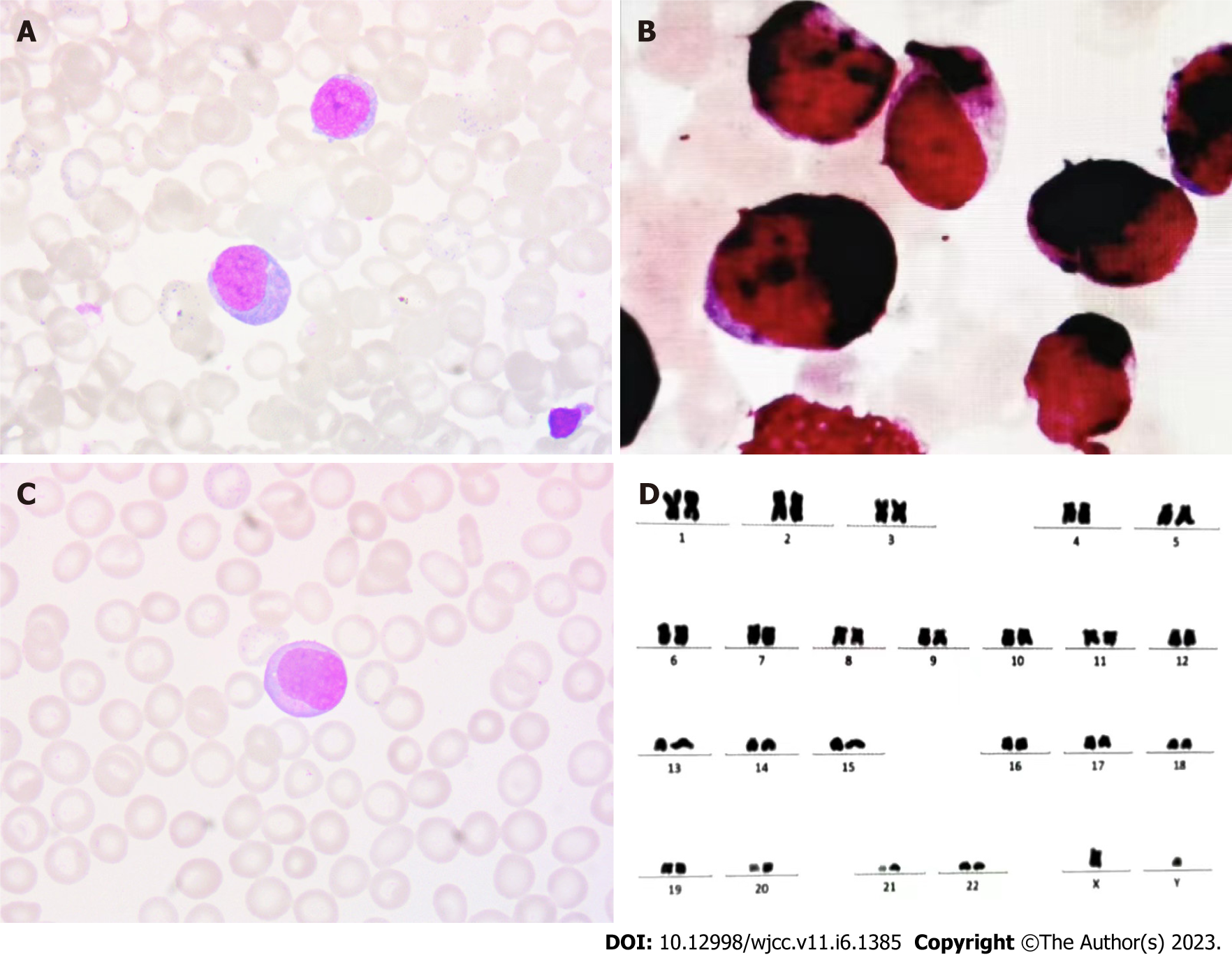
The significant decrease in platelet count could not be explained based on pancreatitis and infection, thus, blood system diseases must be considered. Bone marrow cell morphology (Figure 1A) revealed active bone marrow hyperplasia with increased myeloblasts (approximately 41%). The size of myeloblasts varied, and most of them were nearly round. There was medium cytoplasmic volume, stained blue, dark edge color. The nuclei were slightly irregular, pitted, and folded, and the nucleolus was fine-granular, with mostly 2-4 nucleolus. The proportion of red blood cells was normal, mainly polychromatic normoblasts and metarubricytes, and the size of mature red blood cells was different. The proportion of lymphocytes was normal, and they were mature lymphocytes. There were rare platelets. Myeloperoxidase staining was strongly positive (Figure 1B). Peripheral blood film depicted no significant increase in WBC count. Myeloblasts were more common, with a similar morphology as bone marrow (Figure 1C). FAB AML-M2 type seemed more likely, taking acute leukemia into account. Cytogenetic analysis of the bone marrow showed a 46 XY karyotype (Figure 1D). On flow cytometry, myeloblasts (68.93%) were positive for CD34, CD117, CD38, CD64, CD11c, CD33, CD13, and CD7 and negative for CD15, CD36, CD14, CD16, CD19, CD3, CD20, CD56, and CD 10. Monoblasts (19.36%) were positive for CD14, CD15, CD38, CD7, CD64, CD36, CD11c, CD33, and CD13 and negative for CD20, CD19, CD3, CD56, CD7, CD9, and CD10. The gene mutation tests about the myeloid malignancies showed CEBPA double mutations, CSF3R mutation, and STAG2 mutation. CEBPA and CSF3R mutations were associated with negative prognostic factors in AML. Gene tests were also performed with probes specific for more than 50 genes, including RUNX-1 fusion and JAK2 fusion genes, and the results of these studies were normal.
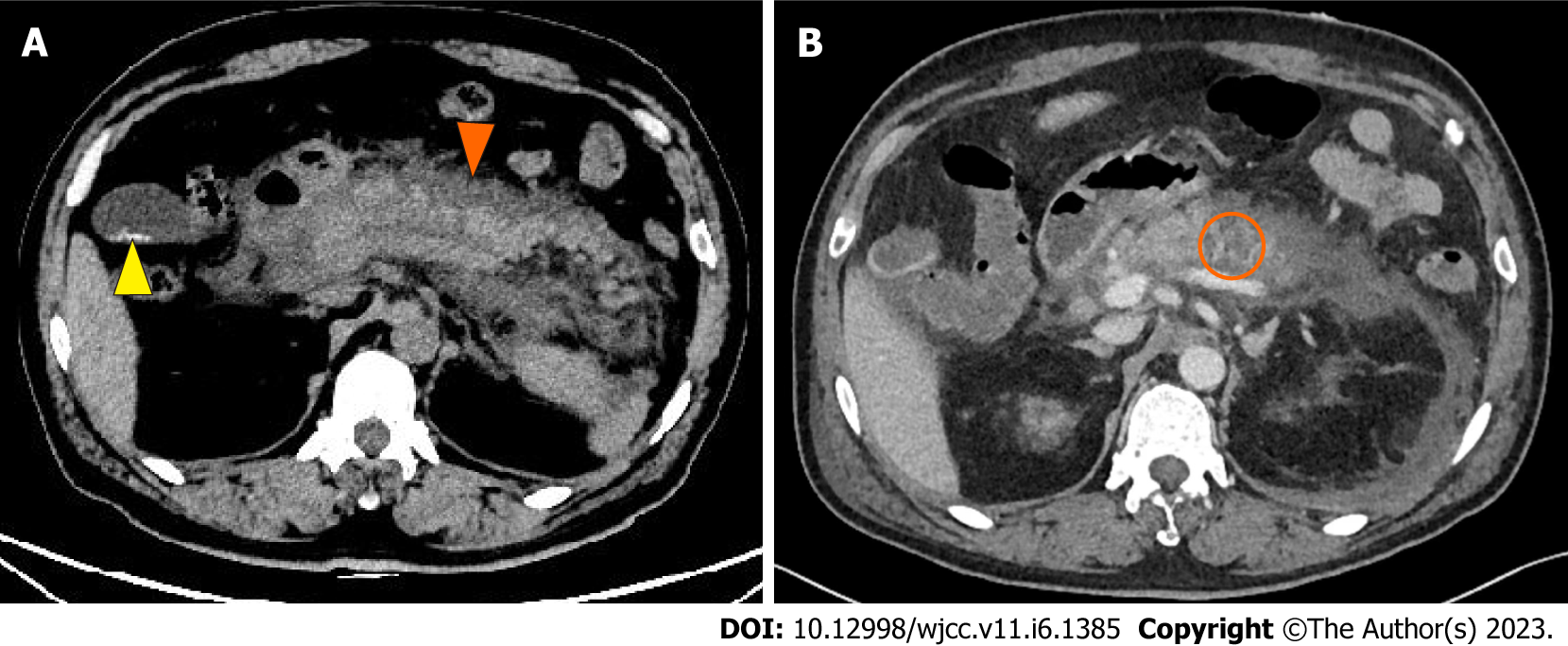
Computed tomography (CT) of the abdomen demonstrated pancreatitis with diffuse edema of the pancreas, peripancreatic effusion, and gallbladder stones (Figure 2A).
Contrast-enhanced CT of the thorax and abdomen in the next day suggested: (1) Bilateral pleural effusion and adjacent atelectasis; (2) Pancreatitis accompanied by peripheral inflammatory exudation and uneven enhancement of pancreatic parenchyma. There were also hypodense lesions infiltrating the pancreas and a slightly thicker adjacent duodenal wall; and (3) Ascites (Figure 2B).
Based on the patient’s medical history, clinical characteristics, and diagnostic test results, the final diagnosis was AML with SAP (extramedullary infiltration).
After infection control, fluid resuscitation, blood component transfusion, and symptomatic antipyretic treatment, the inflammatory indicators decreased, and abdominal pain and bloating were alleviated, with the resumption of oral intake. However, the patient still had intermittent fever with the onset of dyspnea, shortness of breath, and wheezing. Arterial blood gas analysis indicated respiratory failure (PO2 = 6.44 kPa, PCO2 = 5.84 kPa, and oxygenation index = 230 mmHg). After non-invasive ventilator support, the patient’s oxygenation index was above 200 mmHg, and his shortness of breath and wheezing were improved. Pancreatitis remained relatively stable. The patient was hospitalized for 9 d and continued to undergo treatment of AML at another hospital. He received chemotherapy with the idamycin and cytarabine regimen in the hospital.
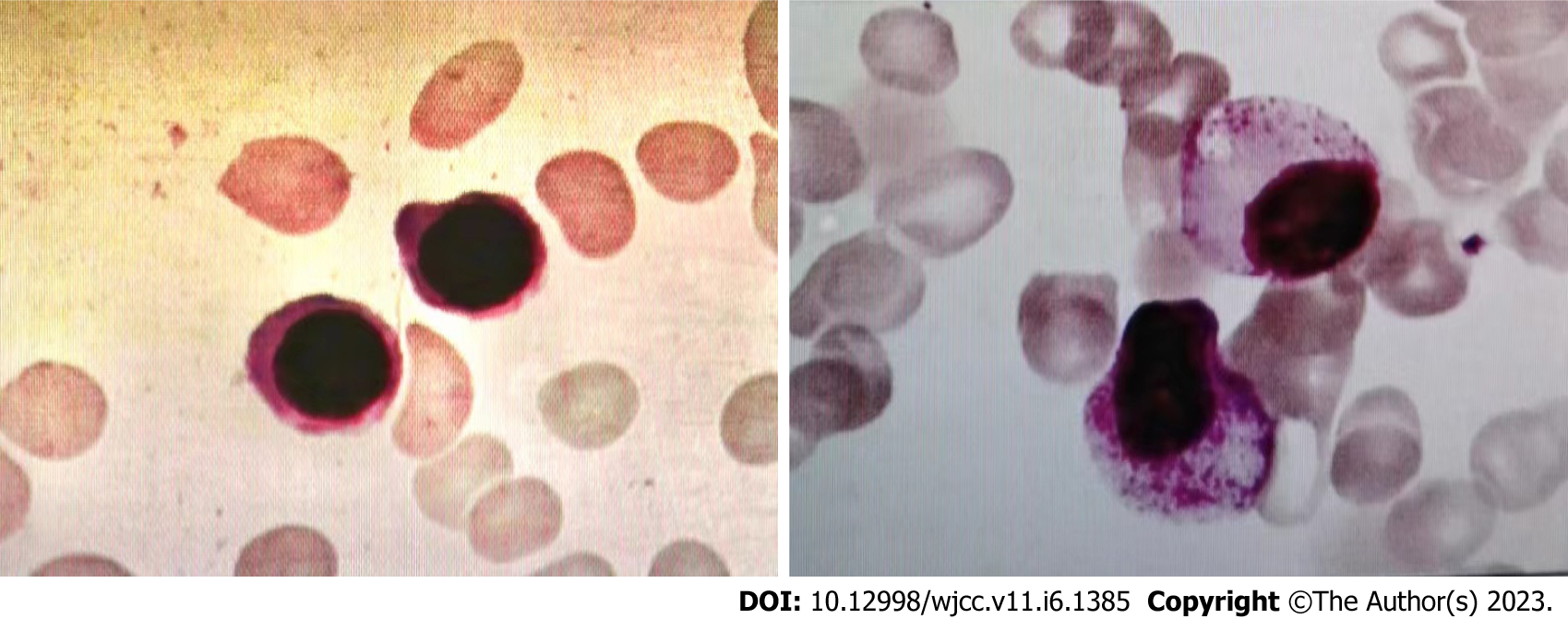
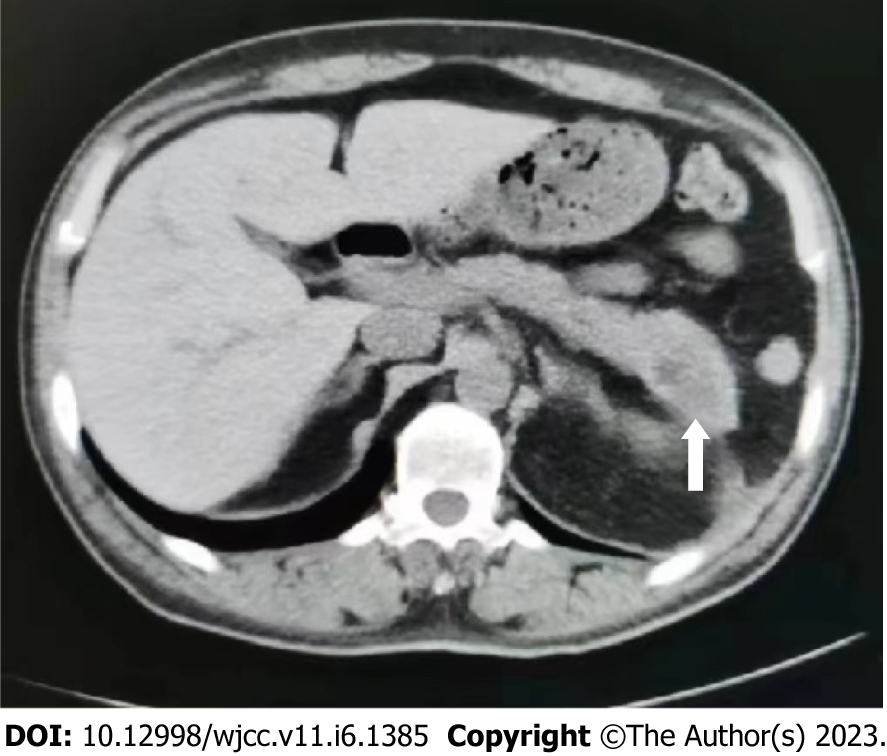
Leukemia and pancreatitis both improved after chemotherapy. After two cycles of chemotherapy, the lesions in his pancreas disappeared (Figure 3). The patient achieved a full recovery and complete remission (Figure 4) with platelet recovery. The patient has been alive for 1 year since the initial development of AML. Pancreatic walled-off necrosis developed in the healed pancreas after the treatment of pancreatitis. There was no significant increase in amylase, no obvious abdominal pain or distension, and no recurrence of pancreatitis.
Pancreatitis associated with acute leukemia has been reported mainly in children with ALL, most of which were linked with the use of asparaginase as a chemotherapeutic drug. Pancreatitis has also been reported in AML patients using cytarabine[3,4] or all-trans retinoic acid[5-7] and other combined chemotherapy regimens[8]. Pancreatitis caused by chemotherapy medications, on the other hand, is usually mild and can be improved if the chemotherapeutic drugs are suspended. In addition, bone marrow transplantation has also been a risk factor for pancreatitis[9-11]. A few cases of AP with adult T-cell leukemia were induced by hypercalcemia[12]. There were cases of AP with direct infiltration of leukemia cells in ALL[13-15], one of which confirmed atypical lymphocytic infiltration at the pathological level using fine-needle aspiration biopsy[13]. Although the other reports were not supported by pathological evidence, with the progress of induction therapy, regression of leukemic infiltration was seen in the pancreas. Acute leukemia, whether direct infiltration or in combination with chemotherapeutic drugs, can result in AP. But in AML, the pancreas is a rare organ for extramedullary infiltration. Only once in the literature, the leukemia cells in AML have been implicated as the cause of AP. In Japan, a patient with AML developed AP mimicking autoimmune pancreatitis[16].
In a previous case report, a patient with AML-M2 type was complicated with extramedullary manifestations[17]. Our patient was initially thought to have biliary pancreatitis because of the gallstone found by abdominal CT, but the patient had no bile duct stones. Serum bilirubin, γ-glutamyltransferase, and alkaline phosphatase were normal, and there was no evidence of cholangitis; therefore, the occurrence was unlikely to be caused by the stone. The patient's blood lipids were not elevated, and he had no prior drinking history; thus, alcohol or hyperlipidemia was ruled out. Then, after a follow-up inspection, he was diagnosed with AML, revealing the underlying reason for pancreatitis. Serum calcium levels at admission were within the normal range; the patient had no history of chemotherapy, so chemotherapy-related adverse reactions were also excluded. On the top of that, after chemotherapy for leukemia, there was no recurrence of pancreatitis. Based on the above details, it was presumable that the leukemic infiltration of the pancreas resulted in pancreatitis in this patient.
The extramedullary manifestations of leukemia can affect any organ, resulting in diversified early manifestations of leukemia, with separate organ damage or prominent local manifestation as the initial symptoms. AP caused by leukemic cell infiltration to the pancreas is rare in AML, and the mechanism is still unclear. Studies have shown that AML cells can accelerate the progression of leukemia by reshaping a supportive malignant microenvironment[18], which results in an invasion of the pancreas and other extramedullary organs. Studies focused maily on CXCR4/CXCL12 signaling axis, matrix metalloproteinases (MMPs), and urokinase-type plasminogen activator system (uPAs) while investigating malignant tumor microenvironment in AML. Higher CXCR4 expression in hematopoietic stem cells suggests an increase in recurrence rate and significantly poor prognosis[19,20]. The bone marrow plasma MMP-9 level is significantly higher in AML patients with extramedullary infiltration, showing that the premature production of MMP-9 may contribute to leukemic cells infiltration to extramedullary organs[21]. The uPAs induces plasminogen activation, which plays a vital role in tissue remodeling, proteolysis, invasion, and metastasis. Lanza et al[22] demonstrated that urokinase plasminogen activator receptor expression increased in patients with AML with invasive manifestations.
There was a high risk of hemorrhage after needle biopsy because of the low platelet count, so typical pathological changes such as leukemic cell infiltration to the pancreas could not be confirmed. But AP should be considered in AML patients with acute, persistent epigastric pain, regardless of whether or not there was amylase elevation. In the absence of fine-needle aspiration biopsy, the highly plausible possibility of direct infiltration by leukemia cells should be sought if other causes were excluded, as early detection and timely treatment of leukemia could improve the outcome of pancreatitis. If not handled properly, pancreatitis caused by a rare cause can develop into severe pancreatitis with systemic inflammatory response syndrome and organ dysfunction with rapid progression, poor prognosis, and high risk of death. It is harmful and difficult to diagnose, so clinicians must pay greater attention to this.
Although extramedullary infiltration of AML is generally regarded as an indicator of poor prognosis, this conclusion is still debatable[23-25]. Due to limited data available, it is difficult to determine the prognostic significance of pancreatic involvement in patients with AML. Therefore, for leukemia patients with extramedullary invasion of uncommon sites such as the pancreas, this may not indicate a more aggressive disease than other common sites, but treatment and diagnosis can be delayed. Especially in the said case, the rare AP as the first extramedullary infiltration manifestation led to misdiagnosis or missed diagnosis.
This case report suggested that AP may be related to AML and highlighted a rare but significant etiology of AP. Many advancements have been made in diagnostic techniques and clinician awareness to identify rare predisposing factors of AP. Early diagnosis and etiological management can help avoid ineffective treatments and improve the outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Agrawal P, United States; Yao J, China; Zhao XC, China S-Editor: Li L L-Editor: Wang TQ P-Editor: Li L
| 1. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-15; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1384] [Article Influence: 115.3] [Reference Citation Analysis (3)] |
| 2. | Newell LF, Cook RJ. Advances in acute myeloid leukemia. BMJ. 2021;375:n2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 240] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 3. | McGrail LH, Sehn LH, Weiss RB, Robson MR, Antin JH, Byrd JC. Pancreatitis during therapy of acute myeloid leukemia: cytarabine related? Ann Oncol. 1999;10:1373-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Murshed F, Wong V, Koning JL, Kuo DJ. Acute Pancreatitis Associated With Cytarabine During the Treatment of Pediatric Acute Myeloid Leukemia. J Pediatr Hematol Oncol. 2020;42:63-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Abou Chacra L, Ghosn M, Ghayad E, Honein K. A case of pancreatitis associated with all-trans-retinoic acid therapy in acute promyelocytic leukemia. Hematol J. 2001;2:406-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Teng HW, Bai LY, Chao TC, Wang WS, Chen PM. Acute pancreatitis during all-trans-retinoic acid treatment for acute promyelocytic leukemia in a patient without overt hypertriglyceridemia. Jpn J Clin Oncol. 2005;35:94-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Abdullah AS, Adel AM, Hussein RM, Abdullah MA, Yousaf A, Mudawi D, Mohamed SF, Nashwan AJ, Soliman D, Ibrahim F, Yassin MA. Hypercalcemia and acute pancreatitis in a male patient with acute promyelocytic leukemia and pulmonary tuberculosis. Acta Biomed. 2018;89:23-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Yang QJ, Zheng J, Dang FT, Wan YM, Yang J. Acute pancreatitis induced by combination chemotherapy used for the treatment of acute myeloid leukemia: A case report. Medicine (Baltimore). 2020;99:e21848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 9. | De Singly B, Simon M, Bennani J, Wittnebel S, Zagadanski AM, Pacault V, Gornet JM, Allez M, Lémann M. [Prolonged acute pancreatitis after bone marrow transplantation]. Gastroenterol Clin Biol. 2008;32:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Kamada Y, Suzukawa K, Taoka K, Okoshi Y, Hasegawa Y, Chiba S. Relapse of Acute Myeloid Leukemia with t(16;21)(p11;q22) Mimicking Autoimmune Pancreatitis after Second Allogeneic Bone Marrow Transplantation. ISRN Hematol. 2011;2011:285487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Ozeki K, Morishita Y, Sakai D, Nakamura Y, Fukuyama R, Umemura K, Yamaguchi Y, Tatekawa S, Watamoto K, Ozeki K, Kohno A. Relapse of acute myeloid leukemia mimicking autoimmune pancreatitis after bone marrow transplantation. Intern Med. 2014;53:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Braun C, Duffau P, Mahon FX, Rosier E, Leguay T, Etienne G, Michaud M. [Acute pancreatitis due to hypercalcemia revealing adult T-cell leukemia]. Rev Med Interne. 2007;28:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Mori A, Kikuchi Y, Motoori S, Watanabe J, Shinozaki M, Eguchi M. Acute pancreatitis induced by diffuse pancreatic invasion of adult T-cell leukemia/Lymphoma cells. Dig Dis Sci. 2003;48:1979-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Malbora B, Avci Z, Alioglu B, Tutar NU, Ozbek N. A case with mature B-cell acute lymphoblastic leukemia and pancreatic involvement at the time of diagnosis. J Pediatr Hematol Oncol. 2008;30:87-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Yadav YK, Mallya V, Ahluwalia C, Gupta O. Secondary pancreatic involvement by precursor T-cell acute lymphoblastic leukemia presenting as acute pancreatitis. Indian J Cancer. 2015;52:465-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Sumitani R, Hori T, Murai J, Kawata S, Oura M, Sogabe K, Takahashi M, Harada T, Fujii S, Miki H, Kagawa K, Abe M, Nakamura S. Acute Myeloid Leukemia Developing with Acute Pancreatitis Mimicking Autoimmune Pancreatitis. Intern Med. 2021;60:1753-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 17. | British Committee for Standards in Haematology; Milligan DW, Grimwade D, Cullis JO, Bond L, Swirsky D, Craddock C, Kell J, Homewood J, Campbell K, McGinley S, Wheatley K, Jackson G. Guidelines on the management of acute myeloid leukaemia in adults. Br J Haematol. 2006;135:450-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Kumar B, Garcia M, Weng L, Jung X, Murakami JL, Hu X, McDonald T, Lin A, Kumar AR, DiGiusto DL, Stein AS, Pullarkat VA, Hui SK, Carlesso N, Kuo YH, Bhatia R, Marcucci G, Chen CC. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia. 2018;32:575-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 323] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 19. | Rombouts EJ, Pavic B, Löwenberg B, Ploemacher RE. Relation between CXCR-4 expression, Flt3 mutations, and unfavorable prognosis of adult acute myeloid leukemia. Blood. 2004;104:550-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 223] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Schelker RC, Iberl S, Müller G, Hart C, Herr W, Grassinger J. TGF-β1 and CXCL12 modulate proliferation and chemotherapy sensitivity of acute myeloid leukemia cells co-cultured with multipotent mesenchymal stromal cells. Hematology. 2018;23:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Aref S, El-Sherbiny M, Mabed M, Menessy A, El-Refaei M. Urokinase plasminogen activator receptor and soluble matrix metalloproteinase-9 in acute myeloid leukemia patients: a possible relation to disease invasion. Hematology. 2003;8:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Lanza F, Castoldi GL, Castagnari B, Todd RF 3rd, Moretti S, Spisani S, Latorraca A, Focarile E, Roberti MG, Traniello S. Expression and functional role of urokinase-type plasminogen activator receptor in normal and acute leukaemic cells. Br J Haematol. 1998;103:110-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Kobayashi R, Tawa A, Hanada R, Horibe K, Tsuchida M, Tsukimoto I; Japanese childhood AML cooperative study group. Extramedullary infiltration at diagnosis and prognosis in children with acute myelogenous leukemia. Pediatr Blood Cancer. 2007;48:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Peled A, Tavor S. Role of CXCR4 in the pathogenesis of acute myeloid leukemia. Theranostics. 2013;3:34-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Cheng CL, Li CC, Hou HA, Fang WQ, Chang CH, Lin CT, Tang JL, Chou WC, Chen CY, Yao M, Huang SY, Ko BS, Wu SJ, Tsay W, Tien HF. Risk factors and clinical outcomes of acute myeloid leukaemia with central nervous system involvement in adults. BMC Cancer. 2015;15:344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |