Published online Dec 26, 2023. doi: 10.12998/wjcc.v11.i36.8434
Peer-review started: November 9, 2023
First decision: November 22, 2023
Revised: December 1, 2023
Accepted: December 6, 2023
Article in press: December 6, 2023
Published online: December 26, 2023
Processing time: 42 Days and 21.6 Hours
Liver cancer is the fifth most common tumor and the second highest death-related cancer in the world. Hepatocarcinoma (HCC) represents 90% of liver cancers. According to the Barcelona Clinic Liver Cancer group, different treatment options could be offered to patients in consideration of tumor burden, liver function, pa
Core Tip: Multifocal hepatocarcinoma (HCC) can benefit from local treatments with curative or down-staging purposes. Trans-arterial chemoembolization (TACE) is the treatment of choice in patients with a diagnosis of HCC not eligible for surgery (resection or liver transplantation) and ablation. TACE is known to be a safe procedure but can lead to both self-limiting mild adverse events and, albeit rarely, severe hepatic and biliary damage due to extensive hepatic ischemia.
- Citation: Cocca S, Carloni L, Marocchi M, Grande G, Bianchini M, Colecchia A, Conigliaro R, Bertani H. Post-trans-arterial chemoembolization hepatic necrosis and biliary stenosis: Clinical charateristics and endoscopic approach. World J Clin Cases 2023; 11(36): 8434-8439
- URL: https://www.wjgnet.com/2307-8960/full/v11/i36/8434.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i36.8434
Hepatocarcinoma (HCC) is the fifth most common and the second highest death-related cancer. It represents almost the totality of liver cancers and its incidence is increasing worldwide, mostly in Eastern countries and Africa[1]. Chronic liver disease, whatever the etiology, represents the major risk factor for HCC occurrence. Ultrasound (US), abdomen magnetic resonance imaging, and serum biochemical marker evaluation represent the validated diagnostic tools for early HCC detection in patients with advanced chronic liver disease[1]. According to tumor burden, liver function, and patient features (performance status, biomarker concentration, and prognostic score value such as Albumin-Bilirubin [ALBI]) Grade and Child-Pugh/MELD scores, several treatment options could be proposed to the patient[2]. Based to the latest Barcelona Clinic Liver Cancer update, liver transplantation (LT) is the treatment of choice when Milan Criteria are met or are achieved after disease down-staging[2,3]. In the case of focal HCC, surgery or local treatments [i.e. radiofrequency ablation, RFA, or microwave ablation (MWA)] could be proposed in consideration of patient surgical fitness. Whereas, in the case of multiple/diffuse HCC, local approaches [i.e. trans-arterial chemoembolization (TACE)] or immune/chemo
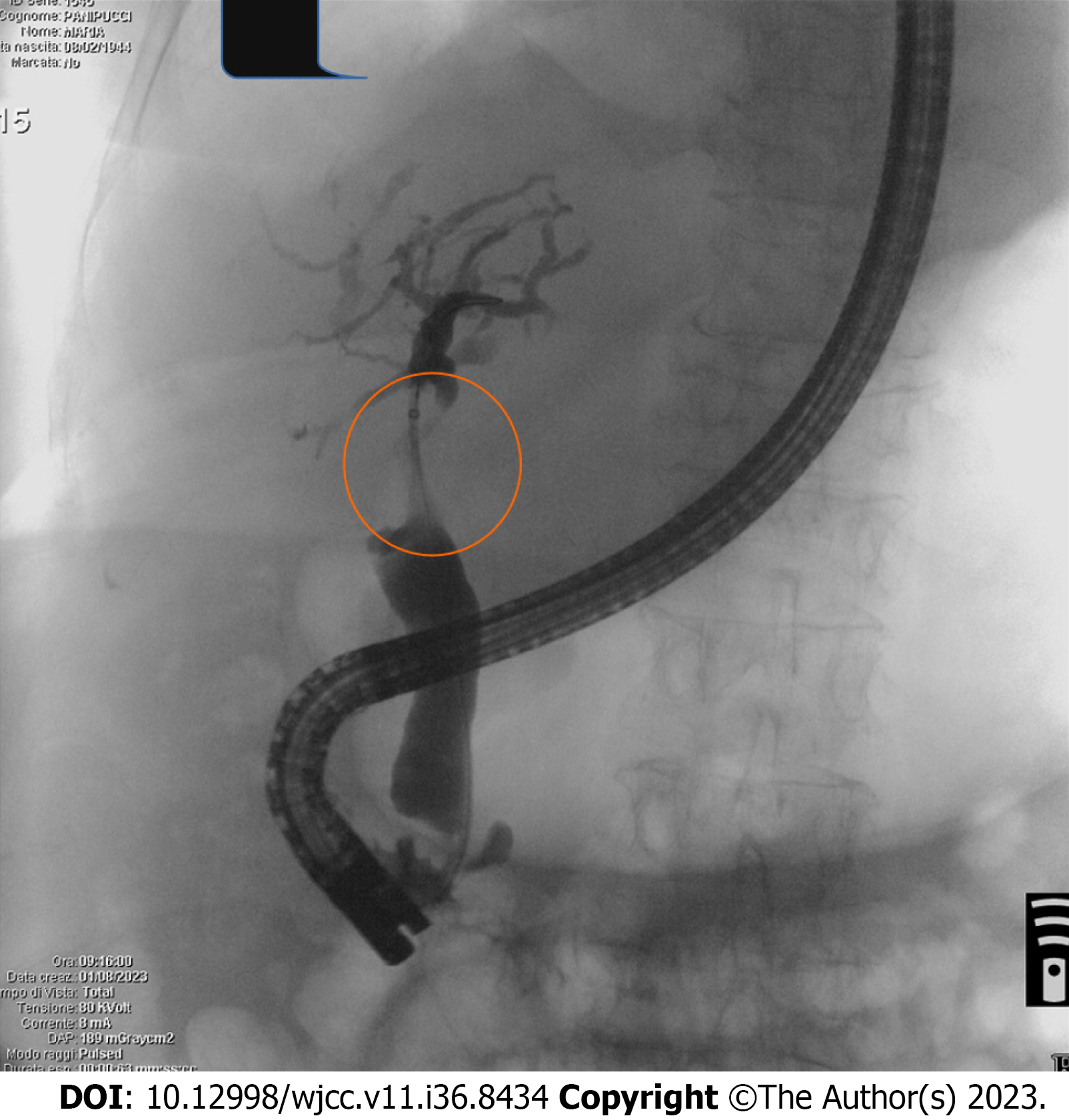
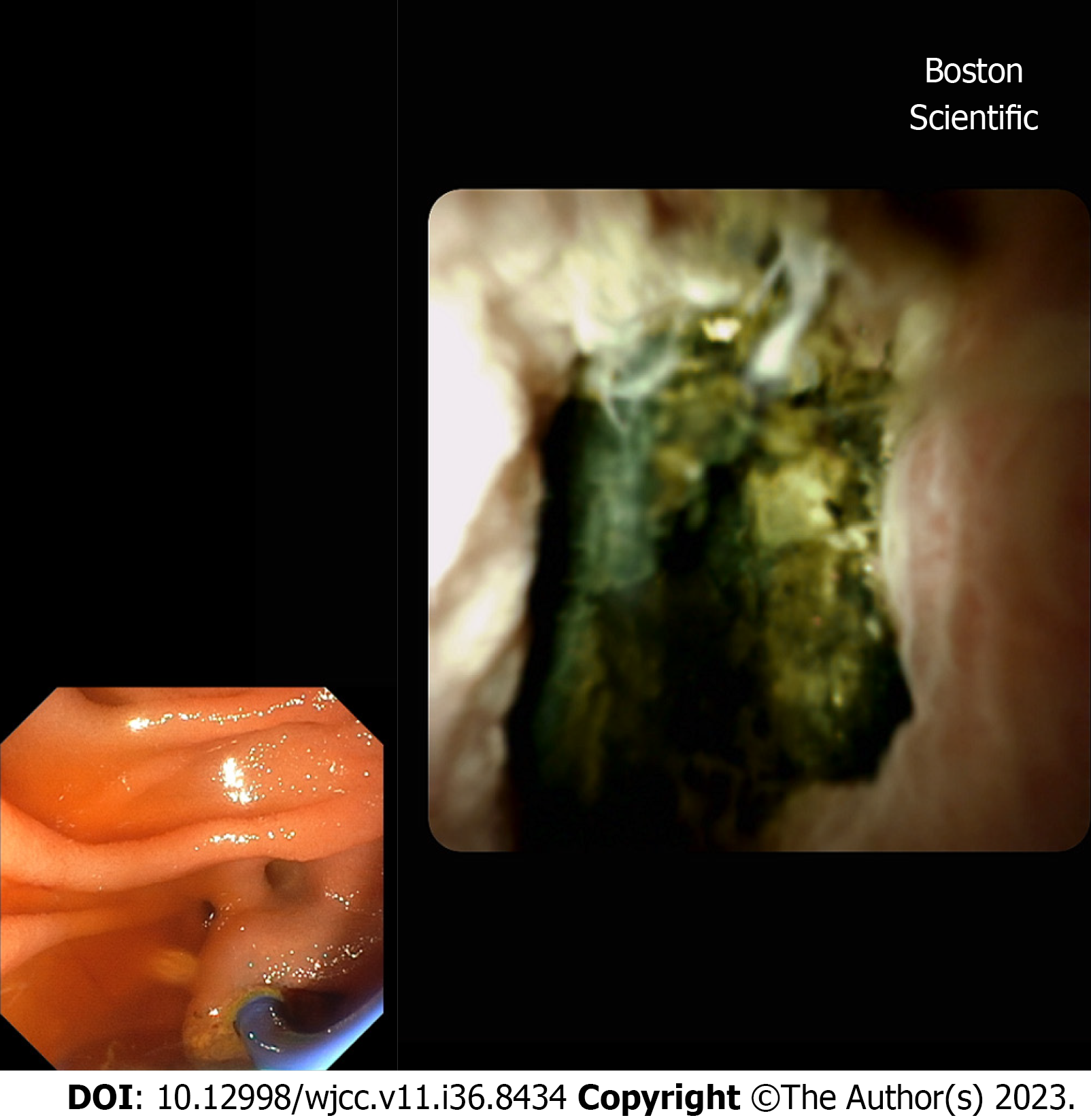
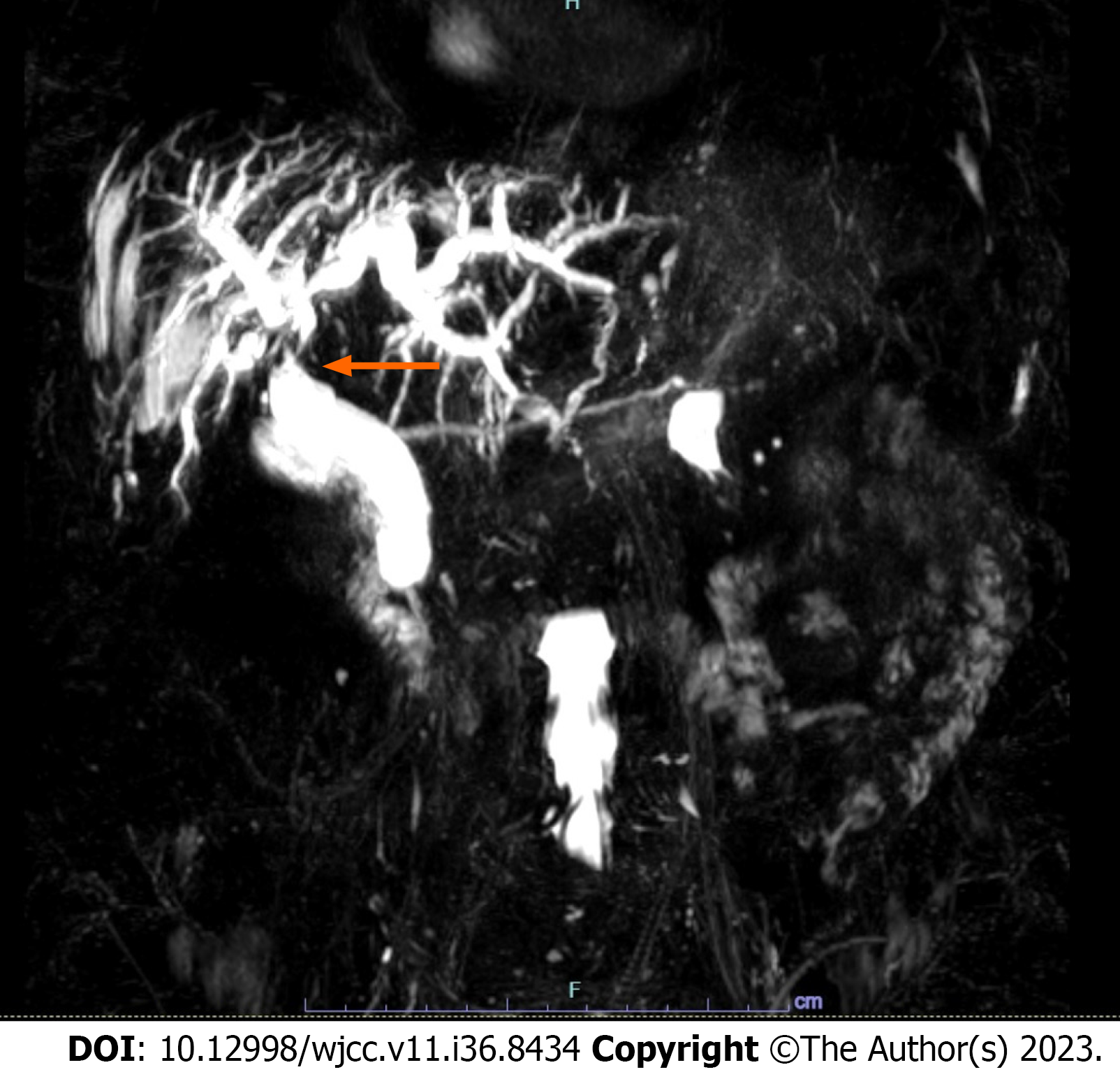
We report a case of a 79-year-old woman with post-TACE hepatic hilar necrosis which resulted in serrated biliary stenosis. The patient was affected by hepatitis C virus related liver cirrhosis (Child-Pugh B7) complicated by hepatic encephalopathy, portal thrombosis, and multifocal HCC. Sustained virological response was achieved in 2016. In January 2023, for unresectable HCC recurrence in the caudate lobe, the patient underwent TACE. One month later, she was admitted to our hospital for persistent fever, abdominal pain, and jaundice. Abdominal imaging (US and CT) showed a cirrhotic liver with intrahepatic biliary dilation with solid tissue at the level of hilar bifurcation with substenosis. An endoscopic retrograde cholangiopancreatography (ERCP) and choledoscopy with Spyglass II was planned to study and characterize the biliary substenosis suitable for recurrence or necrosis and to treat the hilar stenosis. Biliary stenosis were seen during ERCP (Figure 1) and choledoscopy with Spyglass II revealed the presence of firm tissue strongly adherent to the biliary tree in the hepatic hilum (Figure 2). The histology report showed peri-bile duct tissue with necrotic changes, including coagulation necrosis and fibrinoid necrosis of the bile duct. After mechanical (Soehendra Biliary Dilation Catheter 4-6 Fr) and pneumatic dilatation (6 mm) of the intrahepatic ducts, two plastic stents (7 Fr × 15 cm) were placed in the left and right intrahepatic ducts to achieve proper biliary drainage. Moreover progressive pneumatic dilations (up to 8 mm) associated to multiple stenting replacement (up to 8.5 Fr × 12 cm) were subsequently performed to avoid jaundice recurrence and in the attempt to improve hilar stenosis. During follow-up the endoscopic treatment proved to be unsuccessful, given the persistence of biliary strictures together with the intrahepatic biliary dilation (Figure 3), suggesting permanent ischemic biliary damage. However, in light of the already compromised liver function and given the extent of the biliary damage, the patient was not eligible for other therapeutic options. The subsequent clinical, laboratory and radiological evaluations showed a stationary condition, and the patient showed no signs of obstructive jaundice at follow up.
TACE is considered to be safe as well as effective for HCC, but, as already said, nonvascular and vascular AE have been reported[5]. Among the non-vascular complications, post-TACE syndrome presents in almost the totality of cases. It is characterized by fever, hypertransaminasemia, and right upper abdominal pain, but it is a clinical self-limiting condition lasting < 48 h. Beside post-TACE syndrome, other nonvascular complications such as biloma, hepatic and renal failure, sepsis, pancreatitis, and toxicities induced by chemo-agents can occur. Vascular AE include post-TACE hepatic necrosis and perforation. In particular, a rare occurrence of hepatic necrosis in a patient with a metastatic neuroendocrine tumor (NET) was reported by Micallef et al[4]. Kim et al[7] reported post-TACE duodenal perforation and esophageal ischemia in a man with unresectable HCC. Vascular thrombosis was detected on Doppler ultrasound and computed tomography scan (CT) in both reports, likely due to chemo-agents/arterial embolization induced ischemia, which probably plays a pivotal role in post-TACE necrosis and can lead to subsequent irreversible biliary stenosis.
Hepatic arteries are the only blood vessels that supply the bile ducts. When the peribiliary vascular plexus or the tiny hepatic arteries are damaged, or when all arterial blood supplies are cut off, as in the case of a transplanted liver with hepatic artery thrombosis, an ischemic bile duct injury may result. Most causes of bile duct ischemia are iatrogenic: benign biliary stricture (BBS) represents the most frequently observed post-LT biliary complication[3]. However, liver necrosis and subsequent BS is a very rare complication after TACE, as the liver parenchyma receives a dual blood supply via the hepatic arteries and the hepatic portal veins[2]. It has only been reported in a few cases[8,9], therefore its incidence has not been clearly estimated. In the anecdotal cases found in the literature the most common outcome was death.
TACE should be suggested in patients with unresectable HCC[2] not eligible for LT and it is considered a safe procedure. However, in the described case, we have shown how TACE could be linked to severe vascular complications such as hepatic necrosis resulting in biliary tree stenosis. Although this AE is rare and unexpected, it may result in severe sequelae (i.e. jaundice, sepsis). That is why it should be considered if fever and abdominal pain are not self-limiting within 48 h and/or continue after analgesics administration[4,5]. Post-TACE biliary strictures can be diagnosed when signs and symptoms of biliary obstruction (abnormal liver function tests, jaundice, abdominal pain, and cholangitis) occur and in case of biliary dilatation on imaging. Localization and confirmation of the BS ultimately depends on cholangiography, either contrast-enhanced or magnetic resonance cholangiopancreatography (MRCP)[10].
Endoscopy has become the preferred option for treating BBS, of which 85% are located in the common bile duct[11]. Unacceptably high adverse event rates and poor long-term results have led to the discontinuation of BBSs therapy with a single plastic stent or uncovered SEMS[11,12]. Therefore, the use of a single plastic stent as a first line of treatment should only be considered in very specific cases before engaging definite strategies.
Endoscopic treatment consists in multiple plastic stent (MPS) placement, balloon dilation, or a combination of these two techniques. The guidelines of the European Society of Gastrointestinal Endoscopy (ESGE) suggest inserting as many stents as feasible every 3 months for a total period of 12 months[11]. Plastic stents remain the gold standard for management of benign biliary strictures despite recent data demonstrating good and equal effectiveness of fully covered metal stents (FC-SEMS) in selected clinical scenarios[13,14]. With their larger diameters (10 mm vs 3.3 mm, respectively) and technical advantages—they are simpler to insert and take less time to place than MPS—they may be able to overcome some of the drawbacks of plastic stents[15]. However, using FC-SEMS presents a high risk of stent migration (around 9%), which varies significantly depending on the cause of the stenosis[14].
Technically speaking, hepatic and hilary strictures represent greater challenges for endoscopists. Percutaneous transhepatic cholangiography is one of the extra treatments that may be needed for the management of these strictures[10,11]. These kind of strictures are more common in individuals with primary sclerosing cholangitis (PSC). Short-term stents were not better than balloon dilatation in the first and only randomized trial of individuals with PSC with a prominent intrahepatic or hilar stricture, and they might even be harmful. Consequently, a very high rate of treatment-related adverse events, such as cholangitis, post-ERCP pancreatitis, and cholecystitis, necessitated the study's termination. Therefore, due to its ability to lower cholestasis, balloon dilatation ought to be the first treatment option for benign intrahepatic and hilar strictures[15,16].
For patients with intermediate-stage HCC with maintained liver function, TACE is currently the recom
BDN is a stage that precedes overt biloma. When treated promptly, most intrahepatic bilomas have an excellent outcome. Patients who develop bilomas following TACE have a mortality incidence of between 5% and 10%[17]. Therefore, early detection of BDN and biloma on imaging and extra careful management might be mandatory to improve patient prognosis after repeated TACE for liver tumors.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Long X, China S-Editor: Liu JH L-Editor: A P-Editor: Xu ZH
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6019] [Article Influence: 859.9] [Reference Citation Analysis (3)] |
| 2. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2552] [Article Influence: 850.7] [Reference Citation Analysis (59)] |
| 3. | Mehta N, Bhangui P, Yao FY, Mazzaferro V, Toso C, Akamatsu N, Durand F, Ijzermans J, Polak W, Zheng S, Roberts JP, Sapisochin G, Hibi T, Kwan NM, Ghobrial M, Soin A. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 4. | Micallef S, Cortis K, Magri C. Hepatic Necrosis after Trans-Arterial Embolization of Metastatic Neuroendocrine Tumour. Eur J Case Rep Intern Med. 2020;7:001530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Sun W, Xu F, Li X, Li CR. A Case Series of Liver Abscess Formation after Transcatheter Arterial Chemoembolization for Hepatic Tumors. Chin Med J (Engl). 2017;130:1314-1319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Lo YC, Hsu PW, Chew FY, Chen HY. Special presentation of bronchobiliary fistula after transcatheter arterial chemoembolization: A case report. Medicine (Baltimore). 2022;101:e31596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Kim SI, Jin YJ, Cho SG, Shin WY, Kim JM, Lee JW. Duodenal perforation and esophageal ischemia following transarterial chemoembolization for hepatocellular carcinoma: A case report. Medicine (Baltimore). 2016;95:e3987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Abdelrahman H, Ajaj A, Atique S, El-Menyar A, Al-Thani H. Conservative management of major liver necrosis after angioembolization in a patient with blunt trauma. Case Rep Surg. 2013;2013:954050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Loewe C, Schindl M, Cejna M, Niederle B, Lammer J, Thurnher S. Permanent transarterial embolization of neuroendocrine metastases of the liver using cyanoacrylate and lipiodol: assessment of mid- and long-term results. AJR Am J Roentgenol. 2003;180:1379-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Chan CH, Telford JJ. Endoscopic management of benign biliary strictures. Gastrointest Endosc Clin N Am. 2012;22:511-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 476] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 12. | Kaffes A. Benign biliary strictures: how we are evolving to the perfect endoscopic strategy. Endoscopy. 2019;51:1115-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Bill JG, Mullady DK. Stenting for Benign and Malignant Biliary Strictures. Gastrointest Endosc Clin N Am. 2019;29:215-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Coté GA, Slivka A, Tarnasky P, Mullady DK, Elmunzer BJ, Elta G, Fogel E, Lehman G, McHenry L, Romagnuolo J, Menon S, Siddiqui UD, Watkins J, Lynch S, Denski C, Xu H, Sherman S. Effect of Covered Metallic Stents Compared With Plastic Stents on Benign Biliary Stricture Resolution: A Randomized Clinical Trial. JAMA. 2016;315:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 15. | Wong MY, Saxena P, Kaffes AJ. Benign Biliary Strictures: A Systematic Review on Endoscopic Treatment Options. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Ponsioen CY, Arnelo U, Bergquist A, Rauws EA, Paulsen V, Cantú P, Parzanese I, De Vries EM, van Munster KN, Said K, Chazouillères O, Desaint B, Kemgang A, Färkkilä M, Van der Merwe S, Van Steenbergen W, Marschall HU, Stotzer PO, Thorburn D, Pereira SP, Aabakken L. No Superiority of Stents vs Balloon Dilatation for Dominant Strictures in Patients With Primary Sclerosing Cholangitis. Gastroenterology. 2018;155:752-759.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Kobayashi S, Kozaka K, Gabata T, Matsui O, Koda W, Okuda M, Okumura K, Sugiura T, Ogi T. Pathophysiology and Imaging Findings of Bile Duct Necrosis: A Rare but Serious Complication of Transarterial Therapy for Liver Tumors. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |