Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1267
Peer-review started: October 20, 2022
First decision: January 3, 2023
Revised: January 5, 2023
Accepted: February 3, 2023
Article in press: February 3, 2023
Published online: February 26, 2023
Processing time: 127 Days and 8.1 Hours
A noteworthy public health problem, antimicrobial resistance (AMR) has been impeded in many ways by the coronavirus disease 2019 (COVID-19) pandemic. This narrative review discusses the two-sided impact of COVID-19 on the magnitude of AMR. The pandemic has put tremendous strain on healthcare systems, diverting resources, personnel, and attention away from AMR diagnosis and management toward COVID-19 diagnosis and contact tracking and tracing. AMR research has been severely hampered, and surveillance and antimicrobial stewardship (AMS) programs have been de-emphasized, delayed, or halted. Antibiotics, particularly broad-spectrum, were prescribed more frequently without diagnostic confirmation of bacterial infection than before the pandemic. Nonetheless, the COVID-19 pandemic has highlighted the vulnerability of healthcare systems in controlling infectious disease threats and raised awareness of the importance of infection prevention and control. Yet, the pandemic has created opportunities to capitalize on positive effects on AMR management. The review concludes that it is now more important than ever to focus on AMR and strengthen AMS programs to ensure appropriate antibiotic use and other AMR prevention measures in healthcare. We must ensure that one of the COVID-19 legacies is increased support for AMR research, diagnostic implementation, appropriate diagnostic stewardship, and the strengthening of our health systems. The COVID-19 pandemic has demonstrated that prevention is better than cure. Countries will need to step up their efforts to combat AMR as a multidisciplinary community. We must prepare our public health systems to combat multiple threats at the same time.
Core Tip: If given the resources, the globe can continue to develop robust public health and healthcare systems to protect its citizens against antimicrobial resistance (AMR). The findings from this narrative review indicate that the pandemic's overuse of antibiotics highlights the need to strengthen antimicrobial stewardship (AMS) programs so that they can guide disciplines. This review recommends that it is now more important than ever to focus on AMR and strengthen AMS programs to ensure appropriate antibiotic use and other AMR prevention measures in healthcare. Performing rapid and accurate point-of-care tests before an antibiotic prescription is an efficient way to optimize antibiotic administration and prevent the development of antibiotic-resistant bacteria.
- Citation: Rayan RA. Flare of the silent pandemic in the era of the COVID-19 pandemic: Obstacles and opportunities. World J Clin Cases 2023; 11(6): 1267-1274
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1267.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1267
One of the world's most serious public health threats is antimicrobial resistance (AMR). AMR, also known as the silent pandemic, happens when bacteria, viruses, fungi, and parasites change and stop responding to medication. As a result, infections become more challenging to cure, raising the risk of a serious disease and death. Drug resistance renders antibiotics and other antimicrobial medications ineffective, making it more challenging or impossible to treat infections. AMR poses a concern on a worldwide scale, especially in developing nations. Antibiotic and antifungal resistance increased dramatically during the coronavirus disease 2019 (COVID-19) pandemic, reversing previous gains. Antibiotic-resistant bacteria cause 1.3 million direct deaths and five million indirect deaths each year[1]. Estimates were made in 2019 before the COVID-19 pandemic worsened the situation. Unfortunately, those most susceptible to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, which causes COVID-19, are also the most susceptible to drug-resistant infections[2]. People over the age of sixty-five, as well as those with underlying medical conditions such as cardiovascular diseases, diabetes, chronic respiratory diseases, and cancer, are at a higher risk of developing a serious illness, regardless of the cause.
Before the COVID-19 pandemic, the World Health Organization (WHO) classified AMR as one of the top ten most critical global health problems[3]. According to one study, if no action is taken, AMR will cause ten million deaths yearly researching 2050, with a financial effect of over 100 trillion USD[4]. Efforts for addressing AMR as a significant universal health issue have just lately increased. Taking part in the WHO Global Antimicrobial Resistance and Use Surveillance System grew exponentially between 2017 and 2019, aggregating data from over 64000 surveillance sites in sixty-six countries[5]. This surveillance system had ninety-four countries enrolled in August 2020[6]. This level of participation represents a meaningful accomplishment in the global fight against this health threat. Antimicrobial stewardship (AMS) programs and National Action Plans had made considerable progress in many countries before COVID-19 in slowing AMR.
However, there are growing fears that the COVID-19 pandemic has slowed present and upcoming efforts against AMR[7]. Antibiotics, for example, were prescribed more frequently without diagnostic confirmation of bacterial infection than before the pandemic. Because of the COVID-19 emergency in healthcare systems, many planned activities were deprioritized, and already implemented preventive measures were reversed. The pandemic has put tremendous strain on healthcare systems, diverting resources, personnel, and attention away from AMR diagnosis and management toward COVID-19 detection and contact tracing. AMR studies have been largely hampered, and surveillance and AMS programs have been de-emphasized, lagged, or stopped[8]. Furthermore, during the first two years of the COVID-19 pandemic, hard lessons were learned about prioritizing COVID-19 transmission surveillance at the expense of decreased AMR surveillance[9].
To find out the magnitude of AMR considering COVID-19, we carried out a narrative literature review in various distinguished and reliable journals, news, governmental, and organizational websites on Google, Google Scholar, and PubMed. We denoted appropriate studies by searching for reports on One Health, AMR, antimicrobials, antibiotics, and AMS, in relation to the coronavirus pandemic. Findings from studies were considered if they explicitly noted the linkage between AMR and the COVID-19 pandemic. The search was carried out in December 2022 and covered published peer-reviewed studies accessible from the attack of COVID-19 in December 2019 to December 2022.
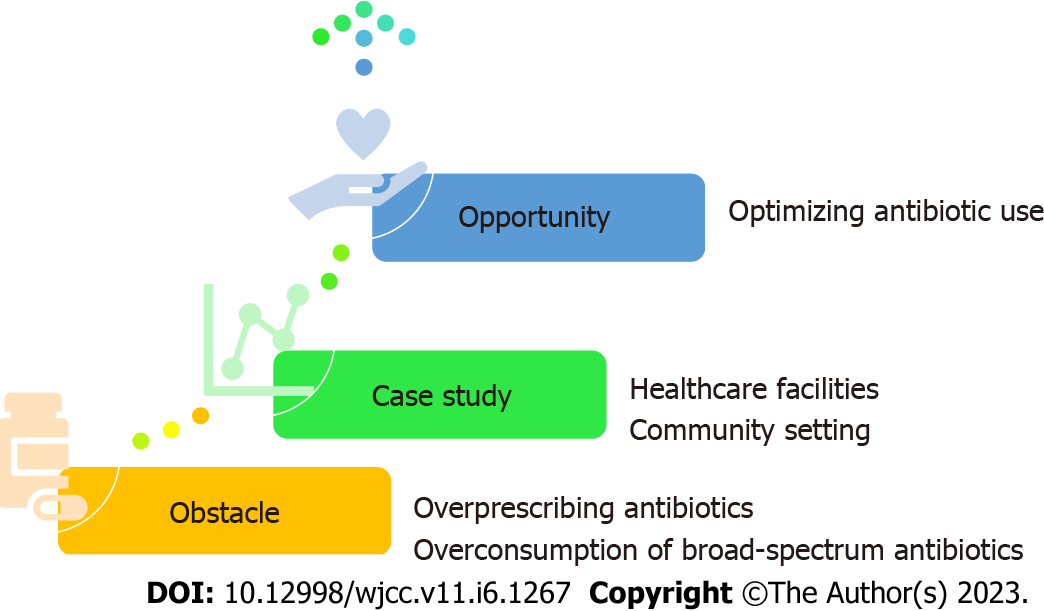
This review aims to discuss the impact of COVID-19 on the magnitude of AMR, as shown in Figure 1. First, it highlights the negative aspect of the situation (obstacles) in terms of overprescribing antibiotics during the pandemic, especially broad-spectrum ones. Besides, it augments the argument with data drawn from the case study of the United States concerning the effect of the COVID-19 pandemic inside and outside healthcare facilities. Next, it shows the positive side (opportunities) highlighting optimizing the use efforts to prevent AMR. Finally, it concludes with a global call for action now to curb the silent pandemic of AMR.
Antibiotics and antifungals can save lives, but they can also contribute to resistance when used in humans, animals, or plants. During the pandemic, antibiotic use differed throughout healthcare settings. Antibiotics were frequently administered to COVID-19 patients, yet antibiotics are ineffective against viruses such as that lead to COVID-19. Preventing infections from starting is vital in both our communities and medical settings. In hospitals, many infection prevention and control (IPC) regulations were impeded by pandemic-related issues, which unfortunately led to some AMR advancements being reversed. Hand hygiene, disinfecting tools, segregating patients, and properly handling personal protective equipment (PPE) are all IPC practices. During the pandemic, there were more and sicker patients who needed catheters and ventilators more frequently and for longer periods. This might increase the risk of hospital-acquired infections (HAIs) and pathogen spread, particularly when combined with PPE and lab supply issues, reduced staff, and longer lengths of stay[9].
A considerable lag in tracking AMR, including detection and reporting data, was brought on by changes in patient care, testing, and treatment, as well as personnel availability at healthcare institutions and health departments, because of the COVID-19 pandemic. Knowing where and how resistance changes occur helps to provide strategies (such as outbreak response) to avoid resistance spread and slow resistance. For instance, 23% fewer specimens or isolates were received by the United States Center for Diseases Prevention and Control AMR Lab Network in 2020 than in 2019 for evaluation. Throughout 2020, it kept gathering isolates using tried-and-true methods, while other isolates went untested because of testing halt. This could be for the fact that health facilities and population health personnel had to direct their efforts to COVID-19. Many AMR systems were used to assist the global response to COVID-19, covering testing and providing surge capacity to overburdened laboratories. The Centers for Disease Prevention and Control (CDC)'s National Tuberculosis Molecular Surveillance Center, for example, studied SARS-CoV-2 using its AMR Lab Network sequencing capabilities[10].
Antibiotics do not cure viral infections like COVID-19, but bacterial coinfections can occur alongside viral infections. Antibiotic treatment of COVID-19 patients was more of a rule than an exception in many countries, particularly during the early pandemic. Concerns about bacterial confections and difficulties distinguishing COVID-19 from community-acquired pneumonia led to the overuse of antibiotics. For instance, antibiotics were prescribed to 80%-100% of COVID-19 hospitalized patients in the United States and China during the first six months of the COVID-19 pandemic, even though they were rarely indicated at the time. Bacterial coinfections were reported in only 7%-8% of hospitalized patients and 14% of intensive care unit (ICU) patients, implying that antibiotics were frequently used inappropriately in the treatment of COVID-19[11]. According to another study’s findings based on data collected from over 30000 patients, the estimated rate of bacterial coinfection was also less than 9%[12].
On the contrary, and because of the reduction in healthcare-seeking behavior during the pandemic, infections that do warrant antibiotics, such as tuberculosis, gonorrhea, and pneumococcus, may have gone untreated. But we do not have the same amount of information on how superbug resistance patterns are developing in the community context as we do in the inpatient situation, where hospitals track the bacteria inside their walls and their susceptibility patterns[13,14].
In both primary care settings and hospitals, the use of broad-spectrum antibiotics has grown in European nations. In addition, broad-spectrum antibiotics were commonly used in hospitalized COVID-19 patients in the United States Antibiotics with a broad spectrum of activity are effective against a wide variety of bacteria. Hospitals were flooded with critically ill patients, particularly early in the pandemic, and those patients stayed for extremely prolonged periods. Those suffering from fever and pneumonia were given broad-spectrum antibiotics. When it was not clear what the course of severe COVID-19 illness would be, there was an impulse to treat severely pneumatic ICU-admitted patients with broad-spectrum antibiotics. And this is despite years of steady decline in HAIs, which AMS committees, IPC programs, and hospitals worked hard to achieve. Broad-spectrum antibiotics should not be used as a first-line treatment and should only be used to treat severe bacterial infections. It is critical to use them correctly to avoid the development of drug resistance.
The COVID-19 pandemic highlights healthcare workers' human desire to intervene, especially when a patient is critically ill, which can lead to a suspension of evidence-based medicine at the bedside. Uncertainty about the COVID-19 diagnosis, combined with a desire to assist patients, concerns about bacterial coinfections, and misleading results from a variety of diagnostic tests, all contributed to an increase in antibiotic overuse early in the pandemic.
In the United States, 29400 people died in the first year of the pandemic from infections that were frequently associated with medical care and were resistant to antibiotics. Nearly 40% of these patients contracted an infection while hospitalized[9,13,14]. Although the overall number of AMR deaths in the United States may be significantly higher, it is impossible to analyze because of data gaps brought on by the epidemic. Since 2013, the United States has been sounding the alarm about AMR, citing the threat it posed to the healthcare system, food supply, environment, and community. Before the pandemic, 50000 Americans died from antimicrobial-resistant infections or Clostridioides difficile infections (often associated with antimicrobial use)[13,14]. According to the CDC's 2019 projections; every year, over 35000 Americans die from at least 2.8 million infections that are resistant to antibiotics[13]. However, significant nationwide investments in improving IPC as well as antimicrobial use resulted in antimicrobial-resistant infections falling by 27% along with reduced antimicrobial-resistant infection deaths by 18% between 2012 and 2017 and by 30% in hospitals[13,14]. These declines persisted until 2020. In contrast, the pandemic led to an increase in antibiotic resistance, a rise in antibiotic usage, and a decrease in data and preventative measures[15].
Antimicrobial-resistant infections in healthcare facilities in the United States increased, particularly in hospitals. Hospitals cared for sicker patients who needed medical devices like catheters and ventilators more frequently and for longer periods. Hospitals also faced issues with PPE supply, staffing shortages, and longer patient visits. From March to October 2020, 80% of COVID-19 patients hospitalized in the United States received an antibiotic[13,14]. Ceftriaxone, which was commonly prescribed with azithromycin, was given to half of the hospitalized patients. This is because of challenges in differentiating COVID-19 from community-acquired pneumonia when patients are admitted in a healthcare facility for evaluation. Both resistant hospital-onset infections and mortality jumped by minimally 15% between 2019 and 2020[13].
Following years of consistent declines in HAIs, in 2010, four of the six kinds of HAIs were significantly higher in United States hospitals. Several such HAIs are resistant to antibiotics. There was a 78% increase in infections caused by the carbapenem-resistant Acinetobacter, a 32% increase in infections caused by the multidrug-resistant Pseudomonas aeruginosa, a 14% increase in infections caused by the vancomycin-resistant Enterococcus, and a 13% increase in infections caused by the methicillin-resistant Staphylococcus aureus (MRSA)[13,14]. Furthermore, antifungal-resistant infections such as Candida Auris (that generally grew 60%) and Candida species (without Candida Auris) surged in 2020, with a rise of 26% in hospital infections. Over twenty outbreaks caused by resistant infections, such as Candida Auris and Acinetobacter, occurred in COVID-19 treatment and observation units. Unknown factors may have a long-term impact on a region's antimicrobial-resistant bacteria outbreak[9,13].
We have limited data on the spread of antimicrobial-resistant pathogens in communities, such as drug-resistant gonorrhea and food-borne germs. Yet, antibiotic use in outpatient clinics fell critically during 2020 in comparison to 2019 because of limited access to outpatient healthcare and lower magnitude of other respiratory conditions, which frequently result in administering antibiotics. Patients had reduced access to care and testing because public health staff were redirected to the global pandemic response. In reaction to COVID-19 issues, several medical institutions and clinics reduced access, received less patients, or shut their doors completely. Prior to the COVID-19 pandemic, data between 2012 and 2017 displayed that numerous resistant infections commonly discovered in the community were on the rise. In 2020, potentially resistant infections spread undetected and untreated in communities. In 2021, outpatient antibiotic consumption increased, yet it remained generally less during 2021 in comparison to 2019. Between 2020 to December 2021, azithromycin was the highest prescribed antibiotic for adults, and increases in azithromycin prescribing followed peaks in COVID-19 cases[9].
Even while antibiotic use in nursing homes has grown in response to COVID-19 outbreaks, it is still very low. However, compared to the same months in 2019, usage of azithromycin rose by 150% in April and 82% in December 2020. Using azithromycin remained high until October 2020. Overall, antibiotic use in 2021 was 5% less than that during 2019. This decline could be attributed to limited residents in nursing homes in this period[9].
Nonetheless, the COVID-19 pandemic has emphasized the fragility of healthcare systems in managing threats of infectious diseases and raised attention of the value of IPC. The pandemic has created opportunities to capitalize on positive effects on AMR management. COVID-19 has had a substantial impact on our social interactions. Subjects are much more aware of protective healthcare measures like washing hands, putting on face masks, and maintaining physical distance[16]. These behavioral shifts will aid in the prevention of infectious diseases, covering those impacted by AMR; however, there is a recidivism risk after the COVID-19 pandemic has passed[17]. Maintaining high-quality IPC training is needed for all healthcare professionals and facilities other than hospitals, such as nursing homes and other long-term care facilities. This includes educating the public on how to prevent the spread of germs and infection in the communities where they live and work.
Because of fewer visits to primary care, overall antibiotic consumption in primary care decreased in many countries during COVID-19. Overall, outpatient antibiotic prescribing trended downward during the first pandemic year. For instance, the Epocrates app, which provides a window into what clinicians are thinking, saw a significant decrease in lookups for antibiotics that are sometimes inappropriately used for upper respiratory infections as early as March 2020. However, during the first year of the pandemic, changes in clinician accessibility and office hours, stay-at-home directives, and mask regulations—all of which we made to stop the spread of COVID-19—had a positive impact on limiting the spread of respiratory infections.
COVID-19 has raised awareness of the importance of laboratory capacity and surveillance[18]. The COVID-19 mitigation response relies heavily on robust diagnostic and laboratory surveillance systems. Repurposing this capacity for AMR will be efficient because such elements are required for the proper detection of infectious conditions and for evaluating the capacity of AMS programs inside the healthcare system, particularly in developing nations[19]. At the same time, the same healthcare authorities’ readiness to spend money on additional diagnostics, including point-of-care testing for rapid bacterial infectious illness and AMR[20]. This investment may encourage healthcare authorities to replicate quickly assembled infrastructure, like polymerase chain reaction and lateral flow examinations, for the detecting COVID-19 at scale, to detect AMR. Expanding electronic data automation would provide healthcare facilities and systems with the information they need on antibiotic use and AMR. This also entails using well-established networks like the AMR Lab Network to share information during emergencies, employing telehealth to track down contacts, and making efforts to guarantee that there are laboratory supplies and tools accessible for IPC and patient care.
AMR is a One Health problem that impacts the wellbeing of people, animals, plants, and the environment. Maneuvers to detect antimicrobial-resistant microorganisms, trace the spread of resistance, and assess the impact of the consumption of antimicrobials need monitoring of human, animal, and plant populations, as well as the environment. Increasing the capacity of the National Wastewater Surveillance Systems to collect AMR data from wastewater treatment plants and healthcare facilities, as well as researching resistance in community and healthcare wastewater on a domestic and global scale. This includes increasing global capacity to combat AMR in the environment and monitoring AMR across One Health.
The COVID-19 pandemic has highlighted the importance of improving IPC and hygiene, as well as a reminder that following IPC protocols is critical in reducing hospitalizations. These protocols, which are required to prevent the transmission of SARS-CoV-2, have the potential to significantly reduce AMR prevalence[18]. The COVID-19 pandemic has raised attention and reaffirmed the importance of a worldwide One Health tract. One Health tract has the possibility to efficiently fight COVID-19 infection[21], and it could combat the growing AMR. Vaccines, which efficiently guard against SARS-CoV-2 will support lowering COVID-19 magnitude and inappropriate antibiotic use, hopefully decreasing the prevalence of AMR worldwide. Encouraging the administration of vaccines for preventable infectious conditions could have a significant effect on the spread of AMR[22].
The future of AMR is dependent on antibiotic prescribing decisions made today, as well as the care teams responsible for IPC. To regain some of the ground lost during the pandemic, we must revisit basic AMS and IPC principles. Vaccinations, both routine, and catch-up, are needed to prevent infections. The COVID-19 pandemic had a significant impact on preventive vaccination uptake rates in a variety of patient populations. Pneumonia caused by MRSA and other bacteria is a leading cause of death in influenza patients, and influenza vaccination may reduce the risk of bacterial superinfection. Antibiotic use should be optimized across all healthcare settings. Additionally, we should promote tracking for companion animals and agriculture, as well as effective antibiotic and antifungal usage[11].
Viral infections should no longer be treated with antibiotics. Using viral diagnostics and procalcitonin measurements may aid in identifying patients who can be weaned off antibiotics. Recognizing the patient's symptoms, providing symptom relief, and educating the patient about the risks associated with inappropriate antibiotic use if a viral infection is suspected. It is also critical to follow the recommendations of specialty societies when treating viral infections. Treatment must be tailored to the antibiotic spectrum. Referring to the most recent and local data on antimicrobial susceptibility when making decisions. When obtaining blood cultures, take precautions to avoid contamination and narrow the spectrum based on the results. Examining the veracity of documented antibiotic allergies, particularly those related to penicillin[23]. We must adhere to the most recent specialist society recommendations for antibiotic treatment durations and employ the shortest durations possible to prevent unwanted antibiotic exposures. A greater emphasis on IPC procedures because of the COVID-19 pandemic has the potential to reduce other HAIs.
Patient education is vital to antibiotic treatment adherence. Years ago, there was an emphasis on “taking the full course” of antibiotics. Now, the focus has shifted to the shortest and clinically appropriate effective duration of therapy. Each additional day of antibiotic treatment increases the risk of patient harm. The length of antibiotic therapy must be optimized at the time of hospital discharge; for instance, most of the unnecessary antibiotic usage for community-acquired pneumonia occurs after discharge.
AMS is our most potent weapon against AMR, and it deserves high priority and investment from healthcare systems. The key components of the initiative include IPC, quick-cycle research, and education activities, reduced diagnostic ambiguity regarding bacterial infections, avoidance of improper antibiotic usage and durations, and rapid de-escalation based on outcomes. New antibiotics alone will not be enough to fix this issue. Antimicrobial drugs with novel modes of action are scarce, and it takes a while for them to reach the market. The post-antibiotic era has already begun because of the disparity between AMR rates and the development of novel antibiotics. Resistance will certainly develop in the presence of new agents. Novel treatment approaches, such as antibody therapy, bacteriophages, and fecal microbiota transplantation, are being investigated.
The shift in trend indicates that AMR prevention efforts must be revisited and reintegrated into healthcare systems. Despite a great desire to assist patients when there are few treatment choices available, medical personnel should use antibiotics and diagnostic tests cautiously in the early phases of an epidemic. We can learn a lot from the COVID-19 pandemic about upcoming viral pandemics. Faced with new infectious disease outbreaks, hospitals and healthcare workers should improve antibiotic prescribing. They should follow the recommendation in cases where an antibiotic should be considered in a respiratory viral epidemic, as well as in cases where diagnostic tests are indicated. The COVID-19 pandemic has demonstrated that prevention is better than cure. We must prepare our public health systems to combat multiple threats at the same time[24].
The strength of this narrative review lies in its findings indicating that the pandemic's overuse of antibiotics highlights the need to strengthen AMS programs so that they can guide disciplines. Yet, the major limitations are basing the argument on data drawn mainly from one country and the lack of a systematic approach to studying the impact of the COVID-19 pandemic on AMR on a global scale.
We must not overlook the possibility that the pandemic will increase AMR globally. Even before the SARS-CoV-2 pandemic, overcoming AMR demanded immediate global action. Understanding the pathogenesis (how a disease develops) of SARS-CoV-2 infection, as well as the possibility of bacterial coinfections, is critical now that we are amid a pandemic. We must ensure that one of the COVID-19 legacies is increased support for AMR research, diagnostic implementation, appropriate diagnostic stewardship, and the strengthening of our health systems. As we emerge from the pandemic, there is a significant opportunity for the healthcare systems to promote cooperation with policymakers, the population, and the mass media to pay more attention to AMR and engagement to complement the legacy of COVID-19 physical separation, washing hands, face masks, producing vaccines, and keeping away from unneeded antibiotic consumption. States will have to step up their capacities to combat AMR in muti-sectoral contexts. Multi-sectoral, coordinated, and targeted research on such resistance, under the One Health approach, will be vital for competent curbing of AMR in the context of the pandemic, as well as for presenting opportunities for addressing AMR. Eventually, more research is needed to study the impact of the COVID-19 pandemic on AMR systematically on a global scale, pooling data from different countries and taking into consideration the economic variations between countries.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Public, environmental and occupational health
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gupta L, Indonesia; Herawati F, Indonesia S-Editor: Wang DM L-Editor: A P-Editor: Wang DM
| 1. | Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8908] [Cited by in RCA: 7275] [Article Influence: 2425.0] [Reference Citation Analysis (0)] |
| 2. | Centers for Disease Prevention and Control. COVID-19 and Your Health. Aug 11, 2022. [cited 20 Oct 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/your-health/understanding-risk.html. |
| 3. | Choudhury S, Medina-Lara A, Smith R. Antimicrobial resistance and the COVID-19 pandemic. Bull World Health Organ. 2022;100:295-295A. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Pierce J, Apisarnthanarak A, Schellack N, Cornistein W, Maani AA, Adnan S, Stevens MP. Global Antimicrobial Stewardship with a Focus on Low- and Middle-Income Countries. Int J Infect Dis. 2020;96:621-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 5. | Harris M, Abad-Vergara D. Record number of countries contribute data revealing disturbing rates of antimicrobial resistance. June 1, 2020. [cited 20 Oct 2022]. Available from: https://www.who.int/news/item/01-06-2020-record-number-of-countries-contribute-data-revealing-disturbing-rates-of-antimicrobial-resistance. [DOI] [Full Text] |
| 6. | World Health Organization. Global antimicrobial resistance and use surveillance system (GLASS) report: 2021. WHO 2021. Available from: https://apps.who.int/iris/handle/10665/341666. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Knight GM, Glover RE, McQuaid CF, Olaru ID, Gallandat K, Leclerc QJ, Fuller NM, Willcocks SJ, Hasan R, van Kleef E, Chandler CI. Antimicrobial resistance and COVID-19: Intersections and implications. Elife. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 195] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 8. | Rodríguez-Baño J, Rossolini GM, Schultsz C, Tacconelli E, Murthy S, Ohmagari N, Holmes A, Bachmann T, Goossens H, Canton R, Roberts AP, Henriques-Normark B, Clancy CJ, Huttner B, Fagerstedt P, Lahiri S, Kaushic C, Hoffman SJ, Warren M, Zoubiane G, Essack S, Laxminarayan R, Plant L. Key considerations on the potential impacts of the COVID-19 pandemic on antimicrobial resistance research and surveillance. Trans R Soc Trop Med Hyg. 2021;115:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 9. | Centers for Disease Prevention and Control. COVID-19 & Antibiotic Resistance. June 2022. [cited 20 Oct 2022]. Available from: https://www.cdc.gov/drugresistance/covid19.html. |
| 10. | Mantzourani E, Cannings-John R, Evans A, Ahmed H. To swab or not to swab? J Antimicrob Chemother. 2022;77:803-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1022] [Cited by in RCA: 1021] [Article Influence: 204.2] [Reference Citation Analysis (0)] |
| 12. | Langford BJ, So M, Raybardhan S, Leung V, Soucy JR, Westwood D, Daneman N, MacFadden DR. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27:520-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 517] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 13. | Newsroom Centers for Disease Prevention and Control. COVID-19 Reverses Progress in Fight Against Antimicrobial Resistance in U.S. July 12, 2022. [cited 20 Oct 2022]. Available from: https://www.cdc.gov/media/releases/2022/s0712-Antimicrobial-Resistance.html. |
| 14. | Tanne JH. Covid-19: Antimicrobial resistance rose dangerously in US during pandemic, CDC says. BMJ. 2022;378:o1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Centers for Disease Prevention and Control. 2019 AR Threats Report. Nov 2019. [cited 20 Oct 2022]. Available from: https://www.cdc.gov/drugresistance/biggest-threats.html. |
| 16. | Collignon P, Beggs JJ. CON: COVID-19 will not result in increased antimicrobial resistance prevalence. JAC Antimicrob Resist. 2020;2:dlaa051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Pires D, de Kraker ME, Tartari E, Abbas M, Pittet D. ‘Fight antibiotic resistance—It’s in your hands’: call from the World Health Organization for 5th May 2017. Clin Infect Dis. 2017;64:1780-1783. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Wellcome Trust. The global response to AMR: momentum, success, and critical gaps. Nov 16, 2020. [cited 20 Oct 2022]. Available from: https://wellcome.org/reports/global-response-amr-momentum-success-and-critical-gaps. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Tomczyk S, Taylor A, Brown A, de Kraker MEA, El-Saed A, Alshamrani M, Hendriksen RS, Jacob M, Löfmark S, Perovic O, Shetty N, Sievert D, Smith R, Stelling J, Thakur S, Vietor AC, Eckmanns T; WHO AMR Surveillance and Quality Assessment Collaborating Centres Network. Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: a global survey. J Antimicrob Chemother. 2021;76:3045-3058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 20. | Hays JP, Mitsakakis K, Luz S, van Belkum A, Becker K, van den Bruel A, Harbarth S, Rex JH, Simonsen GS, Werner G, Di Gregori V, Lüdke G, van Staa T, Moran-Gilad J, Bachmann TT; JPIAMR AMR-RDT consortium. The successful uptake and sustainability of rapid infectious disease and antimicrobial resistance point-of-care testing requires a complex 'mix-and-match' implementation package. Eur J Clin Microbiol Infect Dis. 2019;38:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Ruckert A, Zinszer K, Zarowsky C, Labonté R, Carabin H. What role for One Health in the COVID-19 pandemic? Can J Public Health. 2020;111:641-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Sosler S. Is antimicrobial resistance exacerbating the COVID-19 pandemic? May 11, 2020. [cited 20 Oct 2022]. Available from: https://www.gavi.org/vaccineswork/antimicrobial-resistance-exacerbating-covid-19-pandemic. [DOI] [Full Text] |
| 23. | Centers for Disease Prevention and Control. Core Elements of Antibiotic Stewardship. Apr 7, 2021. [cited 20 Oct 2022]. Available from: https://www.cdc.gov/antibiotic-use/core-elements/index.html. [RCA] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 24. | Barlam TF, Al Mohajer M, Al-Tawfiq JA, Auguste AJ, Cunha CB, Forrest GN, Gross AE, Lee RA, Seo SK, Suh KN, Volk S, Schaffzin JK. SHEA statement on antibiotic stewardship in hospitals during public health emergencies. Infect Control Hosp Epidemiol. 2022;43:1541-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |