Published online Dec 16, 2023. doi: 10.12998/wjcc.v11.i35.8385
Peer-review started: September 5, 2023
First decision: November 13, 2023
Revised: November 25, 2023
Accepted: December 1, 2023
Article in press: December 1, 2023
Published online: December 16, 2023
Processing time: 100 Days and 0.7 Hours
Low-grade myofibroblastic sarcoma (LGMS) is a rare spindle cell sarcoma espe
Herein, we report a case involving the discovery of a pancreatic mass detected during a routine physical examination. Subsequent imaging and pathological tests of the patient led to the diagnosis of LGMS of the pancreas. Following surgical intervention, the patient experienced recurrence and metastasis. Conventional treatment is not effective for postoperative recurrent pancreatic LGMS with multiple metastases. After communicating with the patients and their families, informed consent was obtained for the treatment of anlotinib combined with pembrolizumab. Evaluation of imaging and clinical symptoms post-treatment revealed a relatively favorable response to the combination of anlotinib and pembrolizumab.
Based on the comprehensive literature review, our report aimed to provide evidence for a better understanding of the disease characteristics, diagnostic criteria, imaging findings, and identification of LGMS. And explore novel treatment strategies for this disease.
Core Tip: Low-grade myofibroblastic sarcoma (LGMS) is a rare spindle cell sarcoma with myofibroblastic differentiation. It is predominantly located in the head, neck, and extremities, whereas less frequently in the abdominal cavity, especially the pancreatic region. LGMS is characterized by a low-grade malignancy and low risk of recurrence and metastases. To date, complete surgical resection remains the primary treatment modality. Despite chemoradiotherapy showing anti-tumor activity for the treatment of postoperative recurrence and metastasis, it remains controversial. Until recently, no effective treatment for LGMS patients intolerant to chemoradiotherapy has been well established. This article aims to explore the new idea of LGMS based on literature obtained from PubMed and a clinical case.
- Citation: Wu RT, Zhang JC, Fang CN, Qi XY, Qiao JF, Li P, Su L. Anlotinib in combination with pembrolizumab for low-grade myofibroblastic sarcoma of the pancreas: A case report. World J Clin Cases 2023; 11(35): 8385-8391
- URL: https://www.wjgnet.com/2307-8960/full/v11/i35/8385.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i35.8385
Low-grade myofibroblast sarcoma (LGMS) is an extremely rare type of malignant tumor that originates from mesenchymal spindle cell tumors, showing myofibroblastic differentiation with fibromatoses-like features. LGMS occurs predominantly in the head, neck, and extremities. It was first published in 1998 by Mentzel et al[1] and was classified as a new group of soft tissue and bone tumors by the World Health Organization in 2002[2]. Clinically, LGMS is often poorly differentiated from fibrosarcoma, leiomyosarcoma, and inflammatory myofibroblastic sarcoma because of its low-grade malignancy, uncommonness, and non-specific clinical manifestations, making the diagnosis of LGMS particularly difficult. Complete resection of the primary lesion and local recurrent lesion is considered the main treatment for LGMS according to the previous studies. Nevertheless, to the best of our knowledge, there is currently still a lack of standard-of-care for LGMS patients with postoperative recurrence and metastasis. Here, we report a case of postoperative recurrent pancreatic LGMS with multiple metastases, which was treated with anlotinib combined with pembrolizumab. Based on the systematic review, we further explore new ideas for the treatment of recurrent and metastatic LGMS for patients who cannot tolerate chemoradiotherapy.
A 74-year-old Chinese man presented to the Oncology Department of Integrated Traditional Chinese and Western Medicine of our hospital with abdominal distension and poor appetite for more than 1 mo.
One month earlier, the patient developed abdominal distension and poor appetite.
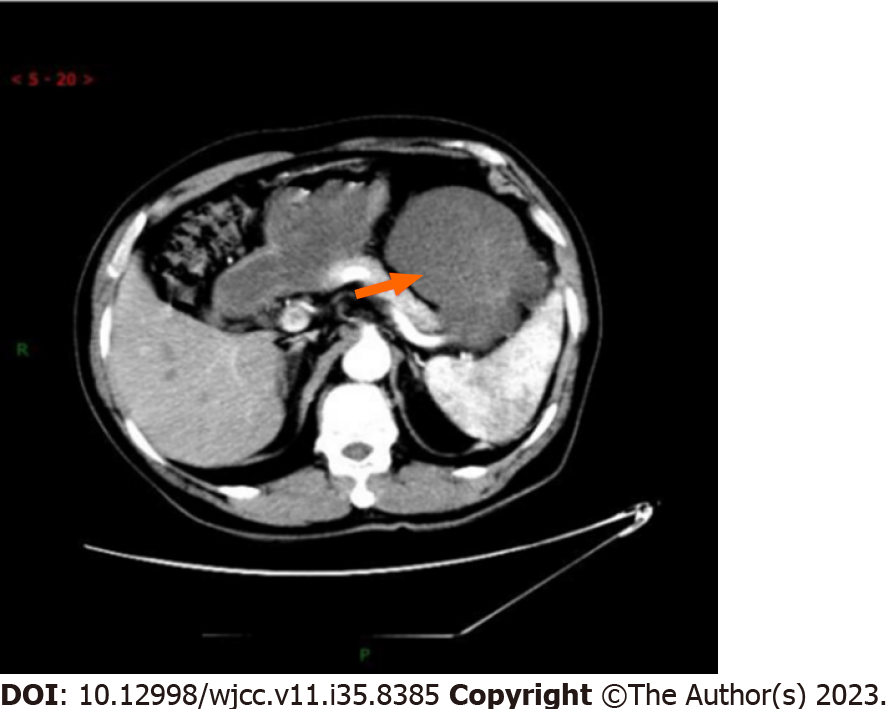
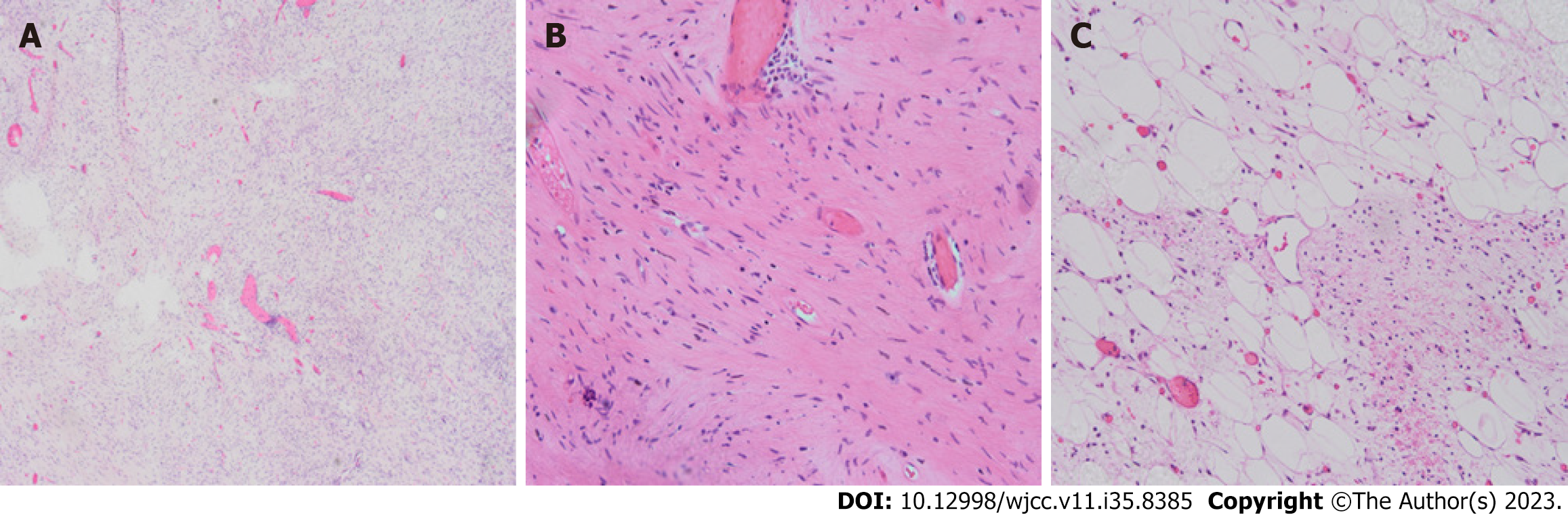
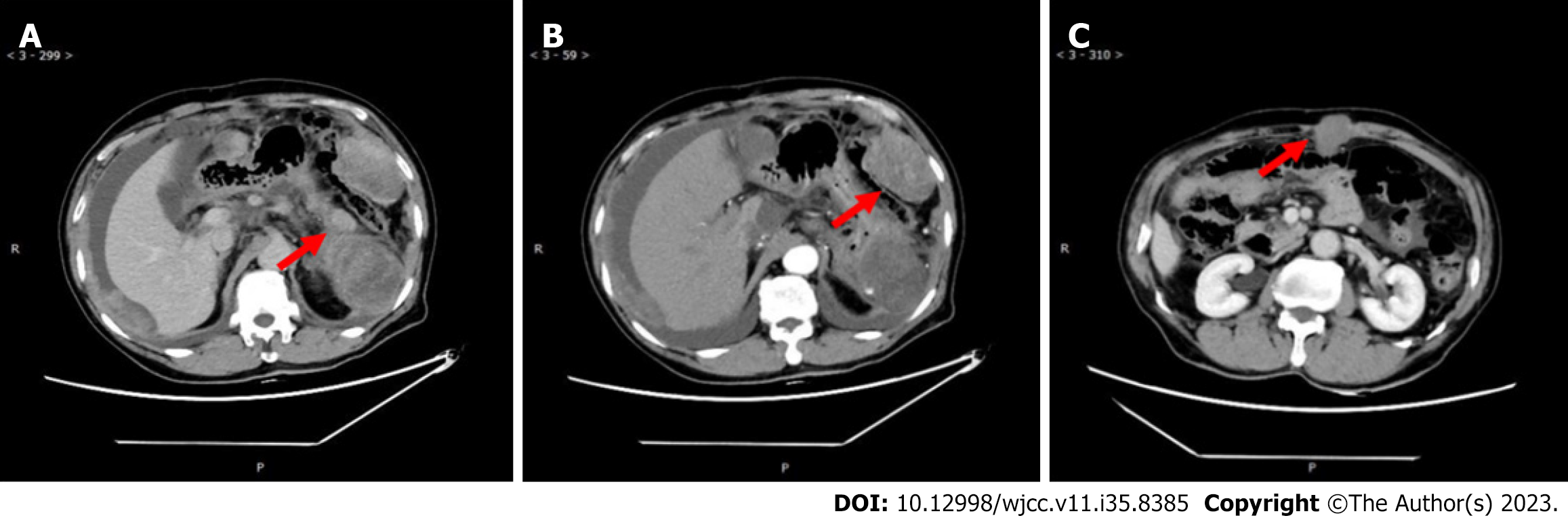
Eleven months ago, the patient was referred to our hospital regarding a low-density space-occupying lesion in the left epigastrium, discovered during a physical examination at another hospital. Physical examination revealed no abnormal findings in the serum tumor marker. Abdominal enhanced computerized tomography (CT) scan revealed a 7.2 cm × 9.7 cm cystic density shadow with clear margins in the left upper abdominal cavity (Figure 1). Simultaneously, the solid component of the lesion demonstrated marked enhancement on enhanced CT images, and a nodular slightly high-density shadow appeared to be seen. The imaging findings confirmed an abdominal tumor before the operation. On January 11, 2021, the patient underwent a procedure involving the distal pancreatectomy and splenectomy in addition to the tension-free repair of the right inguinal mixed hernia. An exploratory laparotomy revealed a tumor (approximately 8 cm × 6 cm) situated at the tail of the pancreas, with a firm texture and local cystic changes. The bottom of the tumor was connected to the pancreatic body through a 2 cm diameter pedicle, showing an infiltration at the pancreatic body and the mesentery of the inferior margin of the pancreas, but not at the fundus of the stomach and the spleen. Firstly, tumor excision from the tumor pedicle followed by rapid intraoperative pathological revealed a myxoma whose malignancy cannot be ruled out definitively. Therefore, a distal pancreatectomy combined with splenectomy with negative margins was performed. After surgery, a solid mass of pancreatic tissue measuring about 5.0 cm × 5.0 cm × 4.8 cm was obtained and used as the surgical specimen. The microscopy showed the obvious proliferation of spindle-shaped and short spindle-shaped cells, which were arranged in interwoven and fascicles with moderate cell density (Figure 2). Immunohistochemistry (IHC) indicated that tumor cells were Vimentin, spinal muscular atrophy (SMA), cluster of differentiation 99 (CD99), b cell lymphoma-2 (BCL-2), β-catenin fraction positive, but were negative for creatine kinase (CK), Epithelial membrane antigen (EMA), S-100, neurofilament protein (NF), Desmin, P53, Calponin, and Signal transducer and activator of transcription 6 (STAT6). The Ki-67 index was approximately 65%. Based on these pathological findings, a diagnosis of pancreatic LGMS was made. A month ago, the abdominal enhanced CT showed changes in the tail of the pancreas, with the characteristics of low-density lesions in the surgical field, and multiple metastases in the abdominal cavity, peritoneum, and abdominal wall (Figure 3). This suggests that the patient developed recurrence and metastasis postoperatively.
The patient denied any family history of malignant tumors.
On physical examination, the vital signs were as follows: Clear mind, spiritual well-being, pupils bilaterally large and round, sensitive to light reflection, and no signs of jaundice on the skin, mucosa, or scleral. There was no enlargement of superficial lymph nodes, and the neck was supple with a centered trachea. Jugular veins were not distended. Chest examination revealed no deformities with decreased breath sounds in both lower lungs and no obvious dry or wet rales. The heart rate was 65 beats/min, with a regular rhythm and no pathological murmurs of the valves. Abdominal examination indicated a soft abdomen with a visible 15 cm surgical scar in the left upper abdomen. Palpation revealed a 4 cm × 4 cm mass in the left upper abdomen with poor mobility, tenderness (+), and no rebound pain. The liver and spleen were not palpable beneath the subcostal area. Shifting dullness was observed (+).
Levels of serum tumour markers were normal (alpha-fetoprotein 1.6 ng/mL, carcinoembryonic antigen 1.7 ng/mL, carbohydrate antigen 19-9 16.02 U/mL, carbohydrate antigen 12-5 20.7 U/mL.)
Postoperative pathology: the microscopy showed the obvious proliferation of spindle-shaped and short spindle-shaped cells, which were arranged in interwoven fascicles with moderate cell density IHC indicated that tumor cells were Vimentin, SMA, CD99, BCL-2, β-catenin fraction positive, but were negative for CK, EMA, S-100, NF, Desmin, P53, Calponin, and STAT6. The Ki-67 index was approximately 65%. Based on these pathological findings, a diagnosis of pancreatic LGMS was made.
2020.12.29 the abdominal enhanced CT scan revealed a 7.2 cm × 9.7 cm cystic density shadow with clear margins in the left upper abdominal cavity (Figure 1). Simultaneously, the solid component of the lesion demonstrated marked enhancement on enhanced CT images, and a nodular slightly high-density shadow appeared to be seen. 2021.10.27 the abdominal enhanced CT showed changes in the tail of the pancreas, with the characteristics of low-density lesions in the surgical field, and multiple metastases in the abdominal cavity, peritoneum, and abdominal wall (Figure 3).
LGMS (postoperative recurrence and metastasis).
The patient did not receive any postoperative systemic therapy. After a period of 9 mo, the abdominal enhanced CT showed changes in the tail of the pancreas, with the characteristics of low-density lesions in the surgical field, and multiple metastases in the abdominal cavity, peritoneum, and abdominal wall (Figure 3). A puncture biopsy of the abdominal wall mass showed a consistent result with the pathological characteristics of pancreatic LGMS. Subsequently, the patient was hospitalized, and RNA sequencing analysis showed no relevant variants in tumor fusion genes using the paraffin-embedded pancreas tissue. Due to the patient's advanced age, poor physical condition, and intolerance to chemotherapy, he was treated with anlotinib (8 mg orally once/day for 2 wk, stopped for 1 wk) plus pembrolizumab (100 mg by intravenous drip) every 3 wk (21-d cycle). Simultaneously, blood pressure was monitored and grade II nursing care was administered.
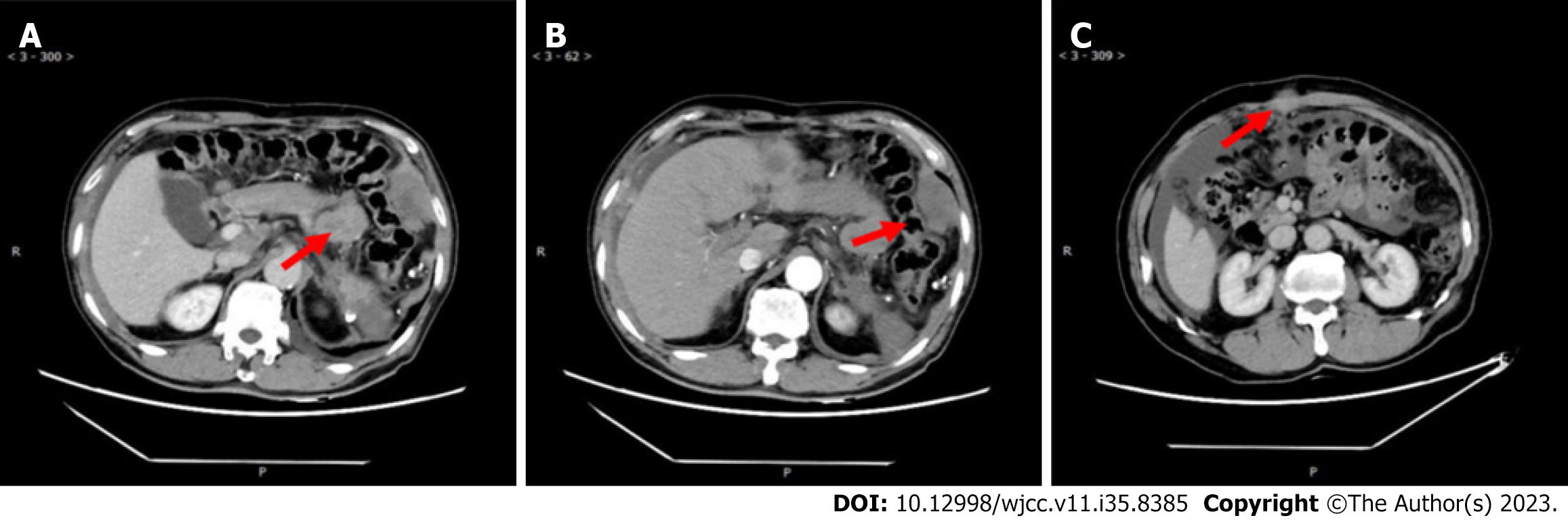
After 7 cycles of treatment, the re-examination of the abdominal enhanced CT clearly showed an obvious regression of the peritoneal and abdominal wall lesions, but an increase in abdominal cavity lesions (Figure 4). Later, the patient's condition progressed again, and he was discontinued in an external hospital, and only received local treatment such as transarterial vascular embolization and radioactive particle therapy for abdominal lesions. After treatment, blood pressure increased, and nifedipine 30 mg qd control was given, but the overall effect was not good, and the patient died of multiple organ failure on August 15, 2022.
LGMS is a clinically rare type of spindle cell sarcoma that is derived from myofibroblasts and has a propensity to develop in the bones or soft tissues of the head and neck, trunk, or extremities[3]. This type of tumor has been reported to occur in many sites, while it is extremely rarely observed in the pancreas of the abdominal cavity. Besides, LGMS is designated as a low-grade malignancy with a typical clinical manifestation of slow enlargement of the mass, and patients may also have clinical symptoms, such as fever, chills, and leucocytosis[4]. Actually, the diagnosis of LGMS is difficult due to the lack of specific clinical manifestations, but imaging examinations may provide valuable information. Several imaging findings of LGMS (e.g., invasiveness, metastasis, and calcification) have been previously revealed by Wang L and other studies[5]. Histopathologically, LGMS is composed of spindle-shaped tumor cells, which are arranged in sheets or strips with diffuse infiltrative growth pattern. In addition, the nuclei of tumor cells are typically narrow and elongated, with neutrophilic or eosinophilic cytoplasm. The fusiform nucleus contains uniformly distributed chromatin and a small nucleolus, with few mitotic figures and slight nuclear pleomorphism. Meanwhile, the previous study has indicated that the interstitial tissue is mostly filled with collagen fibers, which are usually clear and non-necrotic[6]. IHC results indicated that LGMS was positive for Vimentin, α-SMA, and MSA while showing negative for ALK, laminin, S-100, CK, CD34, and EMA[7]. Therefore, it is recommended to make the diagnosis of LGMS based on the histopathologic examination, IHC, and imaging findings.
LGMS has characteristics of slow growth, long course of disease, and nonspecific symptoms, thus it is often difficult to distinguish from various malignant or benign lesions, such as fibrosarcoma, smooth muscle sarcoma, inflammatory myofibroblastic, nodular fasciitis, fibroblastoma[8]. As a result, pathological examination and IHC are the main methods for the identification of this disease.
There is currently still a lack of standard-of-care for LGMS due to its rarity. To date, complete resection of the primary lesion and local recurrent lesion remains the primary treatment modality. Consequently, choosing treatment is particularly important for patients with postoperative recurrence and multiple metastases. Up to now, the efficacy of radiation and chemotherapy in the treatment of LGMS remains unclear and is not recommended by any guidelines[9]. In several case reports, adjuvant chemotherapy has shown obvious clinical benefit, with the improvement in progression-free survival (PFS)[10]. Thereafter, adjuvant chemotherapy is recommended as a potential therapeutic strategy, especially when the tumor is difficult to be completely resected. Besides, several reports are showing a limited role for chemoradiotherapy for LGMS. As described by Xu et al[11], chemoradiotherapy demonstrated a limited improvement in survival rate in 96 patients with LGMS, thereby, chemoradiotherapy should not be recommended as a routine strategy for LGMS patients with negative margins, but might be suitable for patients with positive margins or recurrent metastatic disease. More interestingly, radiotherapy was recommended to be avoided after LGMS excision because it can lead to the recurrence of LGMS[12]. Therefore, further studies are needed to investigate whether radiation or chemotherapy are suitable for patients with recurrence and metastasis of LGMS.
We here reported a case of postoperative recurrent pancreatic LGMS with multiple metastases. Surgery and radiotherapy are not recommended for this patient due to poor physical conditions, intolerance to chemotherapy, and multiple metastases. Then, what are our options for the next step in treatment? With the advancement of targeted therapy and immunotherapy, some studies have already revealed that targeted combination immunotherapy has promising prospects in a variety of tumors[13]. A single-center, single-arm, phase II trial reported by Wilky et al[14] showed the manageable toxicity and preliminary activity of axitinib plus pembrolizumab in advanced sarcoma, but deserves further investigation in randomized controlled trials. Anlotinib is a multitarget tyrosine kinase inhibitor with significant antitumor activity against VEGFR signaling and significant inhibitory effects on FGFR 1-3, PDGFRA, and C-KIT. A single-arm study of anlotinib showed an encouraging activity, with a 12-wk PFS rate of 57.23% (as the primary endpoint), a median PFS of 5.63 mo, and an objective response rate of 11.45% in 154 evaluable soft tissue sarcoma patients. Anlotinib is effective against many pathological subtypes of soft tissue sarcomas, of which alveolar soft tissue sarcomas are the most prominent[15]. Due to its remarkable efficacy, anlotinib was recommended for the treatment of bone and soft tissue sarcomas by the Chinese Society of Clinical Oncology in 2019. Meanwhile, pembrolizumab, a humanized anti-PD-1 monoclonal antibody, has been approved by the United States Food and Drug Administration for the treatment of advanced melanoma and non-small cell lung cancer. Until recently, pembrolizumab has also demonstrated encouraging efficacy in many other malignancies. For instance, in a multicenter, single-arm trial, pembrolizumab showed a manageable safety and tolerability profile, as well as meaningful clinical activity[16]. Therefore, in our study, as a rare type of soft tissue sarcoma, the patient with pancreatic LGMS received anlotinib plus pembrolizumab with the consent of the patient and their families, and achieved expected clinical efficacy.
In general, histopathologic examination and IHC are the gold standards for the diagnosis of LGMS and the main basis for differentiating other lesions. Complete resection of local recurrent lesions and the primary lesion is an important treatment modality for LGMS. For patients with postoperative recurrence and metastases, chemoradiotherapy is a treatment option, but its efficacy still needs further investigation. Furthermore, targeted therapy combined with immunotherapy has achieved encouraging efficacy, such as improved efficacy and longer duration of response in various advanced tumors, which provides a new insight for recurrent metastatic LGMS patients who cannot tolerate chemoradiotherapy and is worthy of further evaluation. Although the patient in this study partially responded to targeted therapy combined with immunotherapy, there is still considerable room for improvement. Subsequent therapy for probable disease progression is the question to be addressed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zeng C, United States S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Mentzel T, Dry S, Katenkamp D, Fletcher CD. Low-grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol. 1998;22:1228-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 192] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 2. | Zambo I, Veselý K. [WHO classification of tumours of soft tissue and bone 2013: the main changes compared to the 3rd edition]. Cesk Patol. 2014;50:64-70. [PubMed] |
| 3. | Keller C, Gibbs CN, Kelly SM, Haller JR, White KS, Coffin CM, Lemons RS. Low-grade myofibrosarcoma of the head and neck: importance of surgical therapy. J Pediatr Hematol Oncol. 2004;26:119-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Niedzielska I, Janic T, Mrowiec B. Low-grade myofibroblastic sarcoma of the mandible: a case report. J Med Case Rep. 2009;3:8458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Wang L, Li LX, Chen DQ, Yang L, Li SK, Cheng C. Low-grade Myofibroblastic sarcoma: clinical and imaging findings. BMC Med Imaging. 2019;19:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Montgomery E, Goldblum JR, Fisher C. Myofibrosarcoma: a clinicopathologic study. Am J Surg Pathol. 2001;25:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Miyazawa M, Naritaka Y, Miyaki A, Asaka S, Isohata N, Yamaguchi K, Murayama M, Shimakawa T, Katsube T, Ogawa K, Fujibayashi M. A low-grade myofibroblastic sarcoma in the abdominal cavity. Anticancer Res. 2011;31:2989-2994. [PubMed] |
| 8. | Thompson LD, Wieneke JA, Miettinen M, Heffner DK. Spindle cell (sarcomatoid) carcinomas of the larynx: a clinicopathologic study of 187 cases. Am J Surg Pathol. 2002;26:153-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 192] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Chan JY, Gooi Z, Wong EW, Ng SK, Tong MC, Vlantis AC. Low-grade myofibroblastic sarcoma: A population-based study. Laryngoscope. 2017;127:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Katalinic D, Santek F. Giant low-grade primary myofibroblastic sarcoma of the posterior chest wall. World J Surg Oncol. 2017;15:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Xu Y, Xu G, Wang X, Mao M, Wu H, Baklaushev VP, Chekhonin VP, Peltzer K, Wang G, Zhang C. Is there a role for chemotherapy and radiation in the treatment of patients with low-grade myofibroblastic sarcoma? Clin Transl Oncol. 2021;23:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Maruyama T, Nakasone T, Nimura F, Matayoshi A, Kawano T, Nishihara K, Arasaki A. Indolent growth of low-grade myofibroblastic sarcoma of the cheek mimics benign lesions: A case report and literature review. Oncol Lett. 2017;13:4307-4314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D, Li Z, Pan CX. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol. 2021;14:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 385] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 14. | Wilky BA, Trucco MM, Subhawong TK, Florou V, Park W, Kwon D, Wieder ED, Kolonias D, Rosenberg AE, Kerr DA, Sfakianaki E, Foley M, Merchan JR, Komanduri KV, Trent JC. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: a single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019;20:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 274] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 15. | Chi Y, Sun Y, Cai J, Yao Y, Hong X, Fang Z, Sun P, Wang G, Wu Q, Qu G, Wang S, Song J, Yu J, Lu Y, Zhu X, Niu X, He Z. Phase II study of anlotinib for treatment of advanced soft tissues sarcomas. J Clin Oncol. 2016;34 (15_suppl):11005-11005. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, D'Angelo S, Attia S, Riedel RF, Priebat DA, Movva S, Davis LE, Okuno SH, Reed DR, Crowley J, Butterfield LH, Salazar R, Rodriguez-Canales J, Lazar AJ, Wistuba II, Baker LH, Maki RG, Reinke D, Patel S. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18:1493-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 1007] [Article Influence: 125.9] [Reference Citation Analysis (0)] |