Published online Dec 6, 2023. doi: 10.12998/wjcc.v11.i34.8126
Peer-review started: October 30, 2023
First decision: November 1, 2023
Revised: November 6, 2023
Accepted: November 24, 2023
Article in press: November 24, 2023
Published online: December 6, 2023
Processing time: 36 Days and 21.4 Hours
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide. Studies have shown a strong association between non-alcoholic steatohepatitis (NASH) cirrhosis and portal vein thrombosis. Spe
To examine the association between the degree of hepatic steatosis and fibrosis, quantified by transient elastography, and the incidence of VTE in patients with NASH.
In our case-control study, we included patients with a documented diagnosis of NASH. We excluded patients with inherited thrombophilia, hemoglobinopathy, malignancy, alcohol use disorder, autoimmune hepatitis, and primary biliary cirrhosis. The collected data included age, demographics, tobacco use, recreational drug use, medical history, and vibration controlled transient elastography scores. VTE-specific data included the location, type of anticoagulant, need for hospital stay, and history of VTE recurrence. Steatosis was categorized as S0-S1 (mild) and S2-S3 (moderate to severe) based on the controlled attenuation parameter score. Fibrosis was classified based on the kilopascal score and graded as F0-F1 (Metavir stage), F2, F3, and F4 (cirrhosis). χ2 and Mann-Whitney U tests were used for the qualitative and quantitative variable analyses, respectively. Furthermore, we performed a logistic regression using VTE as the dependent variable.
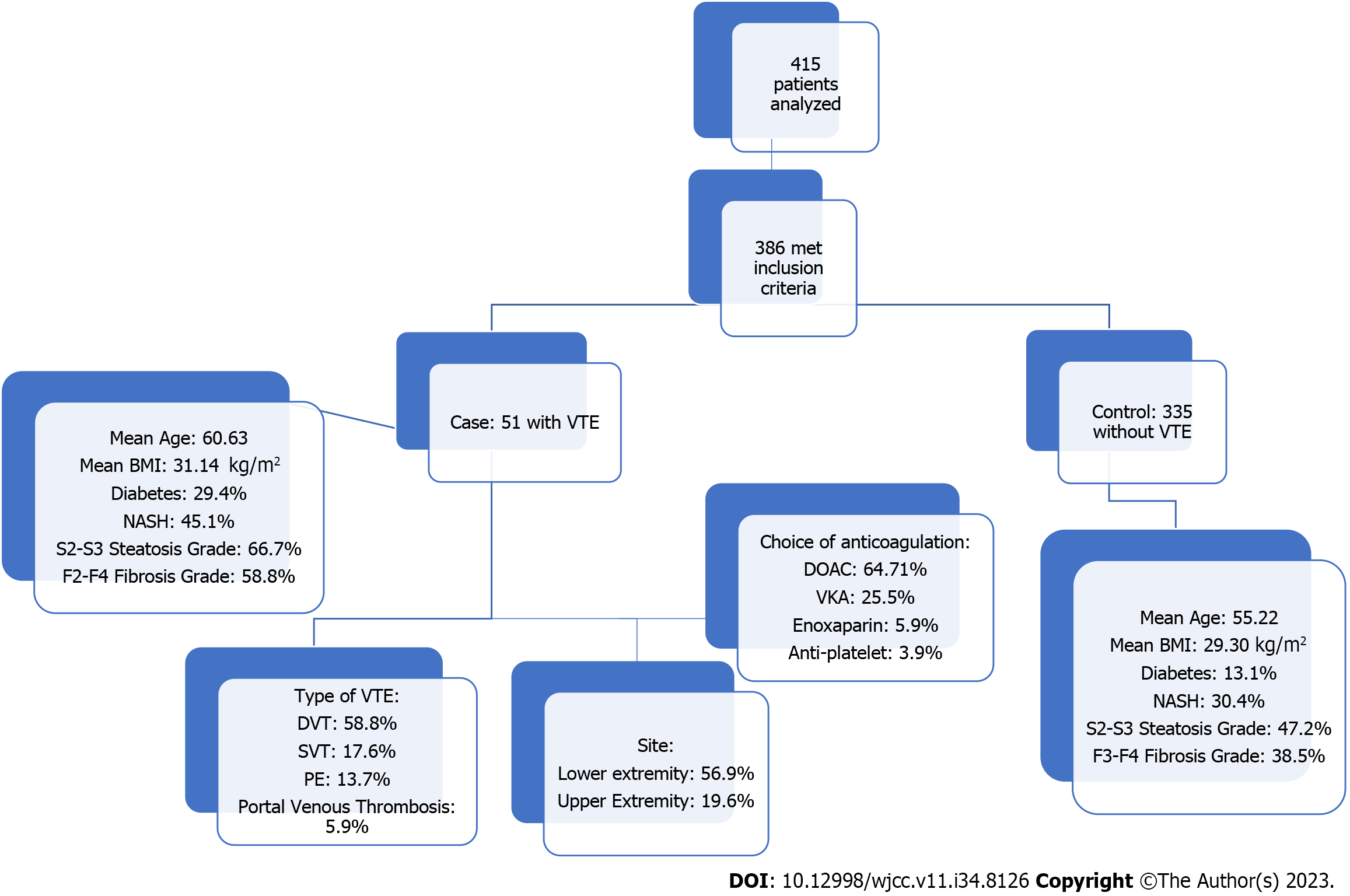
A total of 415 patients were analyzed, and 386 met the inclusion criteria. 51 and 335 patients were included in the VTE and non-VTE groups, respectively. Patients with VTE had a mean age of 60.63 years compared to 55.22 years in the non-VTE group (P < 0.014). Patients with VTE had a higher body mass index (31.14 kg/m² vs 29.30 kg/m²) and a higher prevalence of diabetes mellitus (29.4% vs 13.1%). The history of NASH was significantly higher in the VTE group (45.1% vs 30.4%, P < 0.037). Furthermore, moderate-to-severe steatosis was significantly higher in the VTE group (66.7% vs 47.2%, P < 0.009). Similarly, the F2-F4 fibrosis grade had a prevalence of 58.8% in the VTE group compared to 38.5% in the non-VTE group (P < 0.006). On logistic regression, using VTE as a dependent variable, diabetes mellitus had an odds ratio (OR) =1.702 (P < 0.015), and F2-F4 fibrosis grade had an OR = 1.5 (P < 0.033).
Our analysis shows that NASH is an independent risk factor for VTE, especially deep vein thrombosis. There was a statistically significant association between the incidence of VTE, moderate-to-severe steatosis, and fibrosis. All hospitalized patients should be considered for medical thromboprophylaxis, particularly those with NASH.
Core Tip: Our study delineates the intricate relationship between nonalcoholic fatty liver disease (NAFLD) and venous thromboembolism (VTE), highlighting the increased risk and prevalence of VTE in patients with NAFLD-related conditions. Notably, it demonstrates a significant correlation between advanced steatosis and fibrosis grades and the occurrence of VTE, suggesting that patients with more severe NAFLD characteristics require vigilant monitoring for potential thrombotic complications. These insights delineate the necessity for tailored clinical approaches in managing NAFLD patients to mitigate the risk of VTE, marking a substantial step forward in understanding NAFLD’s systemic impacts.
- Citation: Suresh MG, Gogtay M, Singh Y, Yadukumar L, Mishra AK, Abraham GM. Case-control analysis of venous thromboembolism risk in non-alcoholic steatohepatitis diagnosed by transient elastography. World J Clin Cases 2023; 11(34): 8126-8138
- URL: https://www.wjgnet.com/2307-8960/full/v11/i34/8126.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i34.8126
Nonalcoholic fatty liver disease (NAFLD) has emerged as a global health concern, presenting as a prevalent cause of chronic liver disease worldwide. The geographical distribution of NAFLD exhibits substantial variation, with the highest reported rates in South America (31%) and the Middle East (32%), followed by Asia (27%), the United States (24%), and Europe (23%). Notably, Africa reports a lower incidence, with NAFLD affecting 14% of the population in this continent[1]. This distribution pattern calls for a tailored understanding of NAFLD across populations and unveils the rising epidemic in regions with changing lifestyle patterns. NAFLD encompasses a spectrum of liver conditions, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), progressive fibrosis, cirrhosis, and even hepatocellular carcinoma[2].
Steatosis, an early manifestation of NAFLD, has several well-established risk factors, including obesity, diabetes, and hypertriglyceridemia. Beyond metabolic factors, NAFLD can also be influenced by a myriad of factors such as toxins, medications (e.g., amiodarone, diltiazem, highly active antiretroviral therapy, steroids, and tamoxifen), and inborn errors of metabolism, encompassing disorders of lipid metabolism and total parenteral nutrition[3,4].
NASH represents a severe, more advanced form of NAFLD characterized by the accumulation of fat in the liver, accompanied by inflammation and progressive fibrosis[5]. The pathogenesis of NASH is intricate and multifaceted, involving a complex interplay of cellular interactions between liver parenchymal and nonparenchymal cells[6]. This intricate crosstalk extends to diverse immune cell populations within the liver microenvironment[7]. Among the prevailing theories, insulin resistance emerges as a central mechanism, with lipotoxicity taking a pivotal role in driving hepatocellular injury through processes such as oxidative stress and endoplasmic reticulum stress[8].
The clinical presentation of NASH varies widely, ranging from asymptomatic cases to vague complaints of fatigue, malaise, and vague right upper abdominal discomfort[9]. Notably, hepatomegaly is a common physical finding in NASH patients[10]. While elevated levels of liver enzymes such as aspartate aminotransferase and alanine aminotransferase are often observed in patients with NAFLD, it’s essential to recognize that normal aminotransferase levels do not rule out the presence of NAFLD[11]. NASH not only affects the liver but also has systemic complications, putting patients at risk of severe complications such as venous thromboembolism (VTE). Our research aims to fill the gap in medical literature by investigating the link between NASH and VTE.
The diagnosis and assessment of NAFLD severity have evolved to incorporate noninvasive testing methods. These encompass various imaging techniques such as liver ultrasound, computerized tomography (CT), magnetic resonance imaging (MRI), and vibration controlled transient elastography (VCTE)[12-14]. VCTE, commonly referred to as FibroScan®, utilizes shear wave imaging to estimate liver stiffness[15]. This technique involves the application of mechanical vibrations to liver tissue, with the resulting shear waves measured by an ultrasound detector. Through this method, at least ten valid measurements are obtained during the imaging examination. VCTE offers precise diagnostic capabilities, effectively identifying cirrhosis [fibrosis stage 4 (F4)] and distinguishing advanced fibrosis (F2 or higher) from minimal or no fibrosis (F1 or F0)[4]. Notably, as liver fat content affects ultrasound wave propagation, the controlled attenuation parameter (CAP), which is integrated with VCTE, provides a numerical value reflective of the histological severity of liver steatosis[16].
While recent research efforts have increasingly focused on the diagnosis and management of NAFLD, the connection between NASH and VTE remains an underexplored area in the scientific landscape. A noteworthy study in this regard suggested a 2.5-fold heightened risk of VTE in patients with NASH in comparison to individuals with other liver diseases, particularly among those hospitalized[8]. Additionally, multiple epidemiological and case-control investigations have shed light on an association between NAFLD and elevated vascular thrombotic risk, which appears to be independent of conventional cardiometabolic risk factors[17]. These findings emphasize the existence of a graded relationship between the severity of NAFLD and heightened vascular risk[18]. The prevailing hypothesis attributes this phenomenon to hepatocellular injury, which triggers a chronic inflammatory state, leading to the unregulated activation of procoagulant factors[19].
Surprisingly, there exists a significant knowledge gap regarding the degree of steatosis and fibrosis, as assessed using VCTE, in NASH and its potential connection to VTE. This connection between chronic liver inflammation and systemic vascular events remains a pathophysiological puzzle. By methodically assessing steatosis and fibrosis grades to VTE risk, our study breaks new ground in the quest to understand NASH not just as a hepatic problem but as a systemic one, particularly with its propensity to foreshadow VTE, a potentially life-threatening condition. The findings of our study have significant implications for clinical practice, particularly in relation to the use of medical thromboprophylaxis in patients with advanced NASH. This recommendation is not currently included in existing guidelines, but our research suggests that it could be an effective strategy for reducing the risk of VTE. This investigation not only fills a crucial gap in the literature but also highlights the need for heightened surveillance. This study also spotlights the intersection of hepatology and hematology, needing a multidisciplinary approach to managing the rising epidemic.
The comprehensive investigation into the potential link between NASH and VTE was conducted in adherence to a well-defined protocol. This protocol was developed, reviewed, and sanctioned by the joint institutional review board at MetroWest Medical Center under Approval No. 2020-035. The ethical requirement for individual informed consent was appropriately waived by the institutional review board due to the retrospective nature of this case-control study.
In this case-control study, rigorous eligibility criteria were applied to ensure the accuracy, relevance, and validity of the research findings. Patients who met the following criteria were considered eligible for inclusion in the study: (1) Age criteria: Patients were eligible if they were over 18 years of age, indicating that the study focused on adult populations; (2) Diagnosis of NASH: Patients were required to have a well-documented diagnosis of NASH, ensuring that the studied population had the specific medical condition of interest; (3) VCTE scores: Inclusion necessitated the presence of at least one FibroScan® report, confirming the use of VCTE parameters. This included CAP and kilopascal (KPA) scores, which were key indicators in evaluating hepatic steatosis and fibrosis; and (4) Primary care clinic visits: Eligible patients had a history of at least two or more visits to our primary care clinic, ensuring an adequate medical record history for analysis.
To maintain research integrity and accuracy, several exclusion criteria were also applied, including: (1) Thrombophilia or hemoglobinopathy: Patients with a documented history of inherited thrombophilia or hemoglobinopathy were excluded to minimize confounding factors in the analysis of VTE; (2) Active malignancy: Individuals with an active malignancy were excluded, as malignancies can increase the risk of thromboembolism; (3) Alcohol use disorder: Exclusion of patients with alcohol use disorder ensured that the study focused on non-alcoholic factors related to NASH; (4) Hepatitis B or C infection: Patients with concurrent hepatitis B or C infection were excluded ensuring that the study focused on non-alcoholic factors related to NASH; (5) Autoimmune hepatitis and primary biliary cirrhosis: To maintain homogeneity within the study cohort, patients with autoimmune hepatitis or primary biliary cirrhosis were excluded; (6) Age restriction: To adhere to the adult focus of the study, individuals under the age of 18 years were excluded from the analysis; and (7) Incomplete outpatient records: Patients with incomplete outpatient medical records were excluded to maintain data accuracy and completeness.
The data employed in this case-control study were drawn from a dedicated source - patients seen at a single-centered community hospital outpatient clinic. This inclusive dataset covered a substantial time frame, spanning from January 1, 2001, to December 31, 2019. The primary information source for the study consisted of medical records, including crucial imaging reports that confirmed the presence of VTE diagnoses.
The study was inherently retrospective, primarily reliant on the availability of patient data from the designated time frame.
The procedure for selecting patients and categorizing them into the VTE and non-VTE groups was executed with the utmost diligence to eliminate bias. Initial screening for eligibility resulted in the identification of 386 patients who met the above-specified inclusion criteria. From this initial pool, 51 patients were identified as having VTE, constituting the VTE group, while the remaining 335 patients were categorized as the non-VTE group. This clear distinction allowed for precise group comparison, contributing to the robustness of the study findings (Figure 1).
The authors methodically extracted data from a broad array of medical records. The data collection process incorporated a diverse range of variables, each meticulously documented for analysis. Key data elements collected from each patient encompassed demographic information, including age, sex, and race, essential for the contextualization of the findings. Body mass index (BMI), a crucial indicator of patient health, was noted. The presence or absence of diabetes mellitus, human immunodeficiency virus (HIV) status, and substance use - specifically alcohol and tobacco consumption - were accurately documented, allowing for the examination of the potential influence of these factors on VTE in patients with NASH.
Furthermore, the data collection process included the recording of VCTE scores, a pivotal element of the study. The CAP and KPA scores, reflective of hepatic steatosis and fibrosis, were systematically collected, enriching the dataset with specific markers of NASH severity. The scope of this case-control study necessitated the acquisition of VTE-specific data, allowing for a comprehensive analysis of the condition. Variables related to VTE, including the location of VTE events, the type of anticoagulant administered, the duration of treatment, instances of treatment failure, the need for hospitalization, and any history of VTE recurrence, were diligently recorded.
A wide array of data items was meticulously cataloged and incorporated into the dataset. These variables encompassed: (1) Demographic information: Parameters including age, sex, and race were logged to provide an understanding of the patient population’s diversity; (2) Medical history: Crucial medical history elements were documented, such as the presence of diabetes mellitus, HIV status, and substance use (alcohol and tobacco), shedding light on factors that could impact VTE risk in NASH patients; (3) VCTE scores: The CAP and KPA scores were pivotal data points, serving as objective measures of hepatic steatosis and fibrosis, respectively; and (4) VTE specifics: Data on the occurrence of VTE, including location, anticoagulant therapy, treatment duration, instances of treatment failure, need for hospitalization, and VTE recurrence history, provided a comprehensive picture of the VTE aspect of the study. The careful selection and documentation of these data items ensured the robustness and reliability of the dataset.
Unlike prospective studies, where selection and attrition bias can be assessed, this retrospective design leveraged existing patient data and medical records, minimizing the potential for inherent bias in data collection. The primary focus of the study was on the accurate extraction and analysis of data, ensuring that the risk of bias was kept to a minimum.
Within this multifaceted study, a diverse array of summary measures and variables were considered. The primary outcome, which was the presence of VTE, formed the cornerstone of the analysis. In addition to this primary endpoint, a host of secondary variables were included, encompassing demographics, hepatic steatosis (as assessed through the CAP score), and hepatic fibrosis (evaluated using the KPA score). The inclusion of these additional variables allowed for a comprehensive examination of the factors influencing the association between NASH and VTE, ensuring a nuanced and holistic approach to the study’s objectives.
A range of statistical methods were instrumental in the assessment of associations and relationships between variables. These methods included the Mann-Whitney U test, χ2 analysis, and logistic regression, which were chosen to suit the nature of the data and the research questions. The Mann-Whitney U test was employed to compare continuous variables between groups, allowing for the evaluation of differences in patient characteristics, including age, BMI, and VCTE scores. This non-parametric test was selected due to its suitability for this data that might not follow a normal distribution.
χ2 analysis was particularly valuable for assessing associations between categorical variables, such as the presence of diabetes mellitus, HIV status, and substance use, within the study groups. This statistical tool enabled the identification of statistically significant relationships, allowing for a comprehensive understanding of the patient population and their characteristics.
Logistic regression, a more complex statistical technique, was applied to analyze the primary outcome variable, the presence of VTE. This method allowed for the assessment of the influence of NASH, as indicated by hepatic steatosis (CAP score) and hepatic fibrosis (KPA score), on the likelihood of VTE. The results were presented in a clear and informative manner, using tables and figures to succinctly communicate significant findings, associations, and potential risk factors.
The inclusion of these diverse statistical techniques offered a comprehensive approach to data analysis, enabling a nuanced understanding of the complex relationships within the dataset. The resulting findings, supported by statistical evidence, allowed for the vigorous testing of the study’s hypotheses.
A comprehensive analysis involving 415 patients revealed that 386 of these individuals met the inclusion criteria set for this study. Among the study participants, 51 patients were classified into the VTE group, whereas the remaining 335 formed the non-VTE group. The demographic profile of these two groups showcased notable differences. Firstly, patients in the VTE group exhibited a slightly higher mean age, with an average of 60.63 years, in contrast to the non-VTE group, which had a mean age of 55.22 years (P < 0.014). Gender distribution, racial background, tobacco use, and HIV status showed comparable distribution across both groups. However, key disparities came to light regarding specific health parameters. Patients in the VTE group presented with a higher average BMI (31.14 kg/m²), notably exceeding the 29.30 kg/m² seen in the non-VTE group (P < 0.041). Additionally, the prevalence of diabetes mellitus was significantly more pronounced among patients with VTE, accounting for 29.4%, in stark contrast to the non-VTE group’s prevalence of 13.1% (P < 0.002) (Tables 1 and 2).
| Variables | Thromboembolism | Total | P value1 | ||
| No | Yes | ||||
| Gender | Male | 194 | 33 | 227 | 0.358 |
| 57.9% | 64.7% | 58.8% | |||
| NASH | Yes | 102 | 23 | 125 | 0.037 |
| 30.4% | 45.1% | 32.4% | |||
| Smoking | No | 228 | 26 | 254 | |
| 68.1% | 51.0% | 65.8% | |||
| Yes | 76 | 17 | 93 | ||
| 22.7% | 33.3% | 24.1% | |||
| Former | 31 | 8 | 39 | ||
| 9.3% | 15.7% | 10.1% | |||
| Alcohol consumption | Yes | 55 | 6 | 61 | 0.396 |
| 16.4% | 11.8% | 15.8% | |||
| Ethnicity | Caucasian | 268 | 39 | 307 | NA |
| 80.0% | 76.5% | 79.5% | |||
| Hispanic | 39 | 10 | 49 | ||
| 11.6% | 19.6% | 12.7% | |||
| Asian | 11 | 1 | 12 | ||
| 3.3% | 2.0% | 3.1% | |||
| African American | 15 | 1 | 16 | ||
| 4.5% | 2.0% | 4.1% | |||
| Others | 2 | 0 | 2 | ||
| 0.6% | 0.0% | 0.5% | |||
| Diabetic status | Non DM | 281 | 32 | 313 | 0.002a |
| 83.9% | 62.7% | 81.1% | |||
| Type 1 DM | 10 | 4 | 14 | ||
| 3.0% | 7.8% | 3.6% | |||
| Type 2 DM | 44 | 15 | 59 | ||
| 13.1% | 29.4% | 15.3% | |||
| HIV status | Reactive | 9 | 1 | 10 | 0.761 |
| 2.7% | 2.0% | 2.6% | |||
| CAP (steatosis) grade | S0 | 145 | 14 | 159 | 0.031a |
| 43.3% | 27.5% | 41.2% | |||
| S1 | 32 | 3 | 35 | ||
| 9.6% | 5.9% | 9.1% | |||
| S2 | 34 | 4 | 38 | ||
| 10.1% | 7.8% | 9.8% | |||
| S3 | 124 | 30 | 154 | ||
| 37.0% | 58.8% | 39.9% | |||
| KPA (fibrosis) grade | F0 | 87 | 6 | 93 | 0.03a |
| 26.0% | 11.8% | 24.1% | |||
| F0-F1 | 116 | 14 | 130 | ||
| 34.6% | 27.5% | 33.7% | |||
| F1 | 3 | 1 | 4 | ||
| 0.9% | 2.0% | 1.0% | |||
| F2 | 32 | 2 | 34 | ||
| 9.6% | 3.9% | 8.8% | |||
| F3 | 25 | 7 | 32 | ||
| 7.5% | 13.7% | 8.3% | |||
| F4 | 72 | 21 | 93 | ||
| 21.5% | 41.2% | 24.1% | |||
Of particular interest in the primary outcome was the history of NASH, a condition intricately linked to NAFLD. Here, a notable distinction arose between the VTE group and the non-VTE group. In the VTE group, the history of NASH was significantly more prevalent, with 45.1% of patients carrying this diagnosis, while the non-VTE group reported a history of NASH in 30.4% of cases (P < 0.037). Moreover, steatosis, a critical parameter characterizing liver fat accumulation, exhibited distinct variations between the two groups. The VTE group displayed a notably higher steatosis score, averaging 279.04, in comparison to the non-VTE group, which had an average score of 249.54 (P < 0.005). An even more compelling contrast emerged concerning the fibrosis grade, an essential measure of liver health. In this regard, the VTE group showcased a considerably higher fibrosis grade, quantifying at 15.29, whereas the non-VTE group exhibited a lower average of 12.67 (P < 0.001). An in-depth exploration of the data revealed that moderate to severe steatosis (classified as S2-S3) was strikingly more frequent in the VTE group, constituting 66.7% of this patient cohort, compared to 47.2% in the non-VTE group (P < 0.009). A similar trend emerged regarding the fibrosis grade. Within the VTE group, fibrosis grades F2-F4 were notably more prevalent, accounting for 58.8% of patients, whereas in the non-VTE group, this grade represented 38.5% of cases (P < 0.006). Utilizing logistic regression as an analytical tool, considering VTE as the dependent variable, two critical predictors emerged. Patients diagnosed with diabetes mellitus exhibited an odds ratio (OR) of 1.702 (P < 0.015), highlighting a significant association between diabetes and the presence of VTE. Furthermore, a robust association was noted between the F2-F4 fibrosis grade and VTE, with an OR of 1.5 (P < 0.033) (Table 3).
Within the VTE group, a comprehensive analysis of the distribution of VTE types was conducted. Results indicated that 58.8% of these patients presented with deep vein thrombosis (DVT), 17.6% with superficial vein thrombosis, 13.7% with pulmonary embolism, 5.9% with portal vein thrombosis, and 3.9% with embolic stroke (Table 4).
| Type of thromboembolism | Number | Percentage |
| Deep vein thrombosis | 30 | 58.8% |
| Superficial venous thrombosis | 9 | 17.6% |
| Pulmonary embolism | 7 | 13.7% |
| Portal vein | 3 | 5.9% |
| Stroke (embolic) | 2 | 3.9% |
| Total | 51 | 100.0% |
Lower extremity emerged as the most common location for VTE, observed in 56.9% of cases, followed by the upper extremity at 19.6%. In terms of laterality, right-sided VTE prevailed, accounting for 66.7% of cases. Regarding the severity of VTE cases, a significant majority of patients, precisely 74.5%, necessitated hospital admission, with 2% of this cohort requiring admission to the intensive care unit (Table 5).
| Location | Frequency | Percentage |
| Lower limb | 29 | 56.9% |
| Upper limb | 10 | 19.6% |
| Upper lobe | 3 | 5.9% |
| Middle lobe | 2 | 3.9% |
| Lower lobe | 2 | 3.9% |
| Others | 5 | 9.8% |
| Total | 51 | 100.0% |
The management of VTE involved the administration of anticoagulants, and the distribution of anticoagulant types revealed that 64.71% of patients were on direct oral anticoagulants (DOAC), while 25.5% were on warfarin (Table 6). The persistence of indefinite anticoagulation therapy was observed in 84.3% of patients. Furthermore, an impressive 96.1% required the full therapeutic dose of anticoagulation. However, 5.9% of these individuals experienced treatment failure, reflecting the complexity and variability in managing VTE in patients with NAFLD. These findings underscore the multifaceted nature of VTE in the context of NAFLD and the importance of precise diagnosis and tailored therapeutic strategies to address this medical challenge effectively.
| Type of anticoagulant used | Frequency | Percentage |
| Direct oral anticoagulants | 33 | 64.7% |
| Vitamin K antagonists | 13 | 25.5% |
| Enoxaparin sodium | 3 | 5.9% |
| Antiplatelet | 2 | 3.9% |
| Total | 51 | 100.0% |
NAFLD is the most prevalent cause of chronic liver disease in the United States and globally[20]. Within the context of NAFLD, a spectrum of liver conditions can be observed, ranging from healthy liver function to the perilous extremes of cirrhosis and primary liver cancer. This characterization underscores the importance of closely monitoring liver health and taking proactive steps to mitigate risk factors associated with NAFLD[21]. NAFLD has been intricately tied with metabolic syndromes like insulin resistance and obesity, but it also affects lean individuals, particularly those with lipodystrophy[22,23]. African Americans appear to have the lowest prevalence, Hispanics the highest, and Caucasians occupying the middle ground[24]. The risk of developing cirrhosis is very low in individuals with isolated steatosis (NAFLD), but the risk increases as steatosis becomes complicated by liver-cell injury and death and the accumulation of inflammatory cells (NASH)[25]. NASH is also a heterogeneous condition that can improve to steatosis or normal histology, remain relatively stable for years, or cause progressive accumulation of fibrous scar that eventuates in cirrhosis (F4). NAFLD-related cirrhosis is the primary predictor of eventual liver-related morbidity and mortality. Once cirrhosis develops, the annual incidence of primary liver cancer can be as high as per year[26]. Heritable factors play a significant role in the development of NAFLD, NASH, liver fibrosis, and liver cancer. Genetic variants on or near TM6SF2, MBOAT7, and PNPLA3 genes increase the risk of NAFLD and its severity. Recent studies have shown that PNPLA3 has a strong influence on hepatic fat accumulation, NASH severity, and liver fibrosis. Epigenetic factors may also play a role in NAFLD pathogenesis and progression[27]. The mechanisms underlying the pathogenesis and progression of NAFLD are not fully understood. Hepatic steatosis occurs when the mechanisms for triglyceride synthesis is altered, leading to the accumulation of fat within hepatocytes. Obesity and insulin resistance also stimulate hepatocyte triglyceride accumulation. Triglyceride precursors and metabolic by-products may damage hepatocytes, leading to lipotoxicity. The liver parenchymal cells then respond with an aim to replace lost hepatocytes, involving the expansion of other cell types and recruitment of immune cells. NASH is the morphologic manifestation of lipotoxicity and resultant wound healing responses. Cirrhosis and liver cancer are potential outcomes of futile repair, i.e., progressive accumulation of wound healing cells, fibrous matrix, and abnormal vasculature (scarring), rather than efficient reconstruction/regeneration of healthy hepatic parenchyma[28-30]. NAFLD diagnosis requires the detection of steatosis in the absence of alternate causes. The determination of NAFLD-related liver injury severity and scarring is crucial for treatment recommendations. Noninvasive and invasive staging approaches are available, with liver biopsy being the gold standard for establishing liver injury and fibrosis severity. Noninvasive approaches, such as long-fiber thermoplastic and imaging (CT, MRI, ultrasonography and FibroScan), are being developed to stage NAFLD and monitor fibrosis progression and regression. Combining these tests enhances their predictive power, and they are being used serially or in combination to monitor fibrosis progression and regression in NAFLD patients[31-33]. Most individuals with NAFLD do not exhibit any symptoms. Typically, the diagnosis is made when a patient undergoes an evaluation for other reasons and abnormal liver aminotransferases or features of fatty liver are detected. In some cases, NAFLD may be diagnosed when a patient experiences vague right upper quadrant abdominal pain, hepatomegaly, or an abnormal-appearing liver during abdominal surgery[9]. Some patients may show subtle symptoms of chronic liver disease, such as spider angiomata, palmer erythema, or splenomegaly. In rare cases, individuals with advanced NAFLD may experience complications of end-stage liver disease, such as jaundice, features of portal hypertension (ascites or variceal hemorrhage)[34]. Currently, there are no United States Food and Drug Administration-approved therapies for the treatment of NAFLD. Hence, the current approach to NAFLD management focuses on treating risk factors for NASH, such as obesity, insulin resistance, metabolic syndrome, and dyslipidemia. Lifestyle changes and dietary modifications that result in weight loss and/or improve insulin sensitivity are the primary treatments for NAFLD. Studies show that loss of body weight improves steatosis, and greater weight loss improves steatohepatitis and hepatic fibrosis[35]. Modifying dietary macronutrient contents, such as low-carbohydrate low-fat diets or saturated unsaturated fat diets, is mainly beneficial since they reduce energy intake and improve obesity[36]. A Mediterranean-type diet has been reported to improve NASH and liver fibrosis independently of weight loss[37]. Excluding foods and beverages high in added fructose and increasing coffee consumption are also recommended[38]. High-fructose diets exacerbate hepatic steatosis, steatohepatitis, and fibrosis, while consuming two or more cups of coffee per day is associated with reduced risk of liver fibrosis[39]. Modifying lifestyle to increase physical activity complements dietary caloric restriction and expedites weight loss. Exercise also improves muscle insulin sensitivity, which improves the metabolic syndrome independent of weight loss. Several large clinical trials designed to identify effective and safe treatments for these conditions are in progress. Statins are an important class of agents to treat dyslipidemia and decrease cardiovascular risk. Although interest in bariatric surgery as a treatment for NAFLD exists, there is a lack of randomized clinical trials or adequate clinical studies to assess the benefits and harms of bariatric surgery as a treatment for NASH[40]. Most studies of bariatric surgery have shown that it is generally safe in individuals with well-compensated chronic liver disease and improves hepatic steatosis and necroinflammation[41]. However, the effects on hepatic fibrosis have been variable. NAFLD-related cirrhosis and, particularly, those with portal hypertension should be excluded as candidates for bariatric surgery[42]. Patients with NAFLD in whom end-stage liver disease develops should be evaluated for liver transplantation. The outcomes of liver transplantation in well-selected patients with NAFLD are generally good, but comorbid medical conditions associated with NAFLD, such as diabetes mellitus, obesity, and cardiovascular disease, often limit transplant candidacy. Our analysis sheds light on NASH as an independent risk factor for VTE, particularly DVT. Furthermore, it underscores a positive association between the degree of steatosis/fibrosis and an increased incidence of VTE. While this finding is significant, the underlying mechanisms connecting NASH and the heightened risk of VTE remain enigmatic. It’s well-documented that NASH contributes to a pro-inflammatory state, evident through elevated levels of biomarkers like high-sensitivity C-reactive protein, plasminogen activator inhibitor-1, and fibrinogen concentrations[43]. Recent studies suggest that NASH patients exhibit elevated factor VIII, a potent pro-coagulant, as well as reduced protein C and anticoagulant levels, which creates a procoagulant imbalance and heightens the risk of cardiovascular events[11]. This imbalance might also explain the increased incidence of VTE. Additionally, increased levels of von Willebrand factor, mean platelet volume, and decreased antithrombin III levels have been suggested as potential contributors to the pro-coagulative state in NASH[4]. The intrahepatic thrombi generated by these changes can induce tissue ischemia, exacerbate the disease by activating stellate cells and fibrogenesis, and further aggravate hemostatic alterations favoring pro-coagulation. This theory provides a plausible explanation for the observed linear association between the degree of steatosis/fibrosis and the increased incidence of VTE[8]. The retrospective design used in our study has certain limitations that can affect the accuracy of our results. Errors in data collection can occur despite our efforts to carefully review the patient records. There is a possibility that some important factors have not been documented. Our study was conducted at a single center, that may affect the generalizability of our findings to the broader population. Socioeconomic differences, healthcare practices, and location can impact the prevalence of NASH and related complications such as VTE. To get a more comprehensive understanding of this issue, studies from various locations and patient groups are needed. The sample size of our study, particularly those with VTE, was relatively small. A larger study with more participants would provide more reliable results and could reveal more detailed connections between NASH, steatosis, fibrosis, and VTE risk. Incomplete data is another limitation that can lead to less precise results, which could influence the strength of our conclusions. Our study found links between VTE and factors such as age, BMI, and diabetes mellitus. However, these factors can independently increase the risk of VTE, which could complicate our analysis. Further research is needed to better understand how these factors relate to VTE in people with NASH. Finally, while our study highlighted a strong connection between NASH and VTE, it does not prove that one causes the other. More research, particularly prospective studies, is necessary to uncover the exact mechanisms underlying this relationship.
In summary, our study reveals that NASH is an independent risk factor for VTE, thereby providing further evidence that NASH is associated with a hypercoagulable state. This risk is particularly pronounced in NASH patients with moderate-to-severe steatosis and F2-F4 fibrosis, with DVT being the predominant manifestation. While our study found a positive association between VTE and age, higher BMI, and diabetes mellitus, this could potentially be attributed to confounding factors. As such, more extensive studies are necessary to validate these findings. The role of VTE prophylaxis for primary prevention in this specific population remains unclear. In our study, we observed that 64.71% of patients were on DOAC, 25.5% were on warfarin, 5.9% were on low molecular weight heparin, and 3.9% were on antiplatelet agents. However, we noted treatment failure in 5.9% of patients during follow-up, indicating that recurrence is a possibility despite treatment. Our study underscores the crucial importance of promptly recognizing and managing individuals at risk of NASH to prevent devastating complications, especially the increased risk of VTE. Further research is warranted to better understand the underlying mechanisms, prevention strategies, and management options for this vulnerable patient population.
To investigate the relationship between nonalcoholic fatty liver disease (NAFLD) and the risk of venous thromboembolism (VTE). The study underscores the need for greater awareness of the risk factors contributing to VTE in the context of NAFLD.
The motivation behind this research is to identify the connection between NAFLD, particularly non-alcoholic steatohepatitis (NASH), and the development of VTE. There is a knowledge gap and a compelling need to understand this association to enhance clinical outcomes and tailor management strategies for patients with NAFLD.
The primary objective was to evaluate the incidence and characteristics of VTE in patients with NAFLD and identify associated risk factors. Achieving this goal is significant for improving the prognosis and management of NAFLD patients by enabling the early detection and treatment of VTE.
The case-control study employed comprehensive patient data analysis, logistic regression, and comparative assessments between patient groups with and without VTE. These methods facilitated a detailed examination of the clinical parameters and outcomes, highlighting the robustness and replicability of the study.
Key findings include the higher prevalence of diabetes mellitus and more severe liver pathology (steatosis and fibrosis) in the VTE group. The study revealed that these patients often required hospitalization and intensive management, including anticoagulation therapy. The results contribute significantly to the current understanding of the relationship between NASH and VTE, emphasizing the importance of monitoring at-risk patients.
The study proposes that NAFLD, particularly with advanced liver pathology from NAFLD, is a significant factor in the development of VTE.
Future research should focus on longitudinal studies to further define the causal relationships and investigate the underlying mechanisms that link advanced NAFLD with VTE. Additionally, there is a need for clinical trials to test targeted interventions aimed at reducing the risk of thrombosis in this patient population.
We thank all medical staff of Saint Vincent Hospital who agreed to participate in this study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Roomi AB, Iraq S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1415] [Cited by in RCA: 1317] [Article Influence: 219.5] [Reference Citation Analysis (0)] |
| 2. | Ahmed A, Wong RJ, Harrison SA. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin Gastroenterol Hepatol. 2015;13:2062-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 238] [Article Influence: 23.8] [Reference Citation Analysis (2)] |
| 3. | Machado MV, Diehl AM. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology. 2016;150:1769-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 394] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 4. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1848] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 5. | Carlsson B, Lindén D, Brolén G, Liljeblad M, Bjursell M, Romeo S, Loomba R. Review article: the emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2020;51:1305-1320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 6. | Redgrove KA, Anderson AL, Dun MD, McLaughlin EA, O'Bryan MK, Aitken RJ, Nixon B. Involvement of multimeric protein complexes in mediating the capacitation-dependent binding of human spermatozoa to homologous zonae pellucidae. Dev Biol. 2011;356:460-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Rao J, Wang H, Ni M, Wang Z, Wei S, Liu M, Wang P, Qiu J, Zhang L, Wu C, Shen H, Wang X, Cheng F, Lu L. FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut. 2022;71:2539-2550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 136] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 8. | Stine JG, Niccum BA, Zimmet AN, Intagliata N, Caldwell SH, Argo CK, Northup PG. Increased risk of venous thromboembolism in hospitalized patients with cirrhosis due to non-alcoholic steatohepatitis. Clin Transl Gastroenterol. 2018;9:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 735] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 10. | Amarapurkar D, Kamani P, Patel N, Gupte P, Kumar P, Agal S, Baijal R, Lala S, Chaudhary D, Deshpande A. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6:161-163. [PubMed] [DOI] [Full Text] |
| 11. | Pouwels S, Sakran N, Graham Y, Leal A, Pintar T, Yang W, Kassir R, Singhal R, Mahawar K, Ramnarain D. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022;22:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 455] [Article Influence: 151.7] [Reference Citation Analysis (0)] |
| 12. | Papatheodoridi M, Cholongitas E. Diagnosis of Non-alcoholic Fatty Liver Disease (NAFLD): Current Concepts. Curr Pharm Des. 2018;24:4574-4586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 13. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4949] [Article Influence: 707.0] [Reference Citation Analysis (9)] |
| 14. | Singh Y, Gogtay M, Gurung S, Trivedi N, Abraham GM. Assessment of Predictive Factors of Hepatic Steatosis Diagnosed by Vibration Controlled Transient Elastography (VCTE) in Chronic Hepatitis C Virus-Infected Patients. J Community Hosp Intern Med Perspect. 2022;12:58-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (2)] |
| 15. | Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V, Bedossa P, Newsome PN. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 968] [Article Influence: 161.3] [Reference Citation Analysis (0)] |
| 16. | Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 638] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 17. | Ren Z, Simons PIHG, Wesselius A, Stehouwer CDA, Brouwers MCGJ. Relationship between NAFLD and coronary artery disease: A Mendelian randomization study. Hepatology. 2023;77:230-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 80] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 18. | Bhatia LS, Curzen NP, Byrne CD. Nonalcoholic fatty liver disease and vascular risk. Curr Opin Cardiol. 2012;27:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15:349-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 663] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 20. | Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 1184] [Article Influence: 394.7] [Reference Citation Analysis (1)] |
| 21. | Juanola O, Martínez-López S, Francés R, Gómez-Hurtado I. Non-Alcoholic Fatty Liver Disease: Metabolic, Genetic, Epigenetic and Environmental Risk Factors. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 177] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 22. | Chen F, Esmaili S, Rogers GB, Bugianesi E, Petta S, Marchesini G, Bayoumi A, Metwally M, Azardaryany MK, Coulter S, Choo JM, Younes R, Rosso C, Liddle C, Adams LA, Craxì A, George J, Eslam M. Lean NAFLD: A Distinct Entity Shaped by Differential Metabolic Adaptation. Hepatology. 2020;71:1213-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 247] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 23. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2118] [Article Influence: 235.3] [Reference Citation Analysis (1)] |
| 24. | Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021;41:1290-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 25. | Pierantonelli I, Svegliati-Baroni G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression From NAFLD to NASH. Transplantation. 2019;103:e1-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 335] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 26. | Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 1682] [Article Influence: 420.5] [Reference Citation Analysis (33)] |
| 27. | Amangurbanova M, Huang DQ, Loomba R. Review article: the role of HSD17B13 on global epidemiology, natural history, pathogenesis and treatment of NAFLD. Aliment Pharmacol Ther. 2023;57:37-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 28. | Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 919] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 29. | Nobili V, Alisi A, Valenti L, Miele L, Feldstein AE, Alkhouri N. NAFLD in children: new genes, new diagnostic modalities and new drugs. Nat Rev Gastroenterol Hepatol. 2019;16:517-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 30. | Dongiovanni P, Stender S, Pietrelli A, Mancina RM, Cespiati A, Petta S, Pelusi S, Pingitore P, Badiali S, Maggioni M, Mannisto V, Grimaudo S, Pipitone RM, Pihlajamaki J, Craxi A, Taube M, Carlsson LMS, Fargion S, Romeo S, Kozlitina J, Valenti L. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283:356-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 31. | Lannerstedt H, Konopski Z, Sandvik L, Haaland T, Løberg EM, Haukeland JW. Combining transient elastography with FIB4 enhances sensitivity in detecting advanced fibrosis of the liver. Scand J Gastroenterol. 2013;48:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VW, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GL, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 838] [Article Influence: 104.8] [Reference Citation Analysis (0)] |
| 33. | Lonardo A, Bellini M, Tondelli E, Frazzoni M, Grisendi A, Pulvirenti M, Della Casa G. Nonalcoholic steatohepatitis and the "bright liver syndrome": should a recently expanded clinical entity be further expanded? Am J Gastroenterol. 1995;90:2072-2074. [PubMed] |
| 34. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2344] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 35. | McGlone ER, Siebert M, Dore M, Hope DCD, Davies I, Owen B, Khoo B, Goldin R, Carling D, Bloom S, Le Gall M, Tan TM. Sleeve gastrectomy causes weight-loss independent improvements in hepatic steatosis. Liver Int. 2023;43:1890-1900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Holmer M, Lindqvist C, Petersson S, Moshtaghi-Svensson J, Tillander V, Brismar TB, Hagström H, Stål P. Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet - a randomised controlled trial. JHEP Rep. 2021;3:100256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 37. | Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol. 2018;24:2083-2094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 38. | Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, Nakagawa T, Kuwabara M, Sato Y, Kang DH, Tolan DR, Sanchez-Lozada LG, Rosen HR, Lanaspa MA, Diehl AM, Johnson RJ. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68:1063-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 637] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 39. | Marventano S, Salomone F, Godos J, Pluchinotta F, Del Rio D, Mistretta A, Grosso G. Coffee and tea consumption in relation with non-alcoholic fatty liver and metabolic syndrome: A systematic review and meta-analysis of observational studies. Clin Nutr. 2016;35:1269-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 40. | Furuya CK Jr, de Oliveira CP, de Mello ES, Faintuch J, Raskovski A, Matsuda M, Vezozzo DC, Halpern A, Garrido AB Jr, Alves VA, Carrilho FJ. Effects of bariatric surgery on nonalcoholic fatty liver disease: preliminary findings after 2 years. J Gastroenterol Hepatol. 2007;22:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 41. | Tai CM, Huang CK, Hwang JC, Chiang H, Chang CY, Lee CT, Yu ML, Lin JT. Improvement of nonalcoholic fatty liver disease after bariatric surgery in morbidly obese Chinese patients. Obes Surg. 2012;22:1016-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Chauhan M, Singh K, Thuluvath PJ. Bariatric Surgery in NAFLD. Dig Dis Sci. 2022;67:408-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 43. | Westfall E, Jeske R, Bader AR. Nonalcoholic Fatty Liver Disease: Common Questions and Answers on Diagnosis and Management. Am Fam Physician. 2020;102:603-612. [PubMed] |