Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.1152
Peer-review started: November 1, 2022
First decision: December 19, 2022
Revised: December 31, 2022
Accepted: January 28, 2023
Article in press: January 28, 2023
Published online: February 16, 2023
Processing time: 101 Days and 16.5 Hours
The incidence of Langerhans cell histiocytosis (LCH) is low, and involvement of the thyroid is even rarer, which results in high missed diagnosis or misdiagnosis rates.
We report a young woman with a thyroid nodule. Thyroid malignancy was suggested by fine needle aspiration, but she was eventually diagnosed with multisystem LCH, thus avoiding thyroidectomy.
The clinical manifestations of LCH involving the thyroid are atypical, and the diagnosis depends on pathology. Surgery is the main method for treating primary thyroid LCH, while chemotherapy is the main treatment method for multisystem LCH.
Core Tip: The incidence of Langerhans cell histiocytosis (LCH) is low. We report a young woman with a thyroid nodule, and thyroid malignancy was considered by fine needle aspiration. But she was eventually diagnosed with multisystem LCH, avoiding thyroidectomy.
- Citation: Shi JJ, Peng Y, Zhang Y, Zhou L, Pan G. Langerhans cell histiocytosis misdiagnosed as thyroid malignancy: A case report. World J Clin Cases 2023; 11(5): 1152-1157
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/1152.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.1152
Langerhans cell histiocytosis (LCH) is a rare disease that can involve the bones, lungs, skin, liver, lymph nodes, and pituitary gland, while thyroid involvement is relatively rare. LCH with thyroid involvement mainly manifests as goitre or thyroid nodules. However, malignant thyroid cancer is very common in the clinic and also manifests primarily as thyroid nodules; therefore, the two diseases can be difficult to distinguish. This paper reports a case of LCH with a thyroid nodule as the first symptom that was eventually diagnosed as multisystem LCH (MS-LCH). A literature review was conducted to improve clinicians' understanding of LCH involving the thyroid.
A right thyroid nodule that was detected and followed for 4 years.
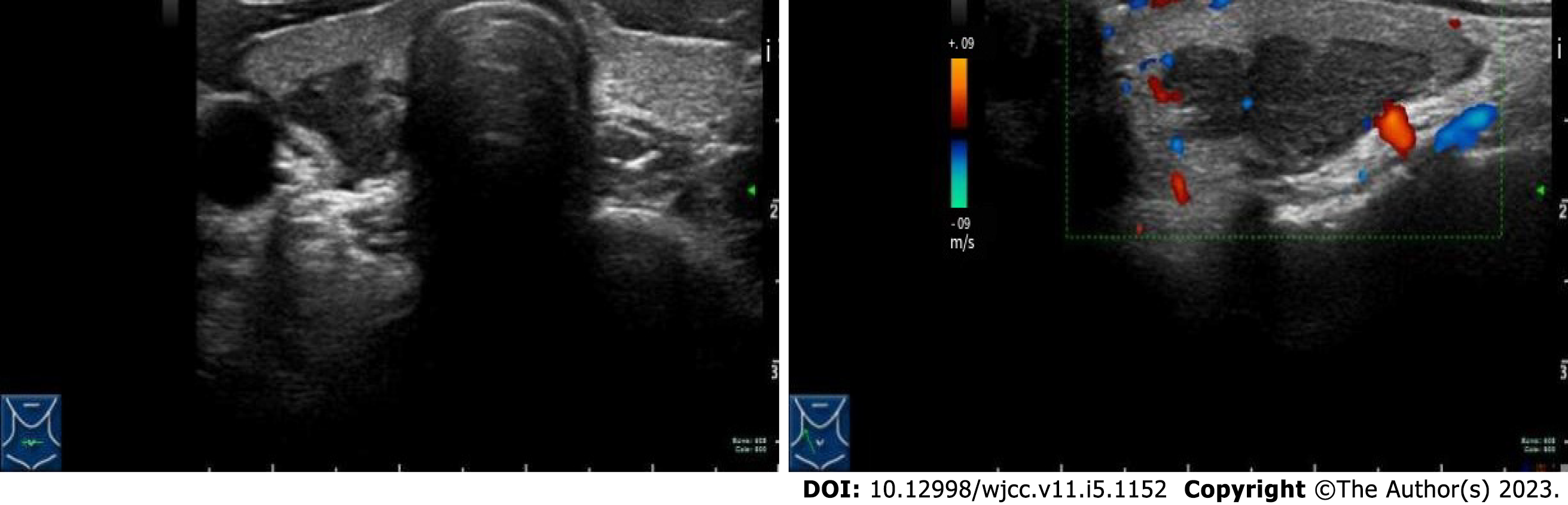
A 26-year-old female patient was admitted to the hospital because of a right thyroid nodule that was found by physical examination and had persisted for over 4 years. At the time the nodule was found, the patient had no discomfort, and she was reexamined regularly in the outpatient department. Recently her thyroid B-ultrasound examination revealed a nodule in the right lobe, approximately 2.1 cm × 0.9 cm × 0.9 cm in size, that was solid hypoechoic, showed horizontal growth, had irregular edges, and was lobulated (Figure 1); a small amount of blood flow signal was seen inside the nodule. Some of the individual right lateral cervical lymph nodes appeared swollen. Fine needle aspiration (FNA) of the thyroid was conducted: Heterotypic cells were found in the bloody fluid, with abundant cytoplasm and nucleoli. Malignancy was initially suspected (Bethesda V). BRAFV600E gene mutation analysis revealed no mutations. Therefore, the patient was admitted to the hospital with an admission diagnosis of a suspected malignant thyroid tumour.
Previous findings included abnormal pituitary function, oestrogen replacement therapy (details unknown), and sublingual gland cyst resection over 4 years prior.
The patient had no relevant personal or family history.
Physical examination showed no obvious nodules of the bilateral thyroid glands, no obvious enlargement of the superficial lymph nodes, and no other special changes.
Routine blood examination and biochemical examination, as well as thyroid function, antibody evaluation, thyroglobulin, and tumour tests, did not reveal any abnormalities.
B-ultrasound of the upper abdomen showed diffuse echogenic changes in the liver.
Lung computed tomography (CT) revealed multiple, small nodular shadows in both lungs with the maximum diameter of 5 mm and patchy shadows in the left lower lobe of the lung with poorly defined boundaries (suggesting inflammation).
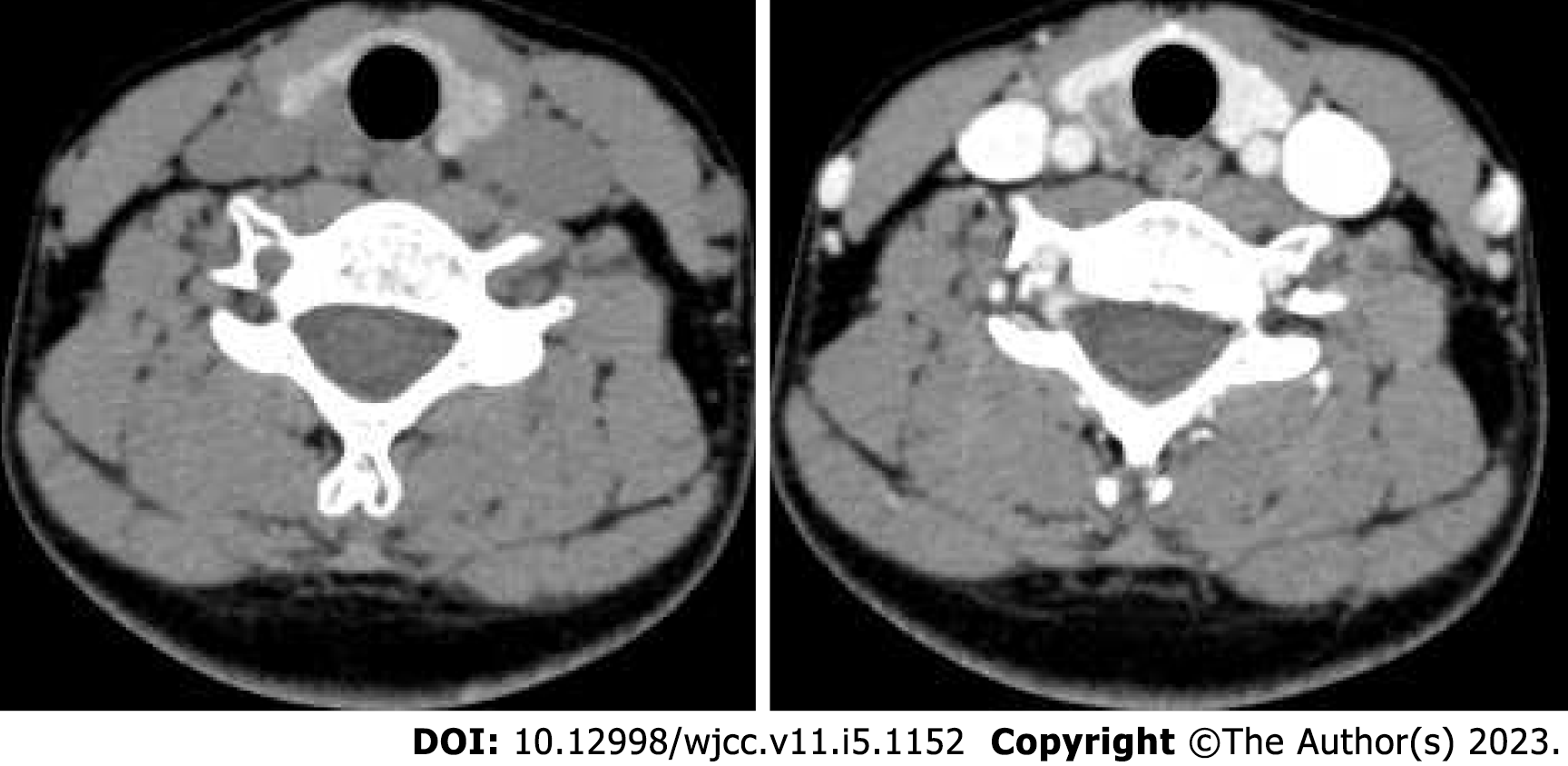
A contrast-enhanced CT scan of the neck revealed a right thyroid nodule, which was suspected to be a malignant lesion, and multiple enlarged lymph nodes in the right neck (Figure 2). Lumbar enhanced MRI showed a low-signal lesion with enhancement in the lumbar 3 vertebra, and metastasis was considered based on the patient’s medical history.
Whole-body PET-CT was performed and revealed the following: (1) The right thyroid nodule was irregular with mixed low-density shadows and increased glucose metabolism, consistent with malignant lesions; (2) Multiple lymph nodes in the left parotid gland, neck, and mediastinum showed increased glucose metabolism; thus, the possibility of metastasis was considered; (3) Minor bone resorption and destruction of the lumbar 3 vertebra was observed surrounded by a few soft tissue shadows, and increased glucose metabolism was present, further indicating the possibility of metastasis; (4) Multiple small nodules and quasi-nodules were seen in both lungs, with no significant increase in glucose metabolism, again indicating the possibility of metastasis; and (5) The liver was swollen with reduced density and vascular separation but did not show increased glucose metabolism, suggesting the possibility of fatty liver.
Ultrasound-guided coarse needle biopsy of the thyroid finally indicated potential LCH. The immunohistochemical results were as follows: S100 (+), CD1а (+), Langerin (+), CK (-), TTF-1 (-), Sy (-), CgA (-), CD56 (-), and KI67 (+, 20%).
The patient received chemotherapy with VP. After two courses of treatment, there was no obvious reduction in the size of the right thyroid nodule. Therefore, the chemotherapy regimen was changed to six cycles of CHOP.
After the eight chemotherapy cycles, the patient's thyroid nodule was significantly reduced. At the last follow-up, the patient was alive and healthy.
Langerhans cells (LCs) are antigen-presenting cells originating from the bone marrow whose function is to present antigens to T cells. Activated LCs are drained out of the epithelial microenvironment from lymphatic vessels to peripheral lymph nodes, where they develop and mature under the control of numerous factors. LCH is characterized by the abnormal proliferation of pathologic LCs. In LCH, the LCs remain in a state of continuous activation and are retained in the peripheral tissues, where they accumulate and induce the excessive secretion of inflammatory factors. LCH is characterized by pathological tissue destruction in multiple sites[1,2].
The incidence of LCH is low but common in children, affecting 2.6/1 million to 5.4/1 million children and approximately 1/1 million to 2/1 million adults[3-5]. Thyroid involvement in LCH is rare in clinical practice, and most studies describing it are case reports. Lieberman PH reported only one case of thyroid involvement among 238 cases of LCH[6]. LCH with thyroid involvement is more common in adults[7]. The clinical manifestations of LCH are diverse, involving the bone, skin, lymph nodes, pituitary gland, liver, spleen, lung, thyroid, lymph nodes, and central nervous system, among which involvement of the bone and skin is the most common. LCH can be divided into single-system LCH (SS-LCH) and MS-LCH. MS-LCH can be further divided into low risk and high risk, with bone marrow, liver, and spleen involvement classified as high risk and the rest (including lung) classified as low risk. Our patient with MS-LCH had involvement of the thyroid, lumbar vertebra, liver, and pituitary gland; thus, the disease was classified as high-risk MS-LCH.
The clinical manifestations of LCH with thyroid involvement are nonspecific. Patten et al[8] reviewed a total of 65 cases of LCH involving the thyroid reported from 1961 to 2011 and found that 59% of LCH cases involving the thyroid presented as diffuse thyroid lesions, 25.8% as nodular enlargement, and 13.6% as heterogeneous enlargement. Zhang et al[9] analysed the clinical data of 29 LCH patients with thyroid involvement reported in the literature from January 2010 to April 2020. A total of 62.07% of the patients presented with goitre or diffuse goitre, and 37.93% presented with single or multiple thyroid nodules. Cai et al[10] reported 228 cases of MS-LCH in adults with thyroid involvement, 35 of which involved the thyroid. MS-LCH with thyroid involvement was found to be more likely to involve the pituitary gland, liver, and lymph nodes but less likely to involve bone than MS-LCH without thyroid involvement.
In terms of thyroid function, Patten et al[8] reported that 40.9% of patients had normal thyroid function, 19.7% had hypothyroidism, 10.6% had subclinical hypothyroidism, and 1.5% had hyperthyroidism. Zhang et al[9] and Cai et al[10] also suggested that thyroid function may show different manifestations in patients with LCH involving the thyroid. Our patient had a thyroid nodule with no clinical discomfort and normal thyroid function, as reported in the literature. Additionally, nodular goitre, adenomatous goitre, and thyroid carcinoma are difficult to identify. Therefore, a missed clinical diagnosis or misdiagnosis can easily occur.
The ultrasonography and cervical contrast-enhanced CT findings of LCH involving the thyroid are also nonspecific. According to the relevant literature, ultrasonography of LCH involving the thyroid mainly shows diffuse goitre or hypoechoic thyroid nodules[11-13]. Cai et al[10] found no calcification in 36 cases of LCH involving the thyroid. The number of reported patients who underwent cervical contrast-enhanced CT is more limited. Chen et al[14] reported that cervical contrast-enhanced CT showed low-density nodules with unclear boundaries and uneven enhancement. In our patient, a lobulated solid hypoechoic nodule with irregular edges was found on ultrasonography, and a flaccid low-density nodule that was less enhanced than the thyroid was found on cervical contrast-enhanced CT, consistent with previous reports.
The efficacy of FNA in diagnosing LCH with thyroid involvement is low. Zhang et al[9] reported that only 37.93% of patients were diagnosed with LCH involving the thyroid by FNA, while 27.59% were diagnosed with papillary or medullary thyroid carcinoma, 6.9% were diagnosed with benign thyroid tumours, and 6.9% were diagnosed with atypical lesions. Chen et al[13] reported that only 5/12 (41.67%) patients were diagnosed by FNA. Most authors have reported that compared with FNA, coarse needle aspiration of the thyroid gland can obtain more tissue, thus improving the diagnostic efficiency[9,11,15]. In our case, FNA revealed the possibility of a malignant tumour. However, more tissue samples were obtained after a coarse thyroid needle puncture to make the final diagnosis and avoid the complications of thyroid surgery. Therefore, we believe that if a patient is suspected of having LCH involving the thyroid, coarse thyroid needle aspiration is preferable. A diagnosis of LCH can be made when the characteristic Birbeck particles are found under electron microscopy or the immunohistochemistry results reveal positivity for CDla, Langerin (CD207), and S-100[16]. S-100 positivity is also present in thyroid sarcoma, but the diagnosis of LCH mainly depends on Birbeck particles and Langerin (CD207)[17].
Due to limited reports on thyroid involvement in LCH, the recommended treatment for LCH remains inconsistent. Surgery is the first choice for the treatment of primary thyroid LCH[8,11]. However, the surgical method is also controversial. Related reports have utilized semithyroidectomy, subtotal thyroidectomy, or total thyroidectomy. There is no clear evidence that postoperative adjuvant radiotherapy and chemotherapy can improve the disease control rate. In MS-LCH patients with thyroid involvement, chemotherapy is the main treatment, and steroids and vincristine are the first-line drugs[11,15]. In addition, systemic chemotherapy drugs, including indomethacin, methotrexate, and cyclophosphamide, can be considered[18]. Laird treated 46 MS-LCH lesions with radiotherapy, including two lesions in the thyroid gland; neither of the two thyroid-involved patients experienced recurrence during the median follow-up of 45 mo[19]. Some studies have found that the BRAFV600E mutation is common in LCH, and the detection rate reaches 50%-70%[20-22]. Therefore, the BRAF inhibitor verofenib has been administered to MS-LCH patients[23,24]. SS-LCH tends to have a good prognosis, with a survival rate of approximately 100% and a 5-year recurrence rate of < 20%[15,25]. The 10-year survival rate in adults with SS-LCH, meanwhile, can reach 86%[26]. The 5-year survival rate of MS-LCH patients is 98%, and that of patients with MS-LCH affecting high-risk organs (liver, spleen, and bone marrow) is ≤ 77%[27].
The main manifestation of LCH involving the thyroid is goitre or thyroid nodules. The clinical manifestation is not nonspecific and thus can easily lead to a misdiagnosis or missed diagnosis. Therefore, thyroid surgeons should be aware of LCH involving the thyroid in clinical work to avoid surgery and surgical complications caused by misdiagnosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cigrovski Berkovic M, Croatia; Shekouhi R, Iran S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Nezelof C, Basset F. An hypothesis Langerhans cell histiocytosis: the failure of the immune system to switch from an innate to an adaptive mode. Pediatr Blood Cancer. 2004;42:398-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | El-Safadi S, Dreyer T, Oehmke F, Muenstedt K. Management of adult primary vulvar Langerhans cell histiocytosis: review of the literature and a case history. Eur J Obstet Gynecol Reprod Biol. 2012;163:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Aricò M, Girschikofsky M, Généreau T, Klersy C, McClain K, Grois N, Emile JF, Lukina E, De Juli E, Danesino C. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39:2341-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 328] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Kim IK, Lee KY. Adult Langerhans cell histiocytosis of skull in a patient with synchronous papillary thyroid carcinoma and Castleman disease. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Zinn DJ, Chakraborty R, Allen CE. Langerhans Cell Histiocytosis: Emerging Insights and Clinical Implications. Oncology (Williston Park). 2016;30:122-132, 139. [PubMed] |
| 6. | Lieberman PH, Jones CR, Steinman RM, Erlandson RA, Smith J, Gee T, Huvos A, Garin-Chesa P, Filippa DA, Urmacher C, Gangi MD, Sperber M. Langerhans cell (eosinophilic) granulomatosis. A clinicopathologic study encompassing 50 years. Am J Surg Pathol. 1996;20:519-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 159] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Xia CX, Li R, Wang ZH, Lv FJ, Tang XQ, Li QF, Zhang SH. A rare cause of goiter: Langerhans cell histiocytosis of the thyroid. Endocr J. 2012;59:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Patten DK, Wani Z, Tolley N. Solitary langerhans histiocytosis of the thyroid gland: a case report and literature review. Head Neck Pathol. 2012;6:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Zhang J, Wang C, Lin C, Bai B, Ye M, Xiang D, Li Z. Spontaneous Thyroid Hemorrhage Caused by Langerhans Cell Histiocytosis: A Case Report and Literature Review. Front Endocrinol (Lausanne). 2021;12:610573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 10. | Cai HC, Liu T, Cai H, Duan MH, Li J, Zhou DB, Cao XX. Adult Langerhans cell histiocytosis with thyroid gland involvement: clinical presentation, genomic analysis, and outcome. Ann Hematol. 2022;101:1925-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Le Y, Wang YY, Peng QZ, Wang BS, Huang B, Zhou JH, Jia GJ, Zhou Y, Xue M. [Langerhans cell histiocytosis involving pituitary and thyroid gland: a case report]. Zhonghua Nei Ke Za Zhi. 2022;61:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Xie J, Li Z, Tang Y. Successful management of multiple-systemic Langerhans cell histiocytosis involving endocrine organs in an adult: A case report and review of literature. Medicine (Baltimore). 2018;97:e11215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Chen ED, Cheng P, Cai YF, Xiang YY, Zheng HM, Xing HX, Li Q. Ultrasonographic features of Langerhans cell histiocytosis of the thyroid. Int J Clin Exp Pathol. 2014;7:1229-1235. [PubMed] |
| 14. | Chen WZ, Lv YX, Xu DB, Chen WZ, Yu JC. Langerhans cell histiocytosis of the thyroid: a case report. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2017; 31: 397-399. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Gui ZQ, Wang ZH, Zhang H. Langerhans cell histiocytosis with papillary thyroid carcinoma: a case report. Zhonghua Fuchanke Zazhi. 2022;42:3. |
| 16. | Rodriguez-Galindo C, Allen CE. Langerhans cell histiocytosis. Blood. 2020;135:1319-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 17. | Seyed-Alagheband SA, Shahmoradi MK, Adeli OA, Shamsi T, Sohooli M, Shekouhi R. Follicular Dendritic Cell Sarcoma of the Thyroid Gland in a Patient with Preexisting Hashimoto's Thyroiditis: A Rare Case Report with a Literature Review. Case Rep Oncol. 2021;14:1698-1705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Minkov M. An update on the treatment of pediatric-onset Langerhans cell histiocytosis through pharmacotherapy. Expert Opin Pharmacother. 2018;19:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Laird J, Ma J, Chau K, Chelius M, Shi W, Zhang Z, Lok BH, Yahalom J. Outcome After Radiation Therapy for Langerhans Cell Histiocytosis Is Dependent on Site of Involvement. Int J Radiat Oncol Biol Phys. 2018;100:670-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Song CC, Li W, Tang GY. Research progress of Langerhans cell histiocytosis. Journal of Clinical Stomatology. 2019;35:4. |
| 21. | McGinnis LM, Nybakken G, Ma L, Arber DA. Frequency of MAP2K1, TP53, and U2AF1 Mutations in BRAF-mutated Langerhans Cell Histiocytosis: Further Characterizing the Genomic Landscape of LCH. Am J Surg Pathol. 2018;42:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Tang X, Guo X, Sun LY, Ai Y, Yang X, Sun JJ, Wu JR, Gao J. [BRAF-V600E mutation and its clinical significance in children with Langerhans cell histiocytosis]. Zhongguo Dang Dai Er Ke Za Zhi. 2018;20:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Gandolfi L, Adamo S, Pileri A, Broccoli A, Argnani L, Zinzani PL. Multisystemic and Multiresistant Langerhans Cell Histiocytosis: A Case Treated With BRAF Inhibitor. J Natl Compr Canc Netw. 2015;13:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Mass E, Jacome-Galarza CE, Blank T, Lazarov T, Durham BH, Ozkaya N, Pastore A, Schwabenland M, Chung YR, Rosenblum MK, Prinz M, Abdel-Wahab O, Geissmann F. A somatic mutation in erythro-myeloid progenitors causes neurodegenerative disease. Nature. 2017;549:389-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 25. | Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: History, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 26. | Tazi A, Lorillon G, Haroche J, Neel A, Dominique S, Aouba A, Bouaziz JD, de Margerie-Melon C, Bugnet E, Cottin V, Comont T, Lavigne C, Kahn JE, Donadieu J, Chevret S. Vinblastine chemotherapy in adult patients with langerhans cell histiocytosis: a multicenter retrospective study. Orphanet J Rare Dis. 2017;12:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |