Published online Jan 26, 2023. doi: 10.12998/wjcc.v11.i3.610
Peer-review started: October 12, 2022
First decision: November 11, 2022
Revised: December 19, 2022
Accepted: January 5, 2023
Article in press: January 5, 2023
Published online: January 26, 2023
Processing time: 106 Days and 16.3 Hours
Propofol is a short-acting, rapid-recovering anesthetic widely used in sedated colonoscopy for the early detection, diagnosis and treatment of colon diseases. However, the use of propofol alone may require high doses to achieve the induction of anesthesia in sedated colonoscopy, which has been associated with anesthesia-related adverse events (AEs), including hypoxemia, sinus bradycardia, and hypotension. Therefore, propofol co-administrated with other anesthetics has been proposed to reduce the required dose of propofol, enhance the efficacy, and improve the satisfaction of patients receiving colonoscopy under sedation.
To evaluate the efficacy and safety of propofol target-controlled infusion (TCI) in combination with butorphanol for sedation during colonoscopy.
In this controlled clinical trial, a total of 106 patients, who were scheduled for sedated colonoscopy, were prospectively recruited and assigned into three groups to receive different doses of butorphanol before propofol TCI: Low-dose butorphanol group (5 μg/kg, group B1), high-dose butorphanol group (10 μg/kg, group B2), and control group (normal saline, group C). Anesthesia was achieved by propofol TCI. The primary outcome was the median effective concentration (EC50) of propofol TCI, which was measured using the up-and-down sequential method. The secondary outcomes included AEs in perianesthesia and recovery characteristics.
The EC50 of propofol for TCI was 3.03 μg/mL [95% confidence interval (CI): 2.83-3.23 μg/mL] in group B2, 3.41 μg/mL (95%CI: 3.20-3.62 μg/mL) in group B1, and 4.05 μg/mL (95%CI: 3.78-4.34 μg/mL) in group C. The amount of propofol necessary for anesthesia was 132 mg [interquartile range (IQR), 125-144.75 mg] in group B2 and 142 mg (IQR, 135-154 mg) in group B1. Furthermore, the awakening concentration was 1.1 μg/mL (IQR, 0.9-1.2 μg/mL) in group B2 and 1.2 μg/mL (IQR, 1.025-1.5 μg/mL) in group B1. Notably, the propofol TCI plus butorphanol groups (groups B1 and B2) had a lower incidence of anesthesia AEs, when compared to group C. Furthermore, no significant differences were observed in the rates of AEs in perianesthesia, including hypoxemia, sinus bradycardia, hypotension, nausea and vomiting, and vertigo, among group C, group B1 and group B2.
The combined use with butorphanol reduces the EC50 of propofol TCI for anesthesia. The decrease in propofol might contribute to the reduced anesthesia-related AEs in patients undergoing sedated colonoscopy.
Core Tip: Propofol target-controlled infusion co-administrated with butorphanol significantly reduces the dose of propofol required for achieving anesthesia in patients undergoing sedated colonoscopy, leading to the enhancement of efficacy, and reduction in anesthesia-related adverse events when using propofol alone. Therefore, these findings may be beneficial for clinicians in inducing anesthesia, eventually improving the care and satisfaction of patients receiving diagnostic or therapeutic colonoscopic procedures for colorectal diseases.
- Citation: Guo F, Sun DF, Feng Y, Yang L, Li JL, Sun ZL. Efficacy and safety of propofol target-controlled infusion combined with butorphanol for sedated colonoscopy. World J Clin Cases 2023; 11(3): 610-620
- URL: https://www.wjgnet.com/2307-8960/full/v11/i3/610.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i3.610
Colonoscopy is an essential endoscopic tool for the screening, early diagnosis, and treatment of colorectal diseases, especially colorectal cancer, which is the second most common cause of cancer-related mortality worldwide[1,2]. It has been noted that conventional colonoscopy has a number of limitations, including high degree of patient discomfort during the colonoscopic procedure, prolonged insertion, and difficult or even failed cecal intubation[3,4]. In order to reduce patient discomfort and facilitate cecal intubation, colonoscopy with anesthesia or performed under sedation has gained increasing acceptance and popularity in recent years[4,5]. Indeed, patients under sedation during diagnostic or therapeutic endoscopic procedures generally experience minimal or even no discomfort[4,5]. Numerous studies have evaluated the efficacy and safety of anesthetics for sedation colonoscopy, such as propofol, which is a short-acting rapid-recovering anesthetic[6-12]. The results of previous studies suggest that propofol, particularly delivered by target-controlled infusion (TCI), which is a drug delivery technique applied to achieve the desired anesthetic drug concentration by using a pharmacokinetic model and considering the patient characteristics [i.e., age, gender and body mass index (BMI)], is an effective anesthetic with rapid onset and short recovery[11,13-18]. Compared to conventional methods of administrating drugs during anesthesia, such as bolus injection with a syringe and continuous infusion with an infusion pump, TCI provides a relatively constant concentration at the target site, and a more rapid recovery time.
Despite the mentioned advantages, various adverse events (AEs) can occur, including hypoxemia, sinus bradycardia, and hypotension[11,12]. These AEs have been associated with the required high dose of propofol for the induction of anesthesia, when used as the sole anesthetic during colonoscopic procedures[11,12]. Hence, propofol co-administrated with other anesthetics has been sought to reduce the dose of propofol required for anesthesia, enhance the efficacy, and improve the satisfaction of patients undergoing endoscopic procedures[19-24]. For instance, butorphanol, a synthetic opioid, has higher affinity for opioid receptors, when compared to opioids. Compared to morphine, butorphanol has higher analgesic potency, a similar duration of action, and lower respiratory depression. Furthermore, butorphanol is a mixed opioid agonist/antagonist, which includes an agonistic action on the kappa-opioid receptor and agonistic/antagonistic effects on the mu-opioid receptor. This exerts an analgesic effect mainly by agonizing the kappa-opioid receptor. In addition, butorphanol can be used to mitigate the respiratory depression of mu-opioid agonists. The advantages of butorphanol include low toxicity and low potential for abuse. Previous studies have revealed that compared to other synthetic opioid analgesic drugs (e.g., sufentanil), butorphanol has less anesthesia-related AEs, such as respiratory depression, decreased gastrointestinal activity and smooth muscle spasm, itchy skin, urinary retention, physical and physiological dependence, nausea, and vomiting[25,26]. Furthermore, butorphanol has been widely used in anesthesia for patients undergoing gastrointestinal endoscopy.
The present controlled clinical trial aimed to determine the effects of different doses of butorphanol on the efficacy of propofol TCI based on the median effective concentration (EC50) and safety, in terms of anesthesia-related AEs, in patients undergoing colonoscopic procedures under sedation. These results may be beneficial for clinicians in inducing anesthesia, eventually improving the care for patients receiving diagnostic or therapeutic colonoscopic procedures for colorectal diseases.
For the present controlled clinical trial, patients who underwent sedated colonoscopy were prospectively recruited from the First Affiliated Hospital of Dalian Medical University (Dalian, Liaoning Province, China) between December 2020 and March 2021. During the patient enrollment, the following inclusion criteria were used: (1) 18-65 years old; (2) Scheduled to undergo sedated colonoscopy; and (3) American Society of Anesthesiologists (ASA) I-II, graded according to the ASA Physical Status (PS) Classification System. Patients who presented with the following clinical conditions were excluded from the clinical trial: (1) Obstructive sleep apnea hypopnea syndrome; (2) Liver failure, kidney failure, or both; (3) Severe cardio/cerebrovascular diseases categorized as class III or higher, according to the New York Heart Association classification system; (4) Medical history of chronic pain or mental illness; (5) Regular intake of sedatives and painkillers; (6) Medical history of vertigo or motion sickness; (7) BMI of ≥ 30 kg/m2; (8) Pregnant patients; and (9) Patients who were unwilling to provide a written informed consent. The enrolled patients were assigned to three groups, based on the time sequence: (1) Group B1, received low-dose butorphanol (propofol TCI plus butorphanol, 5 μg/kg); (2) Group B2, received high-dose butorphanol (propofol TCI plus butorphanol, 10 μg/kg); and (3) Group C, the control group [normal saline (NS)]. The Propofol Medium and Long Chain Fat Emulsion Injection (Batch No. J20160089) was obtained from Beijing Fresenius Kabi Pharmaceutical Co. Ltd. (Beijing, China). The Butorphanol Tartrate Injection (Batch no. h20143106) was manufactured by Jiangsu Hengrui Pharmaceutical Co., Ltd. (Nanjing, Jiangsu, China).
The present study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University, with approval no: PJ-KS-KY-2020-144 (X). A written informed consent was obtained from each participant prior to the initiation of the clinical trial. The clinical trial was registered with the Chinese Clinical Trial Registry (ChiCTR 2000034849, 21/07/2020). The present study was conducted in accordance with the guidelines of the Declaration of Helsinki.
The Propofol Medium and Long Chain Fat Emulsion Injection (Batch no. J20160089) was obtained from Beijing Fresenius Kabi Pharmaceutical Co. Ltd. (Beijing, China). The Butorphanol Tartrate Injection (Trade name: Nuoyang; 2 mL:4 mg; Batch no. h20143106) was manufactured by Jiangsu Hengrui Pharmaceutical Co., Ltd. (Nanjing, Jiangsu, China).
For the anesthesia, at 10 min before the colonoscopy, the patients intravenously received butorphanol at a dose of 5 μg/kg in group B1 and 10 μg/kg in group B2, and patients in group C received NS. Upon arrival in the examination room, an oxygen mask was placed on the patient at a flow rate of 5 L/min. Then, the patient’s routine electrocardiogram, heart rate (HR), non-invasive blood pressure and pulse oximetry (SpO2) were monitored during the procedure. A computer-controlled TCI pump (Guangxi Weili Fangzhou Technology, Guangxi, China) was initiated. Then, propofol TCI was administered with an initial plasma target concentration of 3.0 μg/mL for the first case in each group. This was used as the first dose of propofol. When the target concentration of propofol was achieved, the colonoscopy was performed. The administration of propofol TCI was stopped when the colonoscope reached the ileocecal region, and colonoscopic withdrawal began.
The primary aim of the present study was to determine the EC50 of propofol for TCI, which is necessary for half maximal effectiveness. The EC50 of propofol for TCI in each group was measured using the up-and-down sequential method, which has a widely used sequential design for studies on the EC50 of anesthesia. The initial plasma target concentration designated for each group was 3.0 μg/mL. Purposeful movements (e.g., the head or limbs) were defined as “responsive”, while no purposeful movements were defined as “non-responsive”. If “responsive” was found in a particular patient, the target plasma concentration of propofol was increased for the next patient by 0.5 μg/mL. In contrast, once “non-responsive” was identified in a particular patient, the target plasma concentration of propofol for the next patient was decreased by 0.5 μg/mL. The process was repeated until eight crossover points were attained. The number of patients needed for the present study could not be calculated beforehand. The target propofol concentration in plasma for consecutive patients in each group and the response to the procedures were determined and used to calculate the EC50 and 95% confidence interval (CI).
The secondary outcomes included the following measurements: (1) Amount of propofol used during the colonoscopic procedure; (2) Awakening concentration of propofol, which was referred to as the effect-site concentration of propofol in association with eye opening in response to verbal command; (3) Anesthesia-related AEs in perianesthesia, including hypoxemia, sinus bradycardia, hypotension, nausea and vomiting, and vertigo; and (4) Mean arterial pressure (MAP) and HR at different time points, including prior to anesthesia (T0), immediately after consciousness disappeared in response to the induction of anesthesia (T1), when the colonoscope reached the ileocecal region (T2), and when consciousness was regained (T3).
Statistical analysis was performed using the GraphPad Prism software (San Diego, CA, United States). Quantitative data were expressed as mean ± SD, or median and interquartile range (IQR), as appropriate. Analysis of variance or Kruskal-Wallis test, and post-hoc test with Bonferroni adjustment were performed to compare quantitative data. The numerical data were analyzed by χ2test, while K-test was used to compare the EC50 values of propofol for TCI among the three groups (group C, group B1 and group B2). The logarithm for each target concentration (logx), total number of cases (n), effective rate (P), and difference between the logarithm of two adjacent target concentrations (d) were calculated, as follows: (1) Logarithm of EC50: log EC50 = ΣnlogX/Σn; (2) Standard error of logEC50: SlogEC50 = d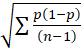 ; and (3) Logarithm of 95%CI of EC50: (logEC50 - 1.96 × Slog EC50, logEC50 + 1.96 × SlogEC50). EC50 and 95%CI were the negative logarithms. A P value of < 0.05 was considered statistically significant between groups.
; and (3) Logarithm of 95%CI of EC50: (logEC50 - 1.96 × Slog EC50, logEC50 + 1.96 × SlogEC50). EC50 and 95%CI were the negative logarithms. A P value of < 0.05 was considered statistically significant between groups.
A total of 106 patients who underwent sedated colonoscopy were enrolled for the present clinical study. The baseline demographic and clinical characteristics of patients in group C (n = 38), group B1 (n = 36), and group B2 (n = 32) are summarized in Table 1. The mean age of patients was 55.9 years old (SD = 4.3 years old) in group C, 56.9 years old (SD = 4.2 years old) in group B1, and 56.6 years old (SD = 4.8 years old) in group B2. There were no significant differences in baseline demographic characteristics among the groups (all, P > 0.05). The mean BMI was 21.8 kg/m2 (SD = 1.4 kg/m2) for patients in group C, 21.3 kg/m2 (SD = 1.4 kg/m2) for patients in group B1, and 21.2 kg/m2 (SD = 1.3 kg/m2) for patients in group B2. No significant difference was observed among the groups (all, P > 0.05). In addition, there was no significant difference in the proportion of patients with ASA I or II, based on the ASA PS Classification System and the operation time among groups (all, P > 0.05).
| Groups | n | Age (yr) | Gender (M/F) | BMI (kg/m2) | ASA (I/II) | Operation time (min) |
| Group C | 38 | 55.9 ± 4.3 | 18/20 | 21.8 ± 1.4 | 26/12 | 12 (10-14) |
| Group B1 | 36 | 56.9 ± 4.2 | 17/19 | 21.3 ± 1.4 | 24/12 | 11 (10-12) |
| Group B2 | 32 | 56.6 ± 4.8 | 15/17 | 21.2 ± 1.3 | 21/11 | 11 (10-12) |
| P value | 0.591 | 0.999 | 0.200 | 0.969 | 0.218 |
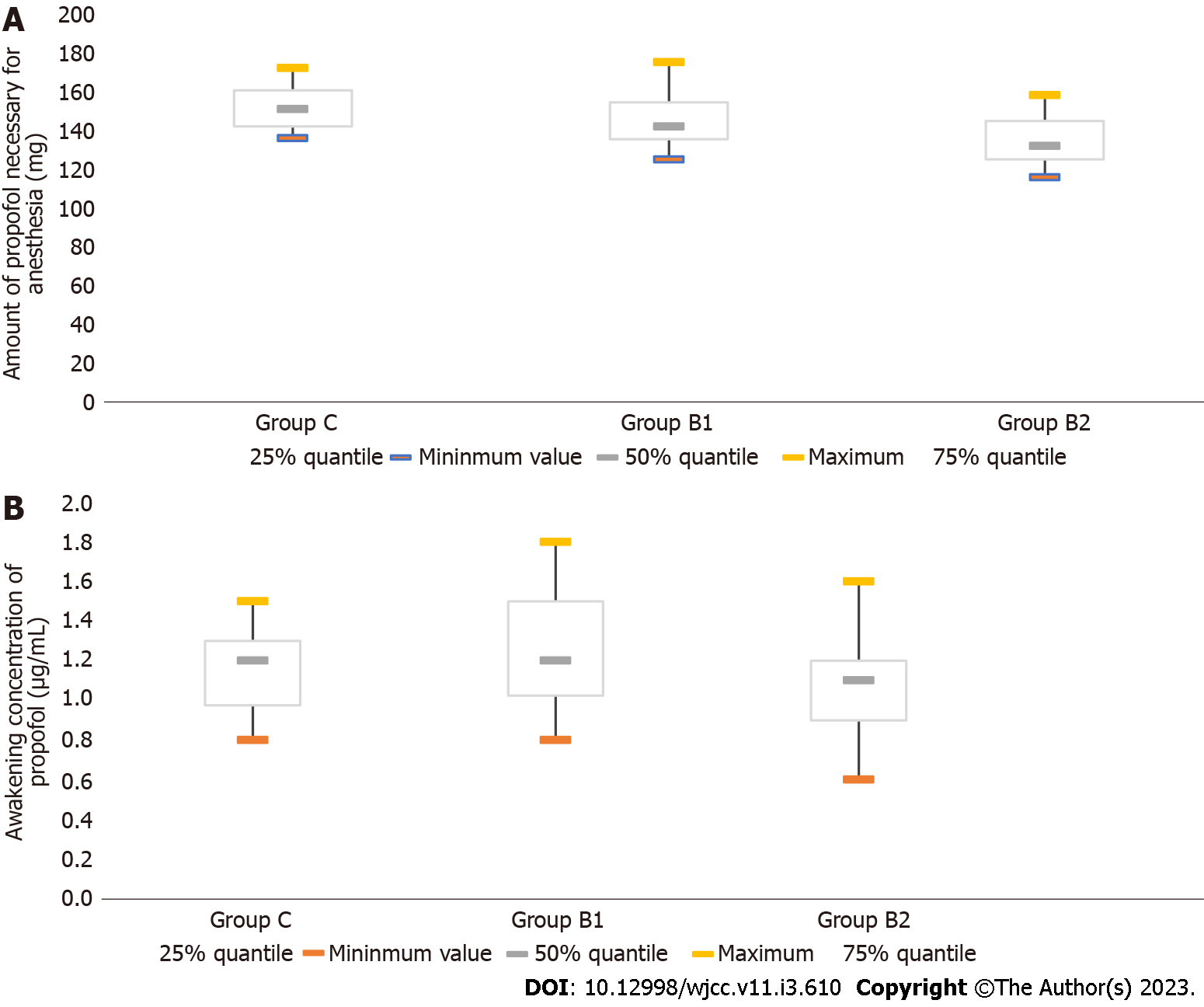
For the EC50 for propofol in TCI, the concentrations needed to achieve half maximal effectiveness were compared among the groups. The target propofol concentrations in the plasma of patients for the calculation of the EC50 are presented in Figure 1. As shown in Table 2, the EC50 for propofol in TCI was 4.05 μg/mL (95%CI: 3.78-4.34 μg/mL) in group C, 3.41 μg/mL (95%CI: 3.20-3.62 μg/mL) in group B1, and 3.03 μg/mL (95%CI: 2.83-3.23 μg/mL) in group B2. The statistical analysis revealed that the EC50 in group B2 was significantly lower, when compared to the EC50 in group B1 and group C (P < 0.05, Table 2). Furthermore, the amount of propofol necessary for anesthesia was determined as 132 mg (IQR, 125-144.75 mg) in group B2, which was significantly lower, when compared to the amount in group B1 (142 mg; IQR, 135-154 mg) (P < 0.05, Table 2, Figure 2). These results suggest that the intravenous infusion of 10 μg/kg of butorphanol reduced the need for high-dose propofol for anesthesia, when compared to 5 μg/kg of butorphanol. In addition, the awakening concentration of propofol was 1.1 μg /mL (IQR, 0.9-1.2 μg/mL) in group B2. This concentration was lower than that in group B1 (1.2 μg/mL; IQR, 1.025-1.500 μg/mL) (P < 0.05, Figure 2).
| Groups | n | EC50 (μg/mL) | 95%CI (μg/mL) | Amount of propofol necessary for anesthesia (mg) | Awakening concentration of propofol (μg/mL) |
| Group C | 38 | 4.05 | 3.78-4.34 | 151.0 (142.0-160.5) | 1.200 (0.975-1.300) |
| Group B1 | 36 | 3.411 | 3.20-3.62 | 142 (135-154)1,3 | 1.200 (1.025-1.500)3 |
| Group B2 | 32 | 3.031,2 | 2.83-3.23 | 132.00 (125.00-144.75)1,2 | 1.1 (0.9-1.2)2 |
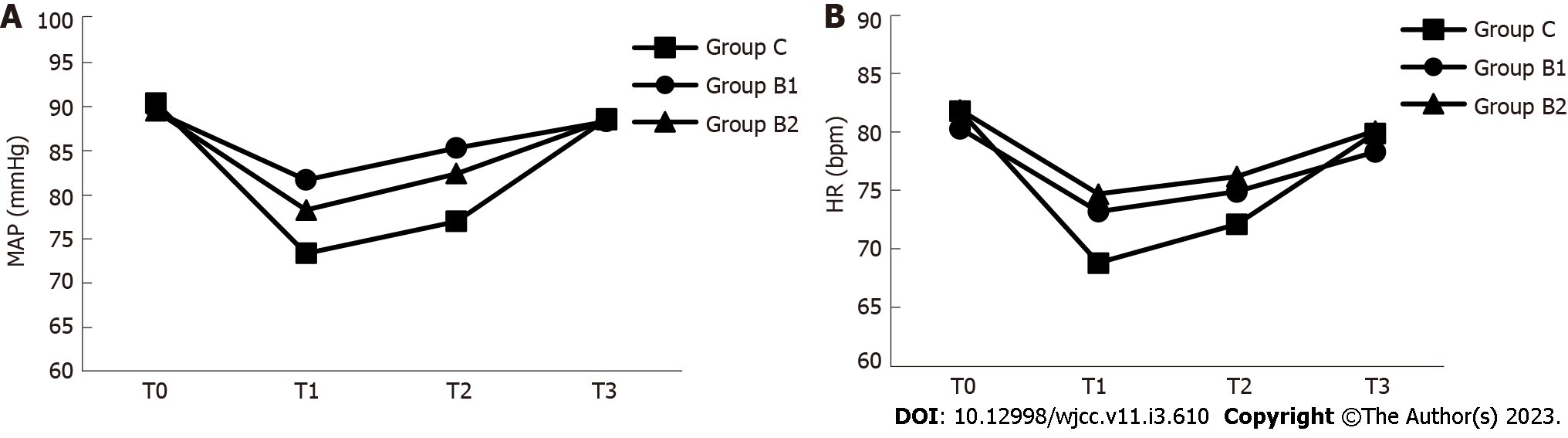
MAP and HR were compared at different time points (T0, T1, T2 and T3) during the procedure among the groups. As shown in Figure 3 and Table 3, the MAP values at time points T1 and T2 were significantly lower in group C, when compared to groups B1 and B2 (P < 0.05). In addition, the HR value at time point T1 was significantly lower in group C, when compared to groups B1 and B2 (P < 0.05).
| Groups | T0 | T1 | T2 | T3 | |
| MAP (mmHg) | Group C | 90.4 ± 6.1 | 73.4 ± 6.11 | 77.0 ± 5.81 | 88.6 ± 5.4 |
| Group B1 | 89.4 ± 5.4 | 81.7 ± 4.1 | 85.3 ± 4.0 | 88.3 ± 3.8 | |
| Group B2 | 89.5 ± 5.1 | 78.3 ± 5.02 | 82.4 ± 4.22 | 88.5 ± 4.1 | |
| HR (bpm) | Group C | 81.8 ± 8.8 | 68.8 ± 7.21 | 72.1 ± 6.9 | 79.9 ± 6.0 |
| Group B1 | 80.3 ± 9.0 | 73.2 ± 8.5 | 74.9 ± 8.0 | 78.3 ± 6.2 | |
| Group B2 | 81.9 ± 9.3 | 74.7 ± 9.8 | 76.2 ± 8.2 | 80.1 ± 6.1 |
Next, a comparison of AEs (e.g., hypoxemia, sinus bradycardia, hypotension, nausea and vomiting, and vertigo) in perianesthesia was performed among the groups, and the resulting data are summarized in Table 4. Overall, the incidence of each AE did not significantly differ among the groups (all, P > 0.05). Notably, the incidence of hypoxemia was higher in group C (13.2%), when compared to group B1 (5.6%) and group B2 (9.4%). In addition, one patient (2.6%) had hypotension in group C, while none of the patients had hypotension in groups B1 and B2. The reduction in anesthesia AEs, especially hypoxemia and hypotension, in groups B1 and B2 could have been attributed to the decrease in propofol required for anesthesia, when used in combination with butorphanol, during the colonoscopic procedure.
| Groups | n | Hypoxemia | Sinus bradycardia | Hypotension | Nausea, vomiting | Vertigo |
| Group C | 38 | 5 (13.2%) | 1 (2.6%) | 1 (2.6%) | 2 (5.3%) | 4 (10.5%) |
| Group B1 | 36 | 2 (5.6%) | 0 (0%) | 0 (0%) | 1 (2.8%) | 3 (8.3%) |
| Group B2 | 32 | 3 (9.4%) | 2 (6.3%) | 0 (0%) | 2 (6.3%) | 3 (9.4%) |
The efficacy and safety of propofol TCI co-administrated with different doses of butorphanol have not yet been evaluated for sedated colonoscopy in the Chinese population. To the best of our knowledge, the present study is the first controlled clinical trial that investigated the effects of different doses of butorphanol on the efficacy and safety of propofol TCI for sedated colonoscopy in Chinese patients. The main novel findings are summarized, as follows. Butorphanol co-administrated with propofol for TCI can significantly improve the efficacy of propofol TCI, as supported by the following evidence: (1) Patients with anesthesia induced by propofol TCI plus high-dose butorphanol had a significantly lower EC50 of propofol for TCI vs patients induced by propofol TCI plus low-dose butorphanol and controls (all, P < 0.05); and (2) The amount of propofol needed for anesthesia was significantly lower in group B2 vs group B1. In addition, the rates for anesthesia-related AEs, including hypoxemia, sinus bradycardia, hypotension, nausea and vomiting, and vertigo, were similar in group C, group B1 and group B2. It is noteworthy that patients in group C had a higher incidence of hypoxemia and hypotension, when compared to the other two groups. Furthermore, there is a possibility that the lower incidence of anesthesia AEs (hypoxemia and hypotension) is associated with the reduced amount of propofol due to the co-administration of butorphanol with propofol.
The primary aim of the present clinical trial was to determine the effects of the co-administration of butorphanol on the required dose of TCI propofol to achieve sedation during colonoscopic procedures. The TCI system can be programmed using either of the two main pharmacokinetic models: The Marsh model and Schnider model[13,14]. The Marsh model has weight as a model parameter, while the Schnider model has multiple parameters (e.g., age, weight, height, and lean body mass). Chen et al[27] examined the performance of the Marsh model and Schnider model for TCI propofol, and suggested that the Marsh model was overall superior to the Schnider model, and more suitable for TCI propofol[27]. Therefore, in the present study, the Marsh model was selected as the pharmacokinetic model to program the TCI system for propofol. It was found that the duration of action and recovery time of propofol were significantly shorter for the TCI of propofol, when compared to the conventional infusion of propofol[11,14,17,18,28]. In addition, the co-administration of propofol with other anesthetics has been shown to reduce the required dose of propofol for targeted anesthesia, and improve patient satisfaction during diagnostic and therapeutic endoscopy[11,19-24,29]. Considering the various advantages of butorphanol, which are mainly associated with less anesthesia-related AEs (e.g., respiratory depression, smooth muscle spasm, skin itches, urinary retention, physical and physiological dependence, nausea and vomiting)[25,26], the co-administration of butorphanol with propofol was examined in the present study. The up-and-down sequential method was used to determine the EC50, which is a commonly used measurement to evaluate the potency of a drug. The results revealed a significant reduction in the required propofol dose, when used in combination with both low-dose (5 μg/kg) and high-dose (10 μg/kg) butorphanol. Given that different anesthetics have various interactions, further studies are needed to determine the optimal combination of different anesthetics, such as propofol and butorphanol, and ensure the effectiveness and safety.
Despite these promising findings, the present study has several limitations. First, the baseline psychological state was not evaluated in the enrolled patients. Considering that preoperative anxiety has been shown to affect the dose of propofol required to achieve anesthesia induction, patients presenting with anxiety may need a higher dose of propofol. Second, the depth of sedation during the colonoscopic procedure was not monitored. Third, although the present study revealed that the butorphanol co-administration with propofol for TCI improved the efficacy without increasing the incidence of anesthesia-related complications, the level of patient satisfaction was not examined. Although propofol and butorphanol exhibited “synergic effects”, the interaction between these two anesthetics should be further assessed, in order to ensure the potency and safety of this anesthesia for patients. Future studies with a larger sample size are presently being performed in our center to gain more insight into the combination of propofol TCI and butorphanol for sedation during colposcopy.
Overall, the present clinical study revealed that butorphanol co-administrated with propofol TCI can reduce the EC50 of propofol for anesthesia without causing additional anesthesia-related complications in patients undergoing sedated colonoscopy. These findings may assist clinicians in performing anesthesia induction for colonoscopy, eventually helping to improve the care for patients who must receive colonoscopic procedures for colorectal diseases.
Propofol, known as an effective anesthetic with rapid onset and short recovery, has been used in sedated colonoscopy. However, when used alone, high-dose propofol is usually needed for anesthesia, and this may cause anesthesia-related adverse events (AEs).
This clinical study was intended to reduce the required dose of propofol by co-administration with butorphanol in patients receiving colonoscopy under sedation.
The objective of this clinical trial was to assess the efficacy and safety of propofol target-controlled infusion (TCI) in combination with butorphanol for sedation during colonoscopy.
One hundred six patients were assigned into three groups to receive different doses of butorphanol before propofol TCI: Low-dose butorphanol group (B1), high-dose butorphanol group (B2), and control group (C). The primary outcome included the median effective concentration (EC50) of propofol TCI, and the secondary outcomes were AEs in perianesthesia and recovery characteristics.
The EC50 of propofol for TCI was 3.03 μg/mL in group B2, 3.41 μg/ in group B1, and 4.05 μg/mL in group C. The amount of propofol necessary for anesthesia was 132 mg in group B2, lower than 142 mg in group B1. Notably, the propofol TCI plus butorphanol groups (groups B1 and B2) had a lower incidence of anesthesia AEs, when compared to group C. Moreover, there were no significant differences among the three groups in the rates of AEs in perianesthesia, including hypoxemia, sinus bradycardia, hypotension, nausea and vomiting, and vertigo.
This study has shown that propofol in combination with butorphanol reduces the EC50 of propofol TCI for anesthesia in patients undergoing sedated colonoscopy, and in turn may decrease anesthesia-related AEs in patients undergoing sedated colonoscopy.
Propofol in combination with butorphanol may improve care for patients undergoing colonoscopic procedures for colorectal diseases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Musa Y, Nigeria; Ubiali MLC, Brazil S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 2. | Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, Anderson RN, Henley SJ, Kohler BA, Penberthy L, Feuer EJ, Weir HK. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 1110] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 3. | Yang C, Sriranjan V, Abou-Setta AM, Poluha W, Walker JR, Singh H. Anxiety Associated with Colonoscopy and Flexible Sigmoidoscopy: A Systematic Review. Am J Gastroenterol. 2018;113:1810-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Ferreira AO, Cravo M. Sedation in gastrointestinal endoscopy: Where are we at in 2014? World J Gastrointest Endosc. 2015;7:102-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Moon SH. Sedation regimens for gastrointestinal endoscopy. Clin Endosc. 2014;47:135-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Schilling D. Propofol-based sedation in gastrointestinal endoscopy: getting safer and safer. Digestion. 2014;89:272-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 345] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 8. | Padmanabhan A, Frangopoulos C, Shaffer LET. Patient Satisfaction With Propofol for Outpatient Colonoscopy: A Prospective, Randomized, Double-Blind Study. Dis Colon Rectum. 2017;60:1102-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Leslie K, Allen ML, Hessian EC, Peyton PJ, Kasza J, Courtney A, Dhar PA, Briedis J, Lee S, Beeton AR, Sayakkarage D, Palanivel S, Taylor JK, Haughton AJ, O'Kane CX. Safety of sedation for gastrointestinal endoscopy in a group of university-affiliated hospitals: a prospective cohort study. Br J Anaesth. 2017;118:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Li XT, Ma CQ, Qi SH, Zhang LM. Combination of propofol and dezocine to improve safety and efficacy of anesthesia for gastroscopy and colonoscopy in adults: A randomized, double-blind, controlled trial. World J Clin Cases. 2019;7:3237-3246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Zhang K, Xu H, Li HT. Safety and efficacy of propofol alone or in combination with other agents for sedation of patients undergoing colonoscopy: an updated meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24:4506-4518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Lundström S, Twycross R, Mihalyo M, Wilcock A. Propofol. J Pain Symptom Manage. 2010;40:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Struys MM, De Smet T, Glen JI, Vereecke HE, Absalom AR, Schnider TW. The History of Target-Controlled Infusion. Anesth Analg. 2016;122:56-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Mu JJ, Jiang T, Deng LP, Choi SW, Irwin MG, Yuen VM. A comparison of two techniques for induction of anaesthesia with target-controlled infusion of propofol. Anaesthesia. 2018;73:1507-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Liu N, Rinehart J. Closed-Loop Propofol Administration: Routine Care or a Research Tool? Anesth Analg. 2016;122:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Swinhoe CF, Peacock JE, Glen JB, Reilly CS. Evaluation of the predictive performance of a 'Diprifusor' TCI system. Anaesthesia. 1998;53 Suppl 1:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Moerman AT, Herregods LL, De Vos MM, Mortier EP, Struys MM. Manual versus target-controlled infusion remifentanil administration in spontaneously breathing patients. Anesth Analg. 2009;108:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Müller T, Ludwig A, Biro P. Two distinct application habits for propofol: an observational study. Eur J Anaesthesiol. 2010;27:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Zhou X, Li BX, Chen LM, Tao J, Zhang S, Ji M, Wu MC, Chen M, Zhang YH, Gan GS, Song XY. Etomidate plus propofol versus propofol alone for sedation during gastroscopy: a randomized prospective clinical trial. Surg Endosc. 2016;30:5108-5116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Haytural C, Aydınlı B, Demir B, Bozkurt E, Parlak E, Dişibeyaz S, Saraç A, Özgök A, Kazancı D. Comparison of Propofol, Propofol-Remifentanil, and Propofol-Fentanyl Administrations with Each Other Used for the Sedation of Patients to Undergo ERCP. Biomed Res Int. 2015;2015:465465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Lonardo NW, Mone MC, Nirula R, Kimball EJ, Ludwig K, Zhou X, Sauer BC, Nechodom K, Teng C, Barton RG. Propofol is associated with favorable outcomes compared with benzodiazepines in ventilated intensive care unit patients. Am J Respir Crit Care Med. 2014;189:1383-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | van den Berg JP, Vereecke HE, Proost JH, Eleveld DJ, Wietasch JK, Absalom AR, Struys MM. Pharmacokinetic and pharmacodynamic interactions in anaesthesia. A review of current knowledge and how it can be used to optimize anaesthetic drug administration. Br J Anaesth. 2017;118:44-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Smith C, McEwan AI, Jhaveri R, Wilkinson M, Goodman D, Smith LR, Canada AT, Glass PS. The interaction of fentanyl on the Cp50 of propofol for loss of consciousness and skin incision. Anesthesiology. 1994;81:820-8; discussion 26A. [PubMed] |
| 24. | Bouillon TW, Bruhn J, Radulescu L, Andresen C, Shafer TJ, Cohane C, Shafer SL. Pharmacodynamic interaction between propofol and remifentanil regarding hypnosis, tolerance of laryngoscopy, bispectral index, and electroencephalographic approximate entropy. Anesthesiology. 2004;100:1353-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 25. | Agarwal A, Raza M, Dhiraaj S, Pandey R, Gupta D, Pandey CK, Singh PK, Singh U. Pain during injection of propofol: the effect of prior administration of butorphanol. Anesth Analg. 2004;99:117-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Zhu X, Chen L, Zheng S, Pan L. Comparison of ED95 of Butorphanol and Sufentanil for gastrointestinal endoscopy sedation: a randomized controlled trial. BMC Anesthesiol. 2020;20:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Chen SL, Lin WW, Wang CL, Lin CL. Comparison of accuracy of Marsh model versus Schnider model for propofol target-controlled infusion system. Chin J Anesthesiol. 2015;35:1466-1469. [DOI] [Full Text] |
| 28. | Xu Z, Liu F, Yue Y, Ye T, Zhang B, Zuo M, Xu M, Hao R, Xu Y, Yang N, Che X. C50 for propofol-remifentanil target-controlled infusion and bispectral index at loss of consciousness and response to painful stimulus in Chinese patients: a multicenter clinical trial. Anesth Analg. 2009;108:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Yoon SW, Choi GJ, Lee OH, Yoon IJ, Kang H, Baek CW, Jung YH, Woo YC. Comparison of propofol monotherapy and propofol combination therapy for sedation during gastrointestinal endoscopy: A systematic review and meta-analysis. Dig Endosc. 2018;30:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |