Published online Jan 26, 2023. doi: 10.12998/wjcc.v11.i3.566
Peer-review started: September 6, 2022
First decision: October 11, 2022
Revised: October 14, 2022
Accepted: December 15, 2022
Article in press: December 15, 2022
Published online: January 26, 2023
Processing time: 142 Days and 10.9 Hours
The recognition of idiopathic membranous nephropathy (IMN) as an autoimmune disease has paved the way for the use of B-cell-depleting agents, such as Rituximab (RTX), which is now a first-line drug for treating IMN with proven safety and efficacy. Nevertheless, the usage of RTX for the treatment of refractory IMN remains controversial and challenging.
To evaluate the efficacy and safety of a new low-dose RTX regimen for the treatment of patients with refractory IMN.
A retrospective study was performed on refractory IMN patients that accepted a low-dose RTX regimen (RTX, 200 mg, once a month for five months) in the Xiyuan Hospital of Chinese Academy of Chinese Medical Sciences’ Department of Nephrology from October 2019 to December 2021. To assess the clinical and immune remission data, we performed a 24 h urinary protein quantification (UTP) test and measured the serum albumin (ALB) and serum creatinine (SCr) levels, phospholipase A2 receptor (PLA2R) antibody titer, and CD19+ B-cell count every three months.
A total of nine refractory IMN patients were analyzed. During follow-up conducted twelve months later, the results from the 24 h UTP decreased from baseline [8.14 ± 6.05 g/d to 1.24 ± 1.34 g/d (P < 0.05)] and the ALB levels increased from baseline [28.06 ± 8.42 g/L to 40.93 ± 5.85 g/L (P < 0.01)]. Notably, after administering RTX for six months, the SCr decreased from 78.13 ± 16.49 μmol/L to 109.67 ± 40.87 μmol/L (P < 0.05). All of the nine patients were positive for serum anti-PLA2R at the beginning, and four patients had normal anti-PLA2R titer levels at six months. The level of CD19+ B-cells decreased to 0 at three months, and CD19+ B-cell count remained at 0 up until six months of follow-up.
Our low-dose RTX regimen appears to be a promising treatment strategy for refractory IMN.
Core Tip: According to the Kidney Disease Improving Global Outcomes 2021 guidelines, Rituximab (RTX) is now the first-line therapy for patients with idiopathic membranous nephropathy (IMN). However, the use of RTX for the treatment of patients with refractory IMN remains challenging. We conducted a retrospective study on nine patients with refractory IMN to explore the efficacy and safety of a new low-dose RTX regimen (RTX, 200 mg, once a month for five months), and conclude that our low-dose RTX regimen is a promising treatment strategy for refractory IMN.
- Citation: Wang YW, Wang XH, Wang HX, Yu RH. Successful treatment of patients with refractory idiopathic membranous nephropathy with low-dose Rituximab: A single-center experience. World J Clin Cases 2023; 11(3): 566-575
- URL: https://www.wjgnet.com/2307-8960/full/v11/i3/566.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i3.566
Idiopathic membranous nephropathy (IMN) is a common pathological type of glomerular disease, and its incidence rate has been continuously increasing yearly[1]. In the past 10 years, the proportion of adult patients with IMN with renal puncture has risen from 12.2%–24.9%, ranking second among those with primary glomerular diseases[2]. Currently, IMN is considered to be an autoimmune disease[3]. The M-type phospholipase A2 receptor (PLA2R) on the cell surface of podocytes is the major target antigen in IMN and can be found in 70%–80% of IMN patients[4]. Additionally, new antigens. Such as thrombospondin type-1 domain-containing 7A, neural epidermal growth factor-like 1 protein, and semaphorin 3b have also been recently discovered[5,6], further rationalizing the use of B-cell-depleting agents.
Rituximab (RTX) is an anti-CD20 monoclonal antibody that is used to treat several autoimmune disorders[7]. Since 2004, some reports have explored the use of RTX in patients with refractory nephrotic syndrome, and the preliminary results showed that RTX may lead to remission and reduce immunosuppressive drug use[8,9]. According to the Kidney Disease Improving Global Outcomes 2021 guidelines, RTX is now the first-line therapy for patients with IMN, and the remission rate can reach 60%–80% at 12 mo[10]. However, 35%–40% of IMN patients still show no response to RTX. Refractory IMN is characterized by recurrence or resistance to RTX-based and other traditional immunosuppressive therapies, including prednisone (Pre), cyclophosphamide (CTX), and calcineurin inhibitors (CNIs)[11]. In Asia, 5%–14% of refractory patients progress to end-stage renal disease (ESRD) within 10–15 years[12]. Therefore, new therapies are urgently needed to treat IMN.
When introduced to IMN treatment, standard doses for RTX (375 mg/m2 every week for four weeks or 1 g fixed-dose with a repeat dose in two weeks) were drawn from the preexisting dosages for treating other autoimmune diseases, such as anti-neutrophil cytoplasmic antibody-associated vasculitis, rheumatoid arthritis (RA), autoimmune cytopenia, and focal segmental glomerulosclerosis[13]. Recently, few case series studies have proven the effectiveness of low-dose RTX regimens with repeated injections in treating patients with refractory IMN and showed that low-dose RTX could effectively improve the remission rate and peripheral blood B-cell elimination[14]. Currently, for the treatment of RA, a low-dose RTX regimen (500 mg twice daily) has replaced the original dose (1000 mg twice daily) and become the new standard. Kurosu et al[15] reported a case of steroid-resistant Nephrotic syndrome, where the kidney pathology showed minimal changes in glomerulopathy. The patient achieved complete remission (CR) with a single dose of RTX of 375 mg/m2. Wang et al[16] reported the case of a 51-year-old man diagnosed with refractory IMN. The patient received a single dose of 100 mg RTX, after which the B-cells declined rapidly, and a gradual reduction in proteinuria was also observed. Katsuno et al[17] reported three patients with refractory IMN who were treated with single-dose RTX (500–600 mg) where two of the patients achieved complete and incomplete remission.
Based on previous studies, we evaluated the efficacy and safety of low-dose RTX in patients with refractory IMN. The regimen included 200 mg RTX once a month for five months. Compared to traditional regimens, our regimen appears to be a promising treatment strategy for refractory IMN.
This was a retrospective case series study that included patients with refractory IMN who were admitted at the Xiyuan Hospital of the Chinese Academy of Chinese Medical Science Department of Nephrology from October 2019 to December 2021 (n = 9).
The inclusion criteria were as follows: (1) Patients with histologically proven IMN and a PLA2R antibody-positive status (Anti-PLA2R titer > 20 RU/mL); (2) Patients diagnosed with refractory IMN, broadly defined, who still presented with the nephrotic syndrome after six months of regular corticosteroid and immunosuppressive therapies, including those with Pre, CTX, cyclosporine, tacrolimus, mycophenolate mofetil, and RTX[10]; (3) Patients who signed the informed consent form for the low-dose RTX regimen and fully understood the risks of treatment; and (4) Patients with a follow-up period of > 1 year with an interval of < three months or > four visits between visits per year. The patients had complete clinical data, including routine blood test results, liver and kidney function test results, 24 h urinary protein quantitation (UTP) test results, serum anti-PLA2R antibody titer, and CD19+ B-cell counts. The exclusion criteria were as follows: (1) Presence of secondary membranous nephropathy, such as lupus nephritis, hepatitis B virus, hepatitis C, and tumors; (2) Glomerular diseases combined with other types; (3) Severe infections; and (4) Allergic reactions constitution.
The low-dose RTX regimen was administered with a dose of 200 mg once a month for five months. Notably, after the patients were treated with RTX, the dosage of the primary therapeutic immunosuppressant was gradually decreased in all patients. If the patient’s blood pressure remained > 130/80 mmHg, an appropriate angiotensin receptor blocker was added to the regime to stabilize blood pressure.
Every three months, clinical data such as 24 h UTP, serum ALB and serum creatinine (SCr) levels, PLA2R antibody titer, and CD19+ B-cell count were evaluated.
The primary outcome was complete clinical remission. A composite remission was defined either as a complete response (CR) or partial response (PR). Proteinuria of < 0.3 g/d is defined as CR, while PR is defined as a 24 h UTP of 0.3 g-3.5 g/d, or 24 h UTP decreased by more than 50% compared to baseline. No reaction was defined as a decrease in proteinuria of less than 30% or renal function deterioration.
The secondary outcomes were 24 h UTP, ALB and SCr levels, Anti-PLA2R titer, CD19+ B-cell count, adverse events, and a composite endpoint of 40% reduction in estimated glomerular filtration rate, doubling of SCr, ESRD, and death.
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, N.Y., United States). Data is presented as the mean ± SD unless otherwise indicated. The comparison of each indicator before and after treatment was performed using a paired sample t-test, and unsatisfactory results were expressed as the median. The paired rank-sum test was used to compare before and after the treatment. Qualitative data was expressed as percentages (%). Comparisons between the groups were performed using the chi-square test, and the test level was set to 0.05.
Nine patients with refractory IMN were treated with low-dose RTX at our center between October 2019 and December 2021. The baseline features of the patients are listed in Table 1. There were five men and four women, with an average age of 44.0 ± 11.7 years. Of these, six patients were diagnosed with IMN by kidney biopsy, and the other three patients were diagnosed with a serum anti-PLA2R titer > 100 RU/mL. Furthermore, all nine (100%) patients tested positive for serum anti-PLA2R at the beginning of the trial. Before the administration of RTX, all nine patients had received regular corticosteroid and immunosuppressive therapy for at least six months prior and still presented with the nephrotic syndrome; therefore, they were diagnosed with refractory IMN. Of these, six patients were steroid-resistant and three patients were steroid-dependent.
| Patient No. | Age (yr) | Sex | PLA2R | Duration (mo) | Previous treatment | Effect | Side effect | Current treatment |
| 1 | 57 | M | + | 12 | (1) RTX 500 mg iv twice in two months; and (2) Pre 40 mg/d + CSA 150 mg/d | SR | Serum glucose up | Pre + TAC |
| 2 | 28 | M | + | 54 | (1) Pre 20 mg/d + TAC 2 mg/d; and (2) Pre 24 mg/d + TAC 2 mg/d | SR | Cushing’s syndrome | Pre + TAC |
| 3 | 40 | M | + | 21 | (1) CSA 150 mg/d; (2) Pre 20 mg/d + CTX 100 mg/d; and (3) Pre 30 mg + TAC 2 mg/d | SR | Hypertension | Pre + TAC |
| 4 | 60 | F | + | 51 | (1) TAC 2 mg/d; and (2) Pre 20 mg + CSA 150 mg/d | SR | Steroid-induced diabetes | Non |
| 5 | 31 | M | + | 9 | (1) CsA 150 mg/d; (2) Pre 48 mg/d + TAC 2 mg/d; and (3) Pre 48 mg/d + CTX 100 mg/d | SD | Steroid-induced diabetes; liver damage; Hypertension | Pre + TAC |
| 6 | 29 | F | + | 31 | (1) Pre 15 mg/d + CSA 150 mg/d; (2) Pre 40 mg/d + LEF 30 mg/d; and (3) Pre 25 mg/d + TAC 2 mg/d | SD | Cushing’s syndrome; Hypertension | Pre |
| 7 | 49 | M | + | 44 | (1) Pre 40 mg/d + CSA 150 mg/d; and (2) Pre 24 mg/d + TAC 2 mg/d | SD | Cushing’s syndrome; Steroid-induced diabetes | Pre + TAC |
| 8 | 49 | F | + | 35 | (1) Pre 50 mg/d + CTX 1000 mg iv; (2) Pre 30 mg/d + CSA 150 mg/d; and (3) TAC 3 mg/d | SR | Cushing’s syndrome; Liver damage; Steroid-induced diabetes | Non |
| 9 | 36 | F | + | 18 | (1) Pre 24 mg/d + CSA 100 mg/d; and (2) TAC 2 mg/d | SR | Non | Non |
Furthermore, at baseline, all nine patients experienced adverse reactions triggered by the above treatment, including two with elevated serum glucose levels, one with hypertension, one with Cushing syndrome; one combined with steroid-induced diabetes, hypertension, and an abnormal liver function; one combined with hypertension and Cushing syndrome; one combined with steroid-induced diabetes and Cushing syndrome; and one combined with steroid-induced diabetes, an abnormal liver function, and an abnormal liver function.
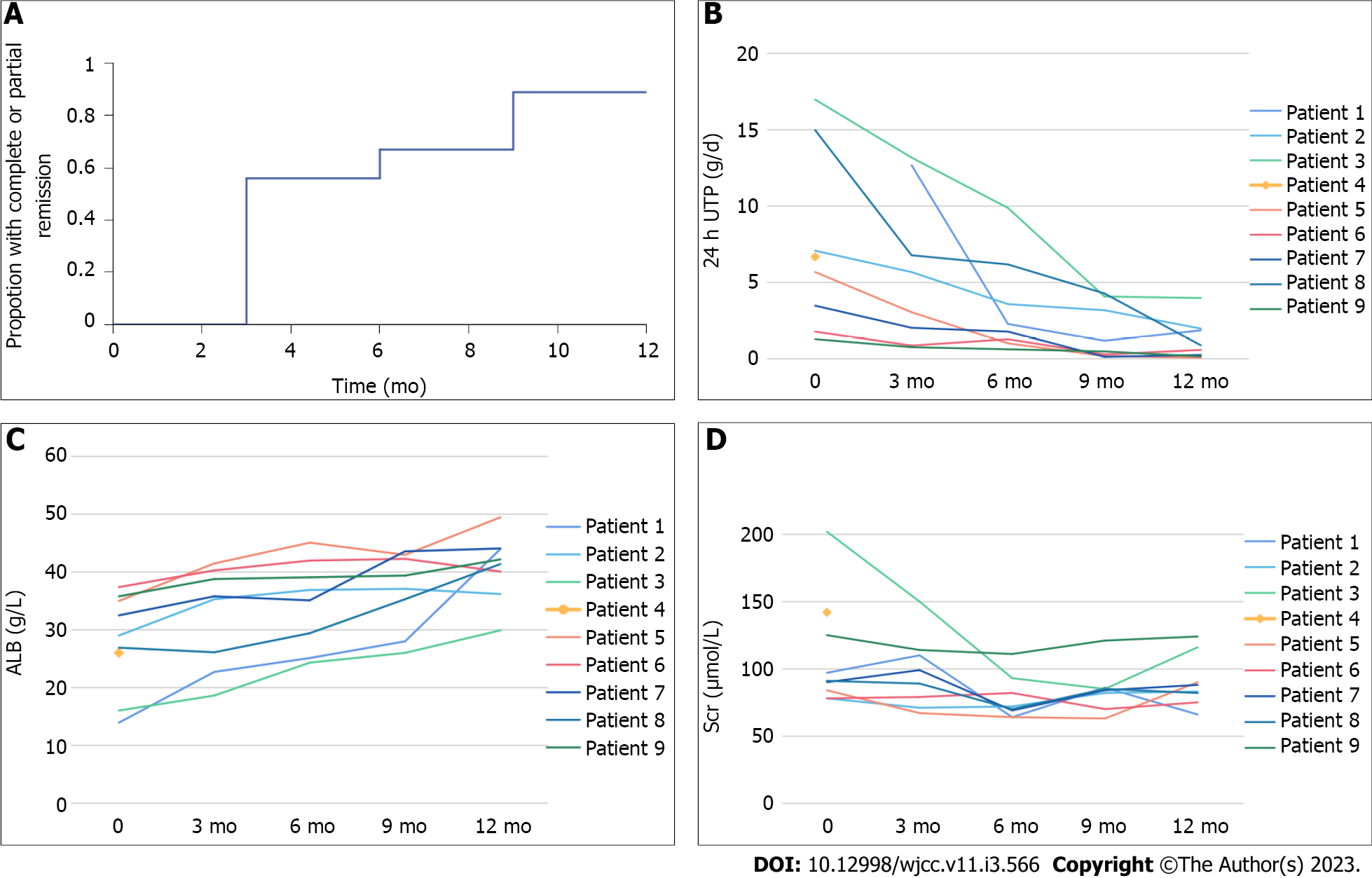
The clinical outcomes of the nine patients during the twelve months of follow-up are listed in Table 2 and Supplementary Table 1. The proportion of patients who achieved CR or PR over time, as well as the trends of the remission rate, 24 h UTP, ALB, and SCr levels are shown in Figure 1. The PR and CR rates gradually increased with a lengthened treatment time. The remission rates were 56% (5/9; PR 5) at three months, 67% at six months (6/9; PR 6), 89% at nine and twelve months (8/9; CR 3 and PR 5). One patient did not complete the course of treatment due to adverse infusion reactions.
| Patient No. | Before administering of RTX (0 mo) | After administering of RTX (6 mo) | Follow-up (12 mo) | ||||||||
| 24 h UTP (g/d) | ALB (g/L) | Scr (μmol/L) | 24 h UTP (g/d) | ALB (g/L) | Scr (μmol/L) | Remission | 24 h UTP (g/d) | ALB (g/L) | Scr (μmol/L) | Remission | |
| 1 | 15.2 | 13.9 | 97 | 2.3 | 25.1 | 64 | PR | 1.88 | 44 | 66 | PR |
| 2 | 7.1 | 29 | 78 | 3.6 | 36.9 | 72 | NR | 2 | 36.2 | 83 | PR |
| 3 | 17 | 16 | 202 | 9.9 | 24.3 | 93 | NR | 4 | 29.9 | 116 | PR |
| 4 | 6.7 | 26 | 142 | - | - | - | - | - | - | - | - |
| 5 | 5.7 | 35 | 84 | 1.03 | 45.1 | 64 | PR | 0.09 | 49.5 | 90 | CR |
| 6 | 1.8 | 37.4 | 78 | 1.3 | 42 | 82 | PR | 0.6 | 40.1 | 75 | PR |
| 7 | 3.5 | 32.5 | 90 | 1.8 | 35.1 | 69 | PR | 0.27 | 44.1 | 88 | CR |
| 8 | 15 | 26.9 | 91 | 6.2 | 29.4 | 70 | PR | 0.9 | 41.4 | 82 | PR |
| 9 | 1.3 | 35.8 | 125 | 0.64 | 39.1 | 111 | PR | 0.175 | 42.2 | 124 | CR |
The comparison of the clinical outcomes at twelve months of follow-up is shown in Table 3. In the eight patients treated with a low dose of RTX for six months, the 24 h UTP and ALB levels showed no significant changes when compared with those before treatment. Nine months later, the 24 h UTP decreased from 8.14 ± 6.05 g/d to 1.74 ± 1.81 g/d (P < 0.05), and the ALB level improved from 28.06 ± 8.42 g/L to 36.84 ± 6.74 g/L (P < 0.05). At the end of the twelve-month follow-up, 24 h UTP decreased to 1.24 ± 1.34, and ALB improved to 40.93 ± 5.85 g/L, which are significant differences compared with those before treatment (P < 0.05 and P < 0.01, respectively). This means that the longer the follow-up period, the higher the remission rate. Notably, after administering RTX, there was a significant difference in the SCr levels when compared with those before treatment (P < 0.05).
| Outcome | 0 (mo) | 3 (mo) | 6 (mo) | 9 (mo) | 12 (mo) |
| 24 h UTP (g/d) | 8.14 ± 6.05 | 5.65 ± 4.99 | 3.35 ± 3.20 | 1.74 ± 1.81a | 1.24 ± 1.34a |
| ALB (g/L) | 28.06 ± 8.42 | 32.39 ± 8.7 | 34.63 ± 7.69 | 36.84 ± 6.74a | 40.93 ± 5.85b,c |
| Scr (μmol/L) | 109.67 ± 40.87 | 97.38 ± 27.34 | 78.13 ± 16.49a | 84.5 ± 16.96 | 90.5 ± 19.81 |
| Remission rate (%) | — | 56% (5/9) | 67% (6/9) | 89% (8/9) | 89% (8/9) |
We used serum PLA2R antibodies and CD19+ B-cells to assess immune remission. As shown in Table 4, all nine patients had elevated serum PLA2R antibody titers at the beginning of the trial. Concerning the anti-PLA2R titers before RTX administration, three patients had low titers of anti-PLA2R (≤ 50 IU/L), two patients had medium titers of anti-PLA2R (50–150 IU/L), and four patients had a high titer of anti-PLA2R (> 150 IU/L). Of them, eight patients were followed up for six months, and the titers of anti-PLA2R decreased after administering RTX. Furthermore, at the three-month follow-up, one patient (No. 6) tested negative for the PLA2R antibody; at the six-month follow-up, four patients (No. 1, 6, 8, and 9) were negative for the PLA2R antibody. The level of CD19+ B-cells decreased to 0 at three months, and remained the same until six months of follow-up.
| Patient No. | Before administering of RTX (0 mo) | Administering of RTX (3 mo) | After administering of RTX (6 mo) | |||
| Anti-PLA2R titer (RU/mL) | CD19+ B (pcs/μL) | PLA2R antibody (RU/mL) | CD19+ B (pcs/μL) | PLA2R antibody (RU/mL) | CD19+ B (pcs/μL) | |
| 1 | 1400 | 418 | 104 | 0 | Negative | 0 |
| 2 | 180 | 6 | 96 | 0 | 82 | 0 |
| 3 | 215 | 169 | 67 | 0 | 48 | 0 |
| 4 | 500 | 422 | - | - | - | - |
| 5 | 32 | 114 | 27 | 0 | 20 | 0 |
| 6 | 45 | 28 | Negative | 0 | Negative | 0 |
| 7 | 52 | 460 | 22 | 0 | 17 | 0 |
| 8 | 130 | 192 | 38 | 0 | Negative | 0 |
| 9 | 36 | 258 | 21 | 0 | Negative | 0 |
During the median (8.7 ± 3.7 mo) period of follow-up, among the nine patients who experienced adverse reactions, one patient presented with shivering. Shivering occurred during the first infusion of RTX, but no dyspnea or fever occurred simultaneously. Symptoms were relieved 10 min after terminating the RTX treatment, and no RTX was infused afterwards; the patient did not complete the treatment. Two patients had a fever, which occurred within 24 h of RTX infusion. The body temperature was 37.5–38.5 °C, but returned to normal within 48 h without administering antibiotics. The fever did not recur.
IMN is the most common cause of primary nephrotic syndrome in adults. The prognosis of patients with IMN varies greatly, with around 1/3 of the patients progressing to ESRD within 10–15 years, and another 1/3 being relieved of symptoms[3]. To date, alkylating agents are the only drugs with a proven efficacy in preventing the development of ESRD[18]. Therefore, corticosteroid therapy combined with alkylating agents has been recognized as the treatment of choice for decades. Other immunosuppressive agents[19], such as CNIs, have been tested only in trials using proteinuria reduction as an alternative endpoint.
Although an effective immunosuppressive treatment scheme for IMN has been established clinically, 20%–30% of IMN patients are resistant to standard immunosuppressive therapy or often relapse[20]; these patients were diagnosed with refractory IMN. In Asia, 5%–14% of refractory IMN patients will progress to ESRD. Therefore, effective and safe therapeutic strategies for refractory IMN must be explored. In the past decade, significant advances in understanding of IMN have established that it is an autoantibody-driven disease[21,22]. As a result, there is a clear choice for treating B-cell depletion. Currently, RTX is the most important immunosuppressive treatment for IMN. The results of the MENTOR trial provide the basis for RTX being the first-line treatment in patients with IMN[23].
Although the results of RTX are promising, it should be noted that approximately 30%–40% of cases experience treatment failure, which means that other treatment regimens are needed. Currently, some studies have focused on the paradigm shift from RTX to new alternatives or combined drugs in treating patients with refractory IMN to overcome drug resistance. However, the second course of RTX may achieve remission even in the setting of resistant RTX after the first course[24].
The natural course of IMN varies greatly; thus, it is not suitable for all patients to receive a unified treatment and the optimal RTX dose for the treatment of IMN remains controversial. Different RTX application regimens were used across various studies, ranging from a single dose of 375 mg/m2 to a repeated dose of 375 mg/m2 for four weeks after six months. A prospective clinical study evaluated the low-dose RTX regimen (375 mg/m2, once) compared with the standard RTX regimen (375 mg/m2, four times)[25]. If the consumption of B-cells is insufficient, the same dose can be used in the low-dose RTX group. The results showed that among the twelve patients who were administered with low-dose RTX, only one needed a second dose to achieve complete B-cell depletion, and at 1 year, the remission rate of the two groups was the same. They also concluded that a single-dose RTX regimen was extremely cost-effective and may limit the production of antichimeric antibodies, lowering the risk of adverse reactions. Similarly, another retrospective study compared forty-two patients administered low-dose RTX (375 mg/m2)[26]. The control group was treated with steroid hormones combined with alkylating agents or a standard RTX regimen (375 mg/m2, four times). At twenty-four months, there was no significant difference in clinical outcomes between the two groups. All patients treated with RTX showed complete B-cell depletion in the first month, but B-cell recovery occurred earlier in the low-dose group than in the standard group. Recently, some case reports and case series studies have proven the effectiveness of low dose but RTX regimens with repeated injections in treating patients with refractory IMN. These regimens seem to extend the inhibition of B-cells. We explored a new RTX regimen (200 mg once a month for five months) for treating patients with refractory IMN in our hospital.
Nine patients with refractory PLAR-associated MN were included in this analysis. In the data provided twelve months after baseline, the remission rate was 56% (5/9; PR 5) at three months, 67% at six months (6/9; PR 6), and 89% at nine and twelve months (8/9; CR 3 and PR 5). Another patient did not complete the treatment because of infusion reactions. In a review of currently available studies, the overall remission rates (complete and partial) of RTX at twelve months of treatment for patients with IMN consistently ranged from 44%–85%. Few studies have reported that among patients with refractory IMN, the efficacy rate of RTX can only reach 40%. The remission rate of the low-dose RTX regimen at our center was significantly higher than that reported in previous studies.
In addition, we observed that clinical remission in patients with refractory IMN was slower than in patients with immune remission. We used serum PLA2R antibody titer and CD19+ B-cell counts to assess immune remission. The level of CD19+ B-cells decreased to 0 at three months, which was maintained until six months of follow-up. At the beginning of the trial, all nine patients were positive for the PLA2R antibody. The titers of anti-PLA2R decreased after administering low-dose RTX, and one patient (No. 6) had a normal anti-PLA2R titer after three months, while four patients (No. 1, 6, 8, and 9) had a normal anti-PLA2R titer after six months. However, when compared with the previous situation, the 24 h UTP and ALB levels showed no significant changes at six months. Until nine months post-treatment, 24 h UTP decreased from 8.14 ± 6.05 g/d to 1.74 ± 1.81 g/d (P < 0.05), while ALB has increased from 28.06 ± 8.42 g/L to 36.84 ± 6.74 g/L (P < 0.05). At the end of the twelve-month follow-up period, the clinical outcomes were significantly enhanced when compared to those before treatment. This means that the longer the follow-up time, the higher the remission rate that was observed. Notably, none of the eight patients had a recurrence until the to date.
Compared with the standardized dose (375 mg/m2 every week for four weeks, or 1 g fixed-dose with a repeat dose in two weeks), our low-dose RTX regimen lasted for a longer time (i.e., B-cell levels remained low for a longer period of time), which meant that the disease was less likely to relapse. Furthermore, when compared to the high-dose RTX and traditional immunosuppressive regimens, our low-dose RTX regimen also has advantages in terms of security. Although effective immunosuppressive regimens for IMN have been established clinically, the preceding therapeutic options have significant disadvantages, which limit their application, especially in patients with renal insufficiency. At the beginning of the trial, eight patients had a side reaction associated with previous immunosuppressive treatment. During the twelve-month follow-up that tracked adverse reactions, there was only one case of shivering. Notably, after administering RTX for six months, the SCr level decreased from 78.13 ± 16.49 μmol/L to 109.67 ± 40.87 μmol/L (P < 0.05). In this study, we found that our low-dose RTX regimen for the treatment of refractory IMN significantly improved the clinical outcomes and further increased the remission rate. Moreover, treatment with our low-dose RTX regimen is more cost-effective than that with high-dose RTX.
However, it is worth noting that this research also has few limitations that must be considered. First of all, since our study was retrospective in nature and the sample size was small (n = 9), the statistical analysis of data may have biases and inadequacies in terms of generalization. Second, the clinical outcomes were monitored after 1 year; however, the data on immunological indicators, including serum anti-PLA2R titer and CD19+ B-cell count, were monitored only after six months. The long-term prognosis of our low-dose RTX regimen in treating refractory IMN patients is still yet to be explored. To confirm these results and deepen our understanding about this treatment option, we need to conduct large-scale clinical trials and further clarify the mechanism of action of RTX in refractory IMN.
The treatment of patients with refractory IMN using RTX remains controversial and challenging. Conventional immunosuppressive treatment strategies have been applied for several years and have clinically established therapeutic efficacy. In conclusion, we found that our low-dose RTX regimen significantly improved clinical outcomes and further increased the remission rate of patients with IMN. Moreover, treatment with our low-dose RTX regimen is more cost-effective than that with high-dose RTX.
The recognition of idiopathic membranous nephropathy (IMN) as an autoimmune disease has paved the way for the use of B-cell depleting agents such as Rituximab (RTX), which is now a first-line drug for IMN patients with proven safety and efficacy.
RTX for the treatment of refractory IMN patients remains controversial and challenging.
To evaluate the efficacy and safety of a new low-dose RTX regimen for the treatment of refractory IMN patients.
A retrospective study was performed on refractory IMN patients that accepted Low-dose RTX regimen (RTX, 200 mg, once a month for five months).
A total of 9 refractory IMN patients were analyzed. At 12 mo of follow-up data from baseline, the 24 h urinary protein quantification was decreased from 8.14 ± 6.05 g/d to 1.24 ± 1.34 g/d (P < 0.05), and the albumin was improved from 28.06 ± 8.42 g/L to 40.93 ± 5.85 g/L (P < 0.01). Notably, after administering RTX for 6 mo, the serum creatinine decreased from 78.13 ± 16.49 μmol/L to 109.67 ± 40.87 μmol/L (P < 0.05). All of the 9 patients were serum anti-phospholipase A2 receptor (PLA2R) positive at the beginning, and 4 patients had a normal anti-PLA2R titer at six months. The level of CD19+ B-cells decreased to 0 at three months, and the count of CD19+ B-cells lasted to 0 until six months of follow-up.
We found that our low-dose RTX regimen for the treatment of refractory IMN significantly improved clinical outcomes and further increased the remission rate.
Treatment with our low-dose RTX regimen is more cost-effective than high-dose RTX.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Doh K, South Korea; Guo X, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Hu R, Quan S, Wang Y, Zhou Y, Zhang Y, Liu L, Zhou XJ, Xing G. Spectrum of biopsy proven renal diseases in Central China: a 10-year retrospective study based on 34,630 cases. Sci Rep. 2020;10:10994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 2. | Xu X, Wang G, Chen N, Lu T, Nie S, Xu G, Zhang P, Luo Y, Wang Y, Wang X, Schwartz J, Geng J, Hou FF. Long-Term Exposure to Air Pollution and Increased Risk of Membranous Nephropathy in China. J Am Soc Nephrol. 2016;27:3739-3746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 327] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 3. | Glassock RJ. Diagnosis and natural course of membranous nephropathy. Semin Nephrol. 2003;23:324-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Kao L, Lam V, Waldman M, Glassock RJ, Zhu Q. Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J Am Soc Nephrol. 2015;26:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Sethi S. New 'Antigens' in Membranous Nephropathy. J Am Soc Nephrol. 2021;32:268-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 6. | Suzuki T, Han W, Watanabe S, Terashita M, Nakata M, Ichikawa D, Shirai S, Shibagaki Y. Clinical characteristics of thrombospondin type-1 domain-containing 7A-associated membranous nephropathy. Ren Fail. 2020;42:966-968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Chisari CG, Sgarlata E, Arena S, Toscano S, Luca M, Patti F. Rituximab for the treatment of multiple sclerosis: a review. J Neurol. 2022;269:159-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 8. | Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, Leung N, Cohen IM, Wochos DN, Bergstralh E, Hladunewich M, Cattran DC. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG, Hogan MC, Dillon JJ, Hickson LJ, Li X, Cattran DC; Mayo Nephrology Collaborative Group. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5:2188-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Waldman M, Beck LH Jr, Braun M, Wilkins K, Balow JE, Austin HA 3rd. Membranous nephropathy: Pilot study of a novel regimen combining cyclosporine and Rituximab. Kidney Int Rep. 2016;1:73-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Hofstra JM, Branten AJ, Wirtz JJ, Noordzij TC, du Buf-Vereijken PW, Wetzels JF. Early vs late start of immunosuppressive therapy in idiopathic membranous nephropathy: a randomized controlled trial. Nephrol Dial Transplant. 2010;25:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Shi B, Zhang RR, Liang Y, Wang XH, Lang R, Yu RH. Efficacy of Traditional Chinese Medicine Regimen Jian Pi Qu Shi Formula for Refractory Patients with Idiopathic Membranous Nephropathy: A Retrospective Case-Series Study. Evid Based Complement Alternat Med. 2018;2018:5854710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Genberg H, Hansson A, Wernerson A, Wennberg L, Tydén G. Pharmacodynamics of rituximab in kidney allotransplantation. Am J Transplant. 2006;6:2418-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Rojas-Rivera JE, Carriazo S, Ortiz A. Treatment of idiopathic membranous nephropathy in adults: KDIGO 2012, cyclophosphamide and cyclosporine A are out, rituximab is the new normal. Clin Kidney J. 2019;12:629-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Kurosu N, Sugiura H, Iwasaki C, Asamiya Y, Kojima C, Moriyama T, Itabashi M, Tsukada M, Takei T, Ogawa T, Yoshida T, Uchida K, Tsuchiya K, Nitta K. Successful use of single-dose rituximab for the maintenance of remission in a patient with steroid-resistant nephrotic syndrome. Intern Med. 2009;48:1901-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Wang XP, Hu ZX, Guo DY, Tao Y. Remission of Refractory Membranous Nephropathy by Low-dose Rituximab: A Case Report. Chin Med J (Engl). 2016;129:871-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Katsuno T, Ozaki T, Kim H, Kato N, Suzuki Y, Akiyama S, Ishimoto T, Kosugi T, Tsuboi N, Ito Y, Maruyama S. Single-dose Rituximab Therapy for Refractory Idiopathic Membranous Nephropathy: A Single-center Experience. Intern Med. 2017;56:1679-1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:S1-S276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 1166] [Article Influence: 291.5] [Reference Citation Analysis (0)] |
| 19. | Couser WG. Primary Membranous Nephropathy. Clin J Am Soc Nephrol. 2017;12:983-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 446] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 20. | Gao S, Cui Z, Wang X, Zhang YM, Wang F, Cheng XY, Meng LQ, Zhou FD, Liu G, Zhao MH. Rituximab Therapy for Primary Membranous Nephropathy in a Chinese Cohort. Front Med (Lausanne). 2021;8:663680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Teisseyre M, Cremoni M, Boyer-Suavet S, Ruetsch C, Graça D, Esnault VLM, Brglez V, Seitz-Polski B. Advances in the Management of Primary Membranous Nephropathy and Rituximab-Refractory Membranous Nephropathy. Front Immunol. 2022;13:859419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Paulina XMR, Anupama P, Xuefei T. New insights into the immunity and podocyte in glomerular health and disease: From pathogenesis to therapy in proteinuric kidney disease. Integr Med Nephrol Androl. 2021;8:5. |
| 23. | Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, Jefferson JA, Gipson PE, Rizk DV, Sedor JR, Simon JF, McCarthy ET, Brenchley P, Sethi S, Avila-Casado C, Beanlands H, Lieske JC, Philibert D, Li T, Thomas LF, Green DF, Juncos LA, Beara-Lasic L, Blumenthal SS, Sussman AN, Erickson SB, Hladunewich M, Canetta PA, Hebert LA, Leung N, Radhakrishnan J, Reich HN, Parikh SV, Gipson DS, Lee DK, da Costa BR, Jüni P, Cattran DC; MENTOR Investigators. Rituximab or Cyclosporine in the Treatment of Membranous Nephropathy. N Engl J Med. 2019;381:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 375] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 24. | Boyer-Suavet S, Andreani M, Lateb M, Savenkoff B, Brglez V, Benzaken S, Bernard G, Nachman PH, Esnault V, Seitz-Polski B. Neutralizing Anti-Rituximab Antibodies and Relapse in Membranous Nephropathy Treated With Rituximab. Front Immunol. 2019;10:3069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 25. | Cravedi P, Ruggenenti P, Sghirlanzoni MC, Remuzzi G. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2007;2:932-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Fenoglio R, Baldovino S, Sciascia S, De Simone E, Del Vecchio G, Ferro M, Quattrocchio G, Naretto C, Roccatello D. Efficacy of low or standard rituximab-based protocols and comparison to Ponticelli's regimen in membranous nephropathy. J Nephrol. 2021;34:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |