Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1287
Peer-review started: October 30, 2022
First decision: November 27, 2022
Revised: December 17, 2022
Accepted: February 3, 2023
Article in press: February 3, 2023
Published online: February 26, 2023
Processing time: 117 Days and 3.4 Hours
New onset hyperglycemia is common in patients with severe coronavirus disease 2019 (COVID-19) infection. Cytokine storm due to COVID-19 infection is an essential etiology for new-onset hyperglycemia, but factors like direct severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced pancreatic β-cell failure have also been postulated to play a role.
We plan to investigate further the mechanisms underlying SARS-CoV-2 infection-induced hyperglycemia, particularly the rationale of the cytokine-induced hyperglycemia hypothesis, by evaluating the association between inflammatory markers and new onset hyperglycemia in non-diabetic patients with COVID-19 infection.
We conducted a retrospective case-control study on adults without diabetes mellitus hospitalized for COVID-19 infection. The serum levels of glucose and inflammatory markers at presentation before initiation of corticosteroid were collected. Hyperglycemia was defined as glucose levels ≥ 140 mg/dL. C-Reactive protein (CRP) ≥ 100 mg/L, ferritin ≥ 530 ng/mL, lactate dehydrogenase (LDH) ≥ 590 U/L, and D-dimer ≥ 0.5 mg/L were considered elevated. We used the χ2 test for categorical variables and the Mann-Whitney U test for continuous variables and calculated the logistic regression for hyperglycemia.
Of the 520 patients screened, 248 met the inclusion criteria. Baseline demographics were equally distributed between patients with hyperglycemia and those who were normoglycemic. Serum inflammatory markers in patients with or without new-onset hyperglycemia were elevated as follows: CRP (58.1% vs 65.6%, P = 0.29), ferritin (48.4% vs 34.9%, P = 0.14), D-dimer (37.1% vs 37.1%, P = 0.76) and LDH (19.4% vs 11.8%, P = 0.02). Logistic regression analysis showed LDH odds ratio (OR) = 1.623 (P = 0.256). We observed significantly higher mortality (24.2% vs 9.1%, P = 0.001; OR = 2.528, P = 0.024) and length of stay (8.89 vs 6.69, P = 0.026) in patients with hyperglycemia.
Our study showed no association between CRP, ferritin, LDH, D-dimer levels, and new-onset hyperglycemia in non-diabetic patients with COVID-19 infection. It also shows an increased mortality risk and length of stay in patients with hyperglycemia. With new-onset hyperglycemia being closely associated with poor prognostic indices, it becomes pivotal to understand the underlying pathophysiological mechanisms behind the SARS-CoV-2 infection-induced hyperglycemia. We conclude that the stress hyperglycemia hypothesis is not the only mechanism of SARS-CoV-2 infection-induced hyperglycemia but rather a multicausal pathogenesis leading to hyperglycemia that requires further research and understanding. This would help us improve not only the clinical outcomes of COVID-19 disease and inpatient hyperglycemia management but also understand the long-term effects of SARS-CoV-2 infection and further management.
Core Tip: Our study suggests that there is no correlation between the inflammatory marker levels and the presence of hyperglycemia in non-diabetic patients with coronavirus disease 2019 (COVID-19) infection. With an increased need to understand the mechanism underlying severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection-induced hyperglycemia, we assessed the validity of the most accepted COVID-19 infection-induced cytokine storm-related stress hyperglycemia theory. However, our study did not show any correlation between inflammatory marker levels that correlate with the cytokine storm and the level of hyperglycemia. This suggests the possibility of other mechanisms playing a role in the SARS-CoV-2 infection-induced hyperglycemia. Our study also demonstrated that new-onset hyperglycemia was an independent risk factor for higher mortality and length of stay, thereby emphasizing the need to understand the mechanisms leading to hyperglycemia.
- Citation: Geetha HS, Singh G, Sekar A, Gogtay M, Singh Y, Abraham GM, Trivedi N. Hyperglycemia in COVID-19 infection without diabetes mellitus: Association with inflammatory markers. World J Clin Cases 2023; 11(6): 1287-1298
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1287.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1287
At the end of 2019, a novel coronavirus rapidly spread worldwide, resulting in a global pandemic. The virus was designated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the illness it caused was coronavirus disease 2019 (COVID-19). The spectrum of COVID-19 in adults ranges from asymptomatic infection to mild respiratory tract symptoms to severe pneumonia with acute respiratory distress syndrome and multiorgan dysfunction.
The presence of hyperglycemia was an independent factor associated with poor outcomes. One study of hospitalized, elderly COVID-19 patients in Wuhan reported that 21.6% had a history of diabetes, 20.8% were newly diagnosed with diabetes [fasting admission glucose ≥ 7.0 mmol/L or glycated hemoglobin (HbA1c) ≥ 6.5%], and 28.4% were diagnosed with dysglycemia (fasting glucose 5.6–6.9 mmol/L or HbA1c 5.7%–6.4%)[1].
Although there is clear evidence demonstrating the association between COVID-19 disease and hyperglycemia, there exists a lack of sufficient literature explaining the mechanism of action of SARS-CoV-2-induced hyperglycemia. The pathophysiological mechanisms of hyperglycemia in COVID-19 patients remain poorly understood. Several complex processes have been hypothesized, including previously undiagnosed diabetes, stress hyperglycemia, steroid-induced hyperglycemia, and direct or indirect effects of SARS-CoV-2 on the pancreatic β-cell[2].
Studies have shown that patients with newly diagnosed diabetes have higher inflammatory markers such as C-reactive protein (CRP), erythrocyte sedimentation rate, and white blood cells. Acute inflammation seen in cytokine storms may worsen insulin resistance. One study shows that neutrophils, D-dimers, and inflammatory markers are significantly higher in those with hyperglycemia than those with normal glucose[3].
We assessed the rationale of the cytokine-induced hyperglycemia hypothesis by evaluating the association between routinely tested inflammatory markers like CRP, ferritin, Lactate dehydrogenase (LDH), and D-dimer and new onset hyperglycemia in non-diabetic patients with COVID-19 infection.
We conducted a retrospective, single-center case-control study of hospitalized patients between Dec 1, 2019, and Jan 1, 2022, at a 329 bed community teaching hospital in central Massachusetts. The study inclusion criteria included: (1) Inpatients diagnosed with SARS-CoV-2 infection; (2) Age > 18 years; and (3) Patients with documented inflammatory markers and glucose levels on admission. The exclusion criteria included: (1) Pregnant patients; (2) Patients with previous diagnoses of Type 1 or Type 2 Diabetes mellitus (DM), and (3) Patients who received steroids before admission. The data were obtained by reviewing the patients’ medical records, including demographic information, past medical history, medication, labs, and course during hospitalization. The Institutional Review Board approved this study.
The primary endpoint measured was hyperglycemia, defined as glucose levels ≥ 140 mg/dL. The patients with hyperglycemia were defined as cases, and the control group included patients with normoglycemia (glucose levels < 140 mg/dL). We assessed the level of inflammatory markers between the two study groups, including CRP, ferritin, LDH, and D-dimer levels. We used prespecified cutoffs for the inflammatory markers described by previous studies[4-6]. The inflammatory markers were categorized as binary variables, i.e. either elevated or normal. A CRP ≥ 100 mg/L, ferritin ≥ 530 ng/mL, LDH ≥ 590 U/L, and D-dimer ≥ 0.5 mg/L were considered elevated. Demographic data collected included age, sex, weight, height, body mass index (BMI), and vaccination status. Relevant clinical data that is associated with hyperglycemia in previous studies which included a family history of diabetes, past medical history of prediabetes, American Diabetes Association (ADA) diabetes risk score, hypertension, chronic liver disease (CLD), chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and chronic kidney disease (CKD) was collected. We also collected data about the outcomes of these patients that included mortality rate, use of steroids, use of remdesivir, and length of hospital stay. The ADA diabetes risk assessment tool that provides a risk stratification index for developing Type II DM was also used.
The data were subjected to the normalcy test (Shapiro-Wilk test) that showed non-normalcy distribution. Hence, non-parametric tests were employed. The baseline demographic characteristics between the two study populations were assessed using χ2 analysis for categorical variables and the Mann-Whitney U test for continuous variables. The association between the covariates and primary endpoints was evaluated using a bivariate logistic regression with hyperglycemia as a dependent variable and other variables significant in the univariate analysis as independent variables. The level of significance was set at 5%.
A total of 520 patients were screened, among which 248 met the inclusion criteria, with 186 patients (75%) being normoglycemic on admission and 62 patients (25%) being hyperglycemic on admission. Patients with hyperglycemia were more likely to have a history of hypertension (58.1% vs 43.5%, P = 0.047) and increased ADA Diabetes risk scoring (66.1% vs 51.6%, P = 0.048) when compared to patients with normoglycemia (Tables 1 and 2). The demographic data like sex distribution, vaccination status, and history of CLD, COPD, CHF, and CKD were similar between the two groups.
| Patient characteristic | Normoglycemia | Hyperglycemia | Total | P value2 |
| Male sex | 102 | 33 | 135 | 0.83 |
| 54.8% | 53.2% | 54.4% | ||
| Vaccinated for COVID | 34 | 10 | 44 | 0.87 |
| 18.3% | 16.1% | 17.7% | ||
| Hypertension | 81 | 36 | 117 | 0.0471 |
| 43.5% | 58.1% | 47.2% | ||
| Family history of diabetes | 16 | 8 | 24 | 0.32 |
| 8.6% | 12.9% | 9.7% | ||
| Prediabetes | 5 | 1 | 6 | 13 |
| 2.7% | 1.6% | 2.4% | ||
| ADA - At risk | 96 | 41 | 137 | 0.05* |
| 51.6% | 66.1% | 55.2% | ||
| Chronic liver disease | 7 | 2 | 9 | 1.00 |
| 3.8% | 3.2% | 3.6% | ||
| Chronic obstructive pulmonary disease | 27 | 7 | 34 | 0.39 |
| 14.5% | 11.3% | 13.7% | ||
| Congestive heart failure | 33 | 7 | 40 | 0.23 |
| 17.7% | 11.3% | 16.1% | ||
| Chronic kidney disease | 31 | 11 | 42 | 0.85 |
| 16.7% | 17.7% | 16.9% |
| Variable | Category | n | Mean | Std dev | Median | IQR | P value1 |
| Age, yr | Normoglycemic | 186 | 63.38 | 18.52 | 68.50 | 23.00 | 0.366 |
| Hyperglycemic | 62 | 66.32 | 15.64 | 65.00 | 29.00 | ||
| Weight, kg | Normoglycemic | 186 | 84.96 | 26.24 | 82.50 | 31.00 | 0.982 |
| Hyperglycemic | 62 | 84.71 | 25.63 | 82.00 | 29.00 | ||
| Height, cm | Normoglycemic | 186 | 168.48 | 10.67 | 166.00 | 18.00 | 0.376 |
| Hyperglycemic | 62 | 167.02 | 10.99 | 168.00 | 17.00 | ||
| BMI | Normoglycemic | 186 | 29.62 | 7.75 | 28.50 | 9.00 | 0.566 |
| Hyperglycemic | 62 | 30.02 | 7.52 | 28.00 | 9.00 |
The association between the levels of inflammatory markers like CRP, ferritin, D-dimer, and LDH in patients with normoglycemia and patients with hyperglycemia was assessed (Tables 3 and 4). Although there was a difference in the mean levels of CRP (106.56 mg/L vs 85.65 mg/L), ferritin (1404.40 ng/mL vs 470.38 ng/mL), D dimer (0.15 mg/L vs 1.71 mg/L) and LDH levels (319.11 U/L vs 224.45 U/L), there were statistically no significant differences between serum inflammatory markers except LDH in patients with or without new-onset hyperglycemia [CRP (58.1% vs 65.6%, P = 0.29), ferritin (48.4% vs 34.9%, P = 0.14), D-dimer (37.1% vs 37.1%, P = 0.76) and LDH (19.4% vs 11.8%, P = 0.02)]. On further binary logistic regression analysis to predict hyperglycemia, there was no difference in LDH levels between the two groups (OR = 1.623, P = 0.256).
| Variable | Category | n | Mean | Std dev | Median | IQR | P value2 |
| CRP levels on admission | Normoglycemic | 186 | 85.65 | 74.12 | 84.00 | 149.00 | 0.278 |
| Hyperglycemic | 62 | 106.56 | 94.09 | 74.00 | 109.00 | ||
| Ferritin levels on admission | Normoglycemic | 186 | 470.38 | 984.06 | 385.50 | 1109.50 | 0.0281 |
| Hyperglycemic | 62 | 1404.40 | 4758.84 | 124.00 | 647.50 | ||
| LDH levels on admission | Normoglycemic | 186 | 224.45 | 245.15 | 276.00 | 319.50 | 0.0411 |
| Hyperglycemic | 62 | 319.11 | 326.57 | 231.00 | 389.00 | ||
| D-dimer levels on admission | Normoglycemic | 186 | 1.71 | 4.63 | 1.00 | 1.25 | 0.470 |
| Hyperglycemic | 62 | 2.15 | 6.26 | 0.00 | 1.00 |
We further analyzed the prognostic indices like mortality rate and length of stay between the two groups (Tables 5 and 6). There was significantly higher mortality (24.2% vs 9.1%, P = 0.001) and length of stay (8.89 d vs 6.69 d, P = 0.026) in patients with hyperglycemia compared to patients with normoglycemia. Further analysis with binary logistic regression shows an increased risk of mortality in patients with hyperglycemia (OR = 2.528, P = 0.024). The administration of remdesivir initially showed a statistically significant difference in patients with hyperglycemia (59.7% vs 44.6%, P = 0.04) but did not show any significant difference in binary logistic regression (OR = 1.620, CI: 0.882 - 2.974) (Table 7). There was also no significant difference in the rate of steroid administration between the two groups.
The SARS-CoV-2 infection that was first noticed in December 2019 has resulted in the worldwide COVID-19 pandemic that has claimed over 6.5 million lives worldwide[7]. Initially thought to be a pathogen that affects only the respiratory system, as we continued to learn more about the virus, its effects on multiple organ systems are being slowly discovered, with several studies assessing its association in different disease states[8,9]. Studies have revealed the association of certain risk factors with increased predisposition and severity of the disease. DM has been shown to increase the risk of morbidity and mortality due to its associated metabolic, microvascular, and macrovascular complications and reactive hyperglycemia being a predictor of severity in previous SARS-CoV-1 and Middle East Respiratory Syndrome coronavirus-CoV infections. We noticed an increased prevalence of hyperglycemia in patients with COVID-19 infection, irrespective of the presence of pre-existing diabetes mellitus. An Italian study of 271 patients admitted for COVID-19 showed that hyperglycemia was independently associated with increased mortality[10]. However, this study failed to elucidate the underlying pathophysiological mechanisms explaining SARS-CoV-2 infection-induced hyperglycemia. In our study, we investigated the association of hyperglycemia at presentation with inflammatory markers and the impact of hyperglycemia on mortality and morbidity in non-diabetic COVID-19 patients. The results showed no association between CRP, ferritin, LDH, and D-dimer levels and new-onset hyperglycemia in non-diabetic patients with COVID-19 infection.
Although age and male sex were the initially associated risk factors, further studies revealed the increased prevalence of hypertension and diabetes mellitus in patients with severe disease compared to those with milder forms of infection[11,12]. Our study showed a similar finding of increased hypertension in patients with hyperglycemia. This was further analyzed by binary logistic regression to account for hypertension as a potential confounding agent, given the increased mortality and length of stay in the hyperglycemia group. However, further analysis demonstrated the absence of a significant difference in the presence of hypertension between the two groups. Other risk factors such as male sex, increased BMI, and vaccination status and comorbidities such as chronic liver disease, congestive heart failure, chronic obstructive pulmonary disease, and chronic kidney disease were similar between the two groups, eliminating potential confounders. Although we accounted for major potential confounders, the demographic prevalence of prediabetes was not accounted for due to the non-availability of baseline HbA1c levels.
With the advent of the COVID-19 pandemic, the utility of inflammatory markers has been on the rise. Biomarkers are quantitative measurements that reflect the pathophysiology of the disease and help gauge the underlying disease severity. Initial studies demonstrated increased levels of inflammatory markers in COVID-19 patients that directly correlated with the disease severity.
Although a milieu of inflammatory markers like Interleukin(IL)-6, IL-1β, and IL-8 exist, these are not routinely assessed in labs. It would not be cost-effective to employ them in the routine monitoring of every patient with SARS-CoV-2 infection, thus necessitating the use of other inflammatory markers that are cost-effective and can be utilized to assess the severity of the disease. This led to further research on the utility of markers like C-reactive protein, ferritin, LDH, and D-dimer. Studies have demonstrated the utility of these biomarkers in correlating with the severity of the disease. The meta-analyses by Malik et al[13] showed an increased association between the elevated levels of different inflammatory markers and the severity of COVID-19 disease. Elevated CRP levels (> 10 mg/L) showed a fourfold increase in poor outcomes. Elevated D-dimer values (≥ 0.5 mg/L) were associated with a threefold higher risk of poor outcomes in COVID-19 patients. Elevated LDH showed a fivefold increased risk of poor outcomes. The Meta-analysis by Huang et al[14] demonstrated that patients with poor composite outcome had higher levels of Ferritin. Although various studies used different values of CRP such as Koozi et al[4] ≥ 1000 mg/L, Ryoo et al[5] ≥ 140 mg/L, and Liu et al[6] ≥ 41.8 mg/L, we uniformly observed increased risk of COVID-19 severity with elevated levels of CRP. Despite its value in predicting a poor outcome in COVID-19, it should be noted that various factors could affect serum CRP levels, including age, sex, smoking status, weight, lipid levels, blood pressure, and liver injury[15].
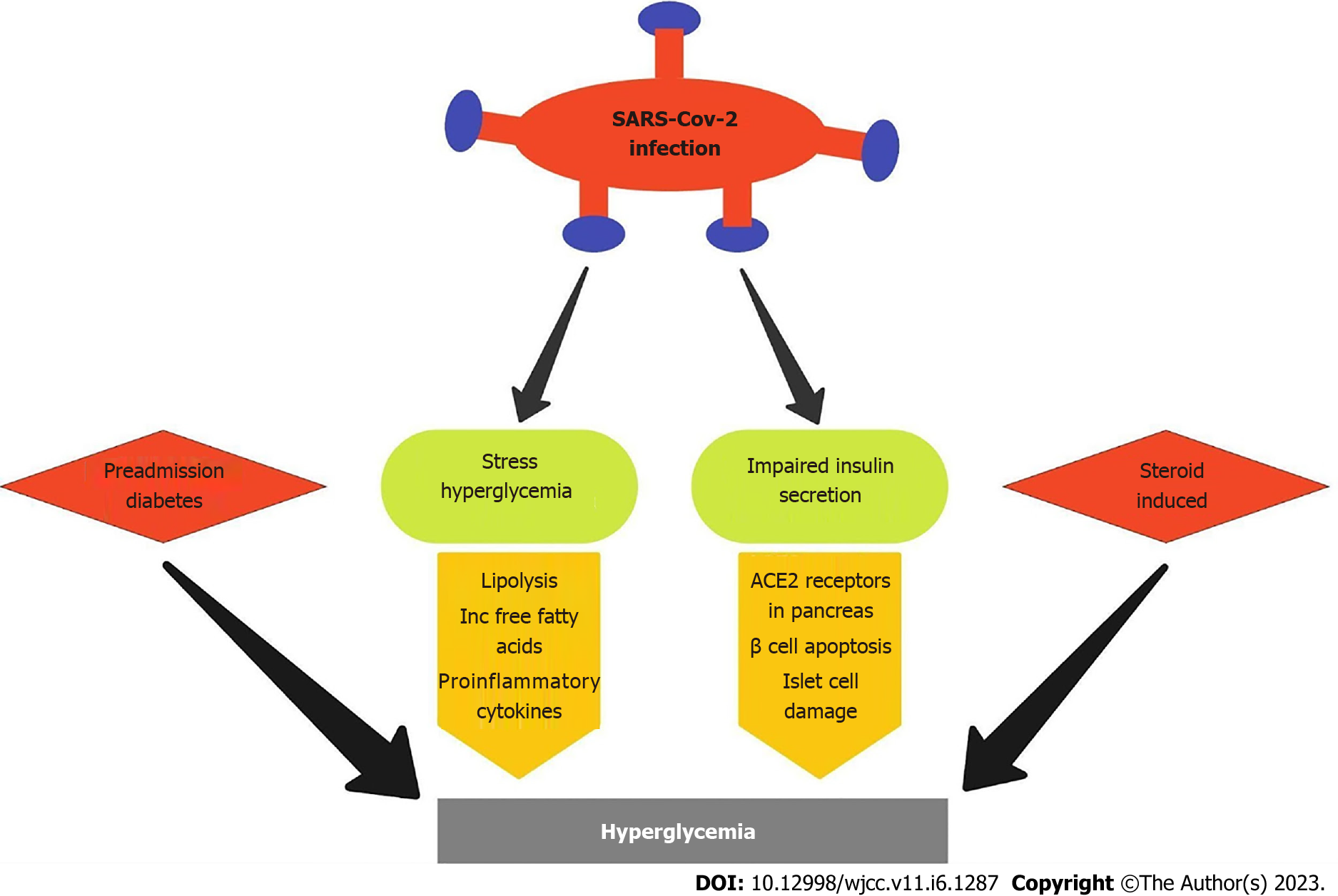
As we continued to discover the close association between COVID-19 disease and hyperglycemia, several theories were postulated on the underlying pathophysiology between SARS-CoV-2 infection and the onset of hyperglycemia (Figure 1).
The most widely accepted hypothesis explaining the mechanism of new-onset hyperglycemia in SARS-CoV-2 infection is the stress hyperglycemia theory. Previous studies have shown that any acute illness, including myocardial infarction or severe infection, tends to drive lipolysis production and an increased number of free fatty acids in the blood[16] which in turn causes production of proinflammatory cytokines. In COVID-19 infection, we expect a similar mechanism or even greater response due to the profound cytokine storm triggered by the SARS-CoV-2 infection. Since previous studies had established the correlation between the levels of inflammatory markers like C-reactive protein, ferritin, LDH, and D-dimer and the severity of SARS-CoV-2 infection, we employed these markers to assess the presence of hyperglycemia in correlation with the disease severity. Our study showed no difference in the levels of C-reactive protein, ferritin, and D-dimer between the two groups. Although initial results showed a significant difference in the LDH levels between the two groups, further binary logistic regression did not reveal any significant change. The mean levels of C-reactive protein, ferritin, LDH, and D-dimer were higher in patients with hyperglycemia. However, no significant difference was observed when a cutoff for severity was imposed based on the established inflammatory marker levels for severe COVID-19 disease.
One of the first theories postulated was the presence of undiagnosed preexisting diabetes in the population that was brought to light when such patients were admitted to the hospital for COVID-19 infection. Studies also supported this hypothesis, such as recently increased weight gain and worsening hyperglycemia due to mandatory isolation, reduced physical activities, social distancing, and poor mental health that led to eating disorders[17]. These changes could potentially lead to metabolic syndrome that culminates in insulin resistance resulting in hyperglycemia. However, our study accounted for such changes by calculating the ADA diabetes risk score, a validated tool by the American Diabetes Association, to detect an individual’s risk of developing diabetes mellitus[18]. A cutoff score of 5 or greater indicates a greater risk of developing diabetes when compared to the general population. In our study, although the initial χ2 analysis showed a significantly greater ADA risk score in the hyperglycemic study group, further analysis with binary logistic regression showed no statistically significant difference in the ADA risk score between the two groups. This finding emphasizes the poor validity of the preexisting undiagnosed diabetes hypothesis.
The first association between SARS-CoV-2 infection and pancreatic damage was postulated when the presence of Angiotensin converting enzyme 2 receptors, the binding site for SARS-CoV-2, was identified in the pancreatic islet cells[19,20]. More recent studies have observed that SARS-CoV-2 can infect pancreatic cells permitting entry of the virus, leading to attenuation of pancreatic insulin levels and consequently leading to β-cell apoptosis[21,22].
A meta-analysis of 13 studies showed that 32.3% of patients treated for COVID-19 infection with dexamethasone developed glucocorticoid-induced hyperglycemia and 18.6% of patients developed new-onset diabetes[23]. Since steroid-induced hyperglycemia would confound our results, we negated the effects of glucocorticoids by obtaining blood glucose levels on admission before administering steroids. We also excluded patients who had received steroids at outside facilities or in the ED before collecting their on-admission blood glucose levels.
There is growing evidence that hyperglycemia in COVID-19 patients bears significant prognostic implications. Hyperglycemia in critically ill patients is a common manifestation directly correlated with increased mortality or morbidity[24]. More importantly, hyperglycemia was found to worsen the progression of respiratory failure.
Platelet activation incited by Fc glycosylated immune complexes is consistent with platelet hyperactivity in severe COVID-19 patients. Excessive macrophage stimulation by enhanced Fc-glycosylated immune complexes is consistent with the macrophage activation syndrome. The resulting hypercoagulability with compromised microperfusion, pulmonary endothelial fluid leakage, and severe respiratory distress syndrome can result in death[25]. In our study, we observed a significantly increased rate of mortality in patients with new onset hyperglycemia compared to those without. This correlated with the findings of existing literature, such as that by Bode et al[26] (2020), who demonstrated that uncontrolled hyper
We also observed a significant increase in the mean length of stay in patients with hyperglycemia compared to patients with normoglycemia. This impact of hyperglycemia in those without an established diagnosis of diabetes is a more concerning matter for clinicians, and research has shown that this could be explained by the acute hyperglycemia causing impairment in innate immunity, leading to a heightened risk of infections and increasing the length of stay (LOS) in hospital. In the study by Bode et al[26], among 493 discharged patients, the median LOS was longer in 184 patients with hyperglycemia compared with 386 patients without diabetes or hyperglycemia (5.7 vs 4.3 d, P < 0.001). Our study results are parallel and support the findings of increased mortality and length of stay in patients with hyperglycemia compared with patients with normoglycemia. These results suggest that disease severity and mortality risk significantly increase with newly diagnosed hyperglycemia after hospital admission.
One of the most important limitations of the study is the small study population. Since the study was based on a community hospital in the United States, the generalizability of the study is limited. Although due consideration was provided regarding the possible confounding factors, due to the study's retrospective nature, it is possible that all potential confounding factors were not adequately adjusted for. Furthermore, the current study was performed during a period of travel limitations and limited physical activity, which could have contributed to the increased risk of dysglycemia in the study population. Our study is also limited by the inflammatory markers used since it did not include markers like IL-6, IL-1β, and IL-8, which better correlate with the level of inflammation. We were also limited by the non-availability of baseline HbA1c levels in the study population in order to negate any pre-existing diabetic conditions.
Further studies are needed to analyze the different potential mechanisms underlying SARS-CoV-2 infection-induced hyperglycemia. Basic science research that would help better understand the underlying pathophysiology and further clinical studies that assess the utility of different treatment strategies in managing hyperglycemia are required to improve the clinical outcomes of COVID-19.
Our study investigated the association of hyperglycemia at presentation with inflammatory markers and the impact of hyperglycemia on mortality and morbidity in non-diabetic COVID-19 patients. The current study showed no association between CRP, ferritin, LDH, and D-dimer levels and new-onset hyperglycemia in non-diabetic patients with COVID-19 infection. It also shows an increased mortality risk and length of stay in patients with hyperglycemia. With new-onset hyperglycemia being closely associated with poor prognostic indices, it becomes pivotal to understand the underlying pathophysiological mechanisms behind the SARS-CoV-2 infection-induced hyperglycemia. This will help us better control the glycemic status and prevent new-onset hyperglycemia, thereby improving the clinical recovery of patients. Although current guidelines recommend the treatment of hyperglycemia using an Insulin sliding scale, these recommendations are based on the hypothesis that hyperglycemia secondary to COVID-19 infection is due to cytokine storm-induced stress hyperglycemia. However, if hyperglycemia arises due to other possible mechanisms, such as viral infection-induced direct suppression of insulin secretion, treatment modalities of achieving glycemic control have to be adjusted to better manage the disease and improve prognosis. Our study results indicate the low probability of the stress hyperglycemia hypothesis being the sole mechanism of SARS-CoV-2 infection-induced hyperglycemia but rather a multicausal pathogenesis leading to hyperglycemia that requires further research and understanding. This would help us improve not only the clinical outcomes of COVID-19 disease and inpatient hyperglycemia management but also understand the long-term effects of SARS-CoV-2 infection and further management.
New onset hyperglycemia is common in patients with severe coronavirus disease 2019 (COVID-19) infection. Cytokine storm and direct severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced pancreatic β-cell failure have been postulated to play a role in new-onset hyperglycemia.
Based on evidence regarding the "Immune-mediated inflammatory storm" during the COVID-19 illness. It has also been proposed that COVID-19 is likely associated with an increased risk of developing diabetes. This motivated us to study the underlying mechanisms contributing to new onset hyperglycemia in hospitalized COVID-19 cases.
To assess the validity of the cytokine-induced hyperglycemia hypothesis by evaluating the association between inflammatory markers and new onset hyperglycemia in non-diabetic patients with COIVD-19 infection.
A retrospective case-control study was conducted on adults without diabetes mellitus hospitalized for COVID-19 infection. The serum levels of glucose and inflammatory markers at presentation before initiation of corticosteroid were collected. Hyperglycemia was defined as glucose levels ≥ 140 mg/dL. Prespecified cutoffs were used for the inflammatory markers. Statistical methods of analysis were used to calculate the logistic regression for hyperglycemia.
Of the 520 patients screened, 248 met the inclusion criteria. Our study showed no association between C-reactive protein, ferritin, Lactate dehydrogenase, D-dimer levels, and new-onset hyperglycemia in non-diabetic patients with COVID-19 infection. We observed significantly higher mortality and length of stay in patients with hyperglycemia.
With new-onset hyperglycemia being closely associated with poor prognostic indices, it becomes pivotal to understand the underlying pathophysiological mechanisms behind the SARS-CoV-2 infection-induced hyperglycemia. This will help us better control the glycemic status and prevent new-onset hyperglycemia, thereby improving the clinical recovery of patients. Our study results indicate the low probability of the stress hyperglycemia hypothesis being the sole mechanism of SARS-CoV-2 infection-induced hyperglycemia but rather a multicausal pathogenesis leading to hyperglycemia that requires further research and understanding.
Basic science research that would help better understand the underlying pathophysiology and further clinical studies that assess the utility of different treatment strategies in managing hyperglycemia are required to improve the clinical outcomes of COVID-19.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Chan ASW, China; Navarro-Alvarez N, Mexico S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Li H, Tian S, Chen T, Cui Z, Shi N, Zhong X, Qiu K, Zhang J, Zeng T, Chen L, Zheng J. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab. 2020;22:1897-1906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 2. | Khunti K, Del Prato S, Mathieu C, Kahn SE, Gabbay RA, Buse JB. COVID-19, Hyperglycemia, and New-Onset Diabetes. Diabetes Care. 2021;44:2645-2655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 179] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 3. | Michalakis K, Ilias I. COVID-19 and hyperglycemia/diabetes. World J Diabetes. 2021;12:642-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | 4 Koozi H, Lengquist M, Frigyesi A. C-reactive protein as a prognostic factor in intensive care admissions for sepsis: A Swedish multicenter study. J Crit Care. 2020;56:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Ryoo SM, Han KS, Ahn S, Shin TG, Hwang SY, Chung SP, Hwang YJ, Park YS, Jo YH, Chang HL, Suh GJ, You KM, Kang GH, Choi SH, Lim TH, Kim WY; Korean Shock Society (KoSS) Investigators. The usefulness of C-reactive protein and procalcitonin to predict prognosis in septic shock patients: A multicenter prospective registry-based observational study. Sci Rep. 2019;9:6579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, Li B, Song X, Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 742] [Cited by in RCA: 707] [Article Influence: 141.4] [Reference Citation Analysis (0)] |
| 7. | Grauhan A. [Combined session of the International Labor organization and the World Health Organization on job and economic conditions of hospital personnel, from 19-30 November, 1973 in Geneva. 1]. Dtsch Krankenpflegez. 1975;28:262-264. [PubMed] |
| 8. | Tripathi K, Godoy Brewer G, Thu Nguyen M, Singh Y, Saleh Ismail M, Sauk JS, Parian AM, Limketkai BN. COVID-19 and Outcomes in Patients With Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Inflamm Bowel Dis. 2022;28:1265-1279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Song D, Geetha HS, Kim A, Seen T, Almas T, Nagarajan VR, Alsaeed N, Cheng JH, Lieber J. Transformation of acute cholecystitis to acute choledocholithiasis in COVID-19 patient. Ann Med Surg (Lond). 2021;71:102946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Coppelli A, Giannarelli R, Aragona M, Penno G, Falcone M, Tiseo G, Ghiadoni L, Barbieri G, Monzani F, Virdis A, Menichetti F, Del Prato S; Pisa COVID-19 Study Group. Hyperglycemia at Hospital Admission Is Associated With Severity of the Prognosis in Patients Hospitalized for COVID-19: The Pisa COVID-19 Study. Diabetes Care. 2020;43:2345-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 11. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18877] [Article Influence: 3775.4] [Reference Citation Analysis (7)] |
| 12. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6660] [Article Influence: 1332.0] [Reference Citation Analysis (0)] |
| 13. | Malik P, Patel U, Mehta D, Patel N, Kelkar R, Akrmah M, Gabrilove JL, Sacks H. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2021;26:107-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 14. | Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 330] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 15. | Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018;9:754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 1716] [Article Influence: 245.1] [Reference Citation Analysis (0)] |
| 16. | Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1485] [Cited by in RCA: 1456] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 17. | World Health Organization. COVID-19 significantly impacts health services for noncommunicable diseases. [Internet] [accessed 12 March 2021]. Available from: https://www.who.int/news/item/01-06-2020-covid-19-significantly-impacts-health-services-for-noncommunicable-diseases. |
| 18. | Chima CC, Anikpezie N, Pongetti LS, Wade BC, Powell T, Beech B. 1443-P: Can the ADA Diabetes Risk Score Be Approximated Using Routine Data in the Electronic Health Record? Diabetes. 2020;69 (Supplement_1):1443-P. [DOI] [Full Text] |
| 19. | Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin Gastroenterol Hepatol. 2020;18:2128-2130.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 473] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 20. | Fignani D, Licata G, Brusco N, Nigi L, Grieco GE, Marselli L, Overbergh L, Gysemans C, Colli ML, Marchetti P, Mathieu C, Eizirik DL, Sebastiani G, Dotta F. SARS-CoV-2 Receptor Angiotensin I-Converting Enzyme Type 2 (ACE2) Is Expressed in Human Pancreatic β-Cells and in the Human Pancreas Microvasculature. Front Endocrinol (Lausanne). 2020;11:596898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 21. | Wu CT, Lidsky PV, Xiao Y, Lee IT, Cheng R, Nakayama T, Jiang S, Demeter J, Bevacqua RJ, Chang CA, Whitener RL, Stalder AK, Zhu B, Chen H, Goltsev Y, Tzankov A, Nayak JV, Nolan GP, Matter MS, Andino R, Jackson PK. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021;33:1565-1576.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 244] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 22. | Shaharuddin SH, Wang V, Santos RS, Gross A, Wang Y, Jawanda H, Zhang Y, Hasan W, Garcia G Jr, Arumugaswami V, Sareen D. Deleterious Effects of SARS-CoV-2 Infection on Human Pancreatic Cells. Front Cell Infect Microbiol. 2021;11:678482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Liu XX, Zhu XM, Miao Q, Ye HY, Zhang ZY, Li YM. Hyperglycemia induced by glucocorticoids in nondiabetic patients: a meta-analysis. Ann Nutr Metab. 2014;65:324-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Plummer MP, Bellomo R, Cousins CE, Annink CE, Sundararajan K, Reddi BA, Raj JP, Chapman MJ, Horowitz M, Deane AM. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med. 2014;40:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 25. | Mamtani M, Athavale AM, Abraham M, Vernik J, Amarah AR, Ruiz JP, Joshi AJ, Itteera M, Zhukovski SD, Madaiah RP, White BC, Hart P, Kulkarni H. Association of hyperglycaemia with hospital mortality in nondiabetic COVID-19 patients: A cohort study. Diabetes Metab. 2021;47:101254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R, Klonoff DC. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 484] [Article Influence: 96.8] [Reference Citation Analysis (0)] |