INTRODUCTION
A comparison of cancer data between China and the United States in 2022, published by the National Cancer Center in the Chinese Medical Journal, showed that colorectal cancer (CRC) was the second most newly added cancer in China, compared to fourth in the United States. At present, the early screening of CRC mainly relies on endoscopy. A large number of practices and studies have shown that early detection and treatment of CRC and its precancerous lesions through screening can effectively reduce the mortality and morbidity of CRC[1]. Due to the shortage of hospital resources, the limited physical conditions of patients, and the existence of many serious complications, its development is limited. Clinically, currently popular biomarkers carcinoembryonic antigen, carbohydrate antigen 724 and carbohydrate antigen 199 for gastrointestinal tumors have been widely used as markers for the diagnosis, screening and monitoring of CRC, but the positive rate is low in the early stage of CRC[2]. Therefore, finding simple and convenient screening tools with high sensitivity and specificity is our primary goal to reduce the case fatality rate of CRC. The expression of genes and proteins is amplified metabolically through complex biochemical processes, and metabolomics has been used to screen for CRC. Amino acids are essential for metabolism in almost all types of cells. The study of amino acids in malignant tumors is of great significance for understanding the metabolic changes in tumors.
GLUTAMINE AND COLORECTAL CANCER
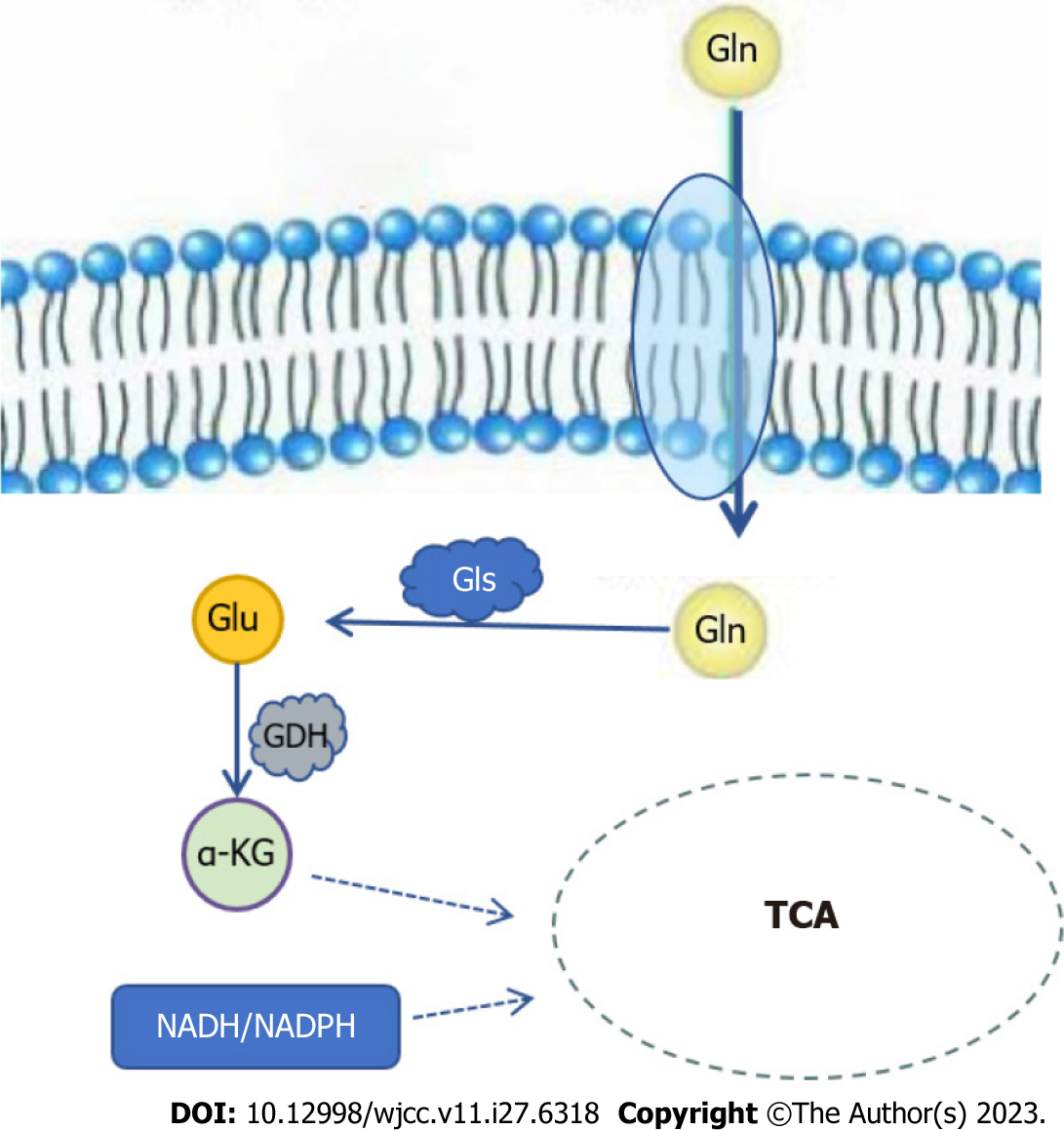
Glutamine (Gln) provides energy to mammalian cells and is a direct nitrogen source for purine and pyrimidine biosynthesis in cancer cells[3]. Glutamine transporters transport Gln into cancer cells, where it is converted to glutamate under the action of glutaminase (GLS) and deaminated by glutamate dehydrogenase (GDH) to form alpha-ketoglutaric acid (α-KG) and nicotinamide adenine dinucleotide/adenine dinucleotide phosphate into the tricarboxylic acid cycle (TCA) to produce adenosine triphosphate (ATP) or converted to glutathione (GSH) by glutamate-cysteine ligase (Figure 1). In the Gln metabolic pathway, GLS, as its key enzyme, is highly expressed in CRC and is positively correlated with the occurrence and development of CRC[4]. When Gln metabolism is inhibited, protective autophagy is produced to degrade excess proteins and organelles in cells to help tumor cells survive the stress process[5]. Li et al[6] found that Gln deprivation significantly reduced the cell growth and viability of SW480 and SW620 cells, while the intracellular levels of ATP and α-KG were also reduced, confirming the importance of Gln on energy metabolism and cell viability of CRC cells. GDH is a key enzyme that also catalyzes the final reaction of the Gln metabolic pathway and has been reported to be involved in tumor growth and metastasis. Liu et al[5] detected the expression of GDH in CRC cells by qRT-PCR and immunohistochemistry. It was found that GDH is upregulated in CRC cells and can induce epithelial-mesenchymal transition (EMT) to improve the proliferation and migration of CRC cells.
Figure 1 Glutamine metabolic pathway.
Gln: Glutamine; Gls: Glutaminase; Glu: Glutamate; GDH: Glutaminase; α-KG: Alpha-ketoglutaric acid; TCA: Tricarboxylic acid cycle; NADH: nicotinamide adenine dinucleotide; NADPH: Adenine dinucleotide phosphate.
Alanine-serine-cysteine transporter 2 (ASCT2) is a Gln transporter[7]. As a medium for Gln uptake and metabolism, ASCT2 affects Gln metabolism and signal transduction. ASCT2 silencing leads to abnormal cell metabolism. ASCT2 was highly expressed in CRC compared with normal colorectal tissue. ASCT2 is essential for the growth and reproduction of CRC cells. microRNAs (miRNAs) belong to a class of small molecular RNAs that block the expression of mRNA degradation regulatory genes, and abnormally regulated miRNAs are involved in the occurrence of tumors. In CRC, there are a large number of abnormally regulated miRNAs, and miR-137 is one of them. Studies have determined that miR-137 regulates CRC Gln metabolism by targeting the 3'UTR region of ASCT2[8]. Amplification of the oncogene c-Myc has been reported in primary CRC, and c-Myc overexpression has been observed in 60% of CRC patients. As key transcription factors, many glucose and Gln metabolizing genes are directly regulated by c-Myc, such as ASCT2 and GLS1[9]. c-Myc can increase Gln metabolism by up-regulating GLS expression, which leads to Gln entering the TCA cycle as α-KG[10]. Tran et al[11] demonstrated that the glutamine-α-KG axis contributes to Wnt signaling and cell differentiation in CRC, providing compelling evidence for the key role of Gln and α-KG homeostasis in regulating carcinogenic Wnt signaling and CRC progression. Many mechanisms have been proposed regarding Gln metabolism in CRC, such as Gln addiction, increased Gln decomposition, and the effect of autophagy activation on cancer cell survival and proliferation[6]. Although the specific mechanism is not clear, it can be confirmed that Gln plays a crucial role in CRC cell metabolism
SERINE AND COLORECTAL
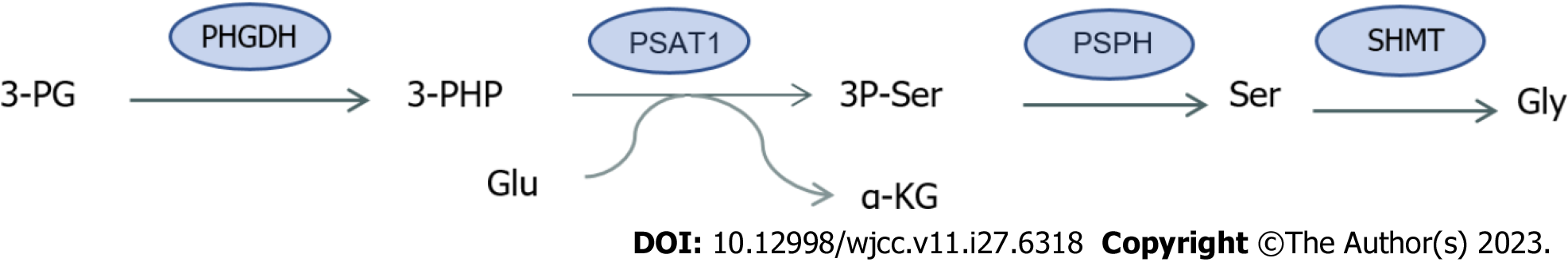
Serine (Ser) is a non-essential amino acid that acts as a major donor of a carbon unit, whose main roles include the synthesis of amino acids, nucleotides, glutathione and participates in biological methylation modification, and the production of NADPH[12] for antioxidant defense. Serine can be taken up from the external environment or synthesized by endogenous pathways, which mainly include de novo synthesis, proteolytic recovery and glycine conversion. Under fasting conditions, most of the intracellular Ser is derived from the de novo synthesis pathway (SSP)[13]. SSPs are produced by 3-phosphoglycerate (3PG), an intermediate product of glycolysis. First, phosphoglycerate dehydrogenase (PHGDH) oxidizes 3PG to form 3-phosphohydroxypyruvate (3PHP), which accepts the amino group of glutamic acid, and 3-phosphatidylinic acid (3P-Ser) and α-KG are produced under the catalysis of phosphoserine aminotransferase 1 (PSAT1). Finally, phosphate phosphatase dephosphorylates 3P-Ser to produce Ser (Figure 2). The established growth-promoting role of Ser in cancer cells has been shown in previous evidence where Ser levels were increased in CRC, and the discovery that increased expression of genes controlling Ser synthesis enhance xenograft growth suggest that this pathway plays a role in the progression of CRC[14].
Figure 2 Synthesis of serine and glycine.
3-PG: 3-phosphoglycerate; PHGDH: Phosphoglycerate dehydrogenase; 3-PHP: 3-phosphohydroxypyruvate; Glu: Glutamate; α-KG: alpha-ketoglutaric acid; PSAT1: Phosphoserine aminotransferase 1; 3P-Ser: 3-Phosphatidylinic acid; PSPH: Phosphate phosphatase; Ser: Serine; SHMT: Serine hydroxymethyltransferase; Gly: Glycine.
As a key enzyme in de novo synthesis of Ser, PHGDH plays an important role in tumor cell proliferation and migration. A study found[15] that CRC patients with high PHGDH expression levels tended to have stage III/IV CRC and larger tumors, and that high PHGDH levels were significantly associated with lower survival rates and were independent predictors of poor prognosis in patients with CRC. Montrose et al[13] demonstrated significant increases in PHGDH and PSAT1 in adenomatous polyps compared to normal tissues, and PSAT1 was the strongest and consistently increased gene compared to PHGDH. PSAT1 levels in the cytoplasm of CRC cells were elevated compared to normal adjacent tissues. Elevated Ser has been found in mouse colorectal tumors and human CRC, and restriction of endogenous and exogenous sources of Ser maximizes inhibition of CRC cell proliferation. Pranzini et al[16] demonstrated that interfering with the availability of Ser enhanced the antitumor effects of 5-fluorouracil (5-FU) in both in vitro and in vivo CRC models.
GLYCINE AND COLORECTAL CANCER
Glycine (Gly), in addition to being a component of glutathione, is the most abundant amino acid in the body and is involved in a variety of metabolic and pathophysiological processes that are important for the growth and survival of proliferating cells, including cancer cells[17]. Gly can be synthesized from a variety of sources and can be synthesized from choline, serine and threonine through a range of pathways. Ser is particularly important for Gly synthesis. The conversion of Ser to Gly is catalyzed by serine hydroxymethyl transferase (SHMT) (Figure 2). SHMT1 and SHMT2 proteins are mainly confined to the cytoplasm and mitochondria, respectively. SHMT2 regulates the activity or expression of proteins that allow tumor cells to survive in the presence of hypoxia or nutrient deficiency. In contrast to normal enzymes, SHMT2 is overexpressed in CRC tissue and is involved in CRC progression[18]. SHMT2 knockdown significantly inhibits a variety of signaling pathways, including TGF-β, Notch and Wnt pathways, which are closely associated with CRC progression, especially the Wnt/β-catenin pathway. In human CRC tissues, both SHMT2 and β-catenin expression are elevated and closely related to CRC progression and metastasis, and SHMT2 expression is negatively correlated with survival[18]. The study by Redalen et al[19] reported an association between pre-treatment tumor metabolic profile and treatment outcome in locally advanced CRC patients undergoing radical surgery after combined neoadjuvant therapy, and provided the first clinical evidence of high tumor Gly concentration as an adverse prognostic factor. Treatment with Gly alone resulted in a significant 43% reduction in tumor volume. This decrease is associated with decreased MVD and proliferation index in tumor tissue. Gly is known to deactivate VEGF-stimulated endothelial cell growth, migration, and angiogenesis through mechanisms involving receptor-dependent pathways[20]. Jacob et al[21] reported that Gly has a protective effect on mesenteric ischemia/reperfusion (I/R) injury, and one of the mechanisms is to down-regulate lethal signaling and eliminate the apoptotic cascade in the I/R injury model. The promising efficacy of Gly supplements in the prevention of several intestinal diseases suggests that Gly could in principle be a target for therapeutic intervention[17].
ARGININE AND COLORECTAL CANCER
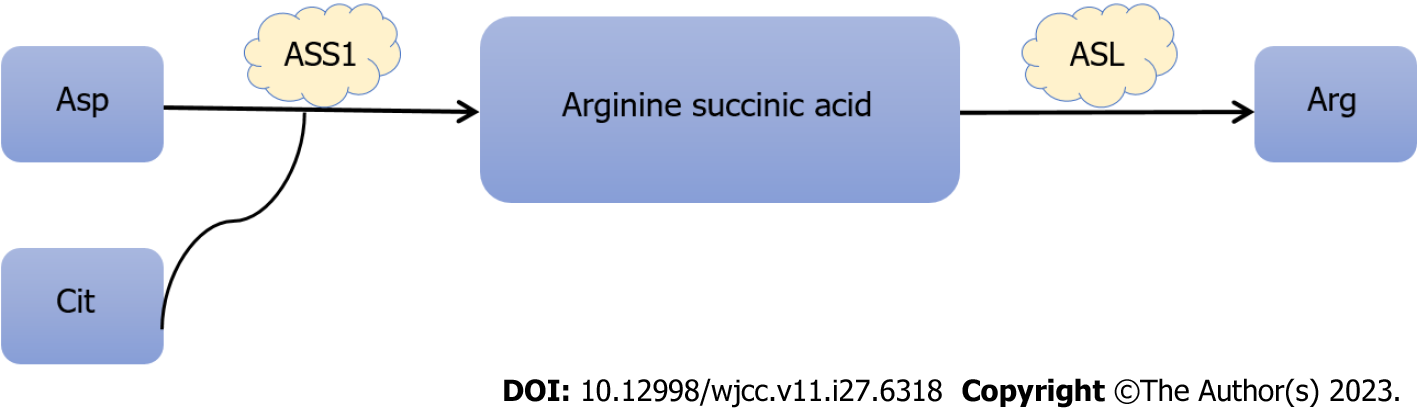
Arginine (Arg) is a semi-essential amino acid and an important precursor for the production of proteins, polyamines, creatinine and nitric oxide, and is involved in all aspects of tumor metabolism[22]. Arg comes from a variety of sources including dietary intake, protein breakdown and endogenous de novo synthesis. Endogenous Arg is mainly produced in the gut and kidney. The conversion of intestinal citrulline to Arg in the kidney mainly involves two steps: Aspartic acid and citrulline under the action of succinic acid synthetase 1 (ASS1) to arginine succinic acid, and rapid conversion to Arg and fumarate by arginine succinic acid lyase (Figure 3). ASS1 is a rate-limiting step in catalytic de novo arginine biosynthesis. Several cancers are characterized by low ASS1 expression, including hepatocellular carcinoma, pleural mesothelioma, and prostate cancer[23]. In these cancers, the promoter of the ASS1 gene is usually epigenetically silent[24]. ASS1 silencing is associated with tumor progression, possibly due to increased pyrimidine synthesis due to higher availability of aspartic acid[25]. In contrast, CRC shows increased expression of ASS1[23]. Histological data from normal and primary CRC tissues indicate that ASS1 is strongly stained in primary CRC and that protein levels in CRC significantly up-regulate ASS1 compared to normal colorectal tissue[26]. A more in-depth study of clinical histology in approximately 600 samples of ASS1 independently supported this finding[27].
Figure 3 Synthesis of arginine.
Asp: Aspartic acid; Cit: Citrulline; ASS1: Succinic acid synthetase 1; ASL: Arginine succinic acid lyase; Arg: Arginine.
Roy et al[28] demonstrated that increased expression of inducible nitric oxide synthetase (iNOS), a key enzyme in Arg metabolism, is an early event in the development of colon cancer, and the induction of iNOS may play a key role in increasing the blood supply of dysplastic mucous membranes, thus promoting colon cancer. Arg deprivation has been used as a cytotoxic method to selectively target a large number of arginine-trophic tumors. Studies have shown that[29] a diet supplemented with Arg increased the growth of metastatic CRC by 55%, while restriction of Arg inhibited the growth by 78%. Consumption of Arg reduced CRC cell mobility and reduced CRC cell invasive growth, suggesting that Arg is trophic to CRC cells and cannot survive in the absence of an external source of Arg[30].
METHIONINE AND COLORECTAL CANCER
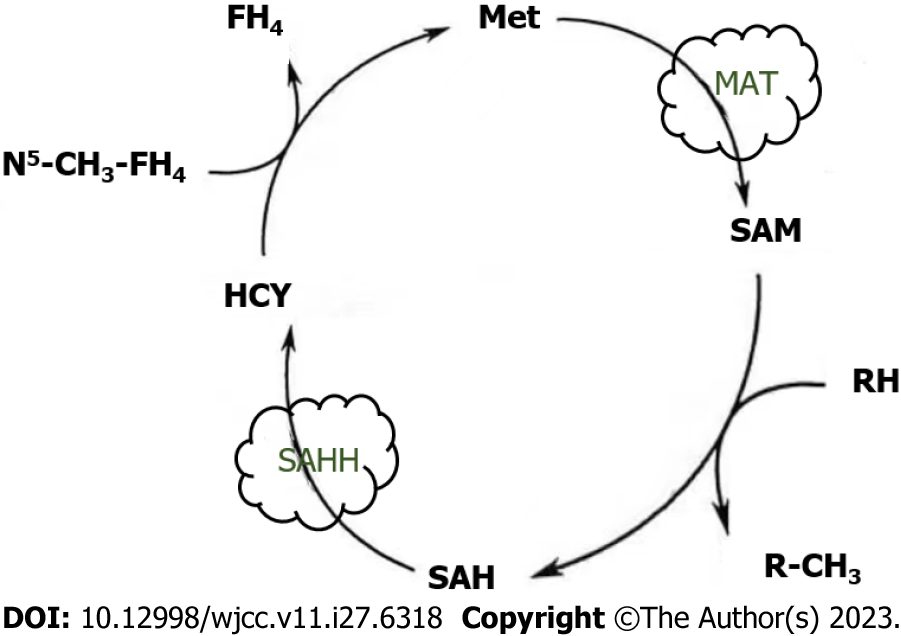
Methionine (Met) is a sulfur-containing amino acid that is generally considered a hydrophobic residue involved in the methylation of deoxyribonucleic acid (DNA), RNA, histones, small molecules, and lipids[31]. Met is synthesized by homocysteine methylation or the polyamine biopathway under betaine cofactor and 5-methyl-tetrahydrofolic acid. The Met cycle is the process by which Met is converted to methyl. Met is used to produce S-adenosine methionine (SAM) by methionine adenosine transferase and demethylated to form S-adenosine homocysteine (SAH). After dehydrogenation of S-adenosine homocysteine hydrolase (SAHH), SAH is converted back to homocysteine, which recombines with methyl groups to regenerate Met and in turn the Met cycle (Figure 4). This circulatory pathway is closely associated with CRC. In recent years, a growing body of literature has accumulated evidence that SAM exerts anti-proliferative and pro-apoptotic activities in CRC cells, demonstrating its ability to regulate genes responsible for cell invasion and metastasis[32]. Metastasis and diffusion of CRC cells is the major cause of death in patients. We found that SAM significantly reduced vimentin levels and promoted the transformation from N-cadherin to E-cadherin expression in HCT-116 and Caco-2 cells, as demonstrated by comparing the strength of corresponding protein bands in SAM treated and untreated cells. This highlights the ability of SAM to slow CRC cell migration by inhibiting the transition from epithelial to mesenchymal states[33]. SAM inhibits CRC cell growth by reversing the hypomethylation of c-Myc and H-ras promoters, thereby inhibiting the expression of these oncogenes[34]. SAM has been shown to overcome 5-FU chemical resistance in CRC cells by targeting autophagy, P-gp expression, and NF-κB signaling activation, providing important implications for the development of new adjuvant therapies to improve CRC treatment and patient outcomes[32]. Komninou et al[35] showed that Met dietary restriction inhibits the development of CRC in rats, an effect that occurs primarily in the post-initiation phase of carcinogenesis, possibly due to inhibition of CRC cell proliferation, suggesting a potential role for Met dietary restriction in CRC prevention.
Figure 4 Methionine cycle.
Met: Methionine; MAT: Methionine adenosine transferase; SAM: S-adenosine methionine; SAH: S-adenosine homocysteine; SAHH: S-adenosine homocysteine hydrolase; HCY: Homocysteine.
CYSTEINE AND COLORECTAL CANCER
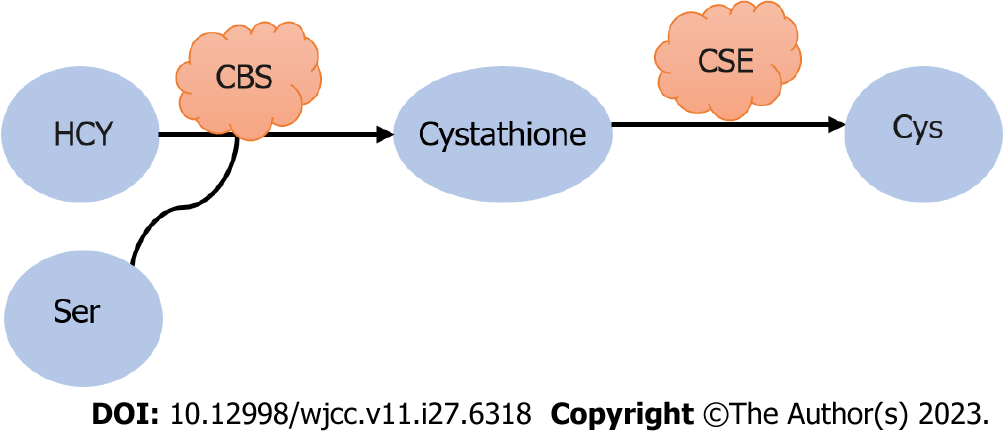
Cysteine (Cys) is a sulfur-containing protein amino acid that plays a uniquely important role in cell response to changes in the REDOX environment. Cys can be derived from protein catabolism or de novo synthesis of Met. Dietary Met is converted to homocysteine in two successive steps: First by condensation of homocysteine with Ser, it is catalyzed to cysteine β-synthetase, and then hydrolyzed to Cys by cysteine gamma-lyase, thus establishing a link between the trans-vulcanization pathway and the TCA cycle (Figure 5). The trans-vulcanization pathway is associated with energy metabolism, tumor angiogenesis, and drug resistance in colon cancer[36]. A recent study has shown that the trans-vulcanization pathway directly benefits cancer cells in maintaining REDOX balance and escaping iron death. Studies have shown that many cells require Cys uptake to prevent the onset of iron death[37]. Cys is an effective regulator of iron death. Cys levels indirectly control cell susceptibility to iron death through their effects on the synthesis of GSH, CoA, Fe/S clusters, and potentially other molecules. These molecules are essential for the continued function of the antioxidant network that prevents iron death, as well as for management of the intracellular distribution of iron, a key iron death catalyst to which a variety of cancer cells are highly susceptible[38]. Many studies have shown that increased Cys uptake is not a universal response to oncogene activation[39]. High levels of Cys input may be an adaptive feature of at least some cancer cells to inhibit iron death or other forms of oxidative stress in the body.
Figure 5 Synthesis of cysteine.
HCY: Homocysteine; Ser: Serine; CBS: Cysteine β-synthetase; CSE: Cysteine gamma-lyase; Cys: Cysteine.
Cys has a major role in cancer cell metabolism: Acting as a precursor to glutathione under the action of glutamylcysteine ligase, contributing to oxidative stress control. Oxidative stress promotes damage to cellular proteins, lipids, membranes, and DNA and plays a key role in the development of cancer[40]. It is well known that patients with CRC have higher oxidative stress and lower antioxidant capacity than healthy subjects[41]. One of the reasons for this is that oxidative stress is an important cause of inflammation and may contribute to the development of CRC. Yang et al[42] observed that Cys may be oxidized more in CRC tissue than in non-tumor tissue, where Cys is usually maintained in the reduced form. If Cys oxidation promotes free radical production and increases oxidative stress in tumor tissue, tumor cells can protect themselves from higher oxidative stress, and the imbalance between oxidative stress and antioxidant capacity is thought to cause CRC[43]. Studies have shown that plasma Cys concentration is inversely associated with the incidence of CRC in postmenopausal women, associated with rectal and proximal tumors, but not distal tumors[40]. In addition, this association was significant for local tumors, but not for metastatic tumors, and was not observed in studies conducted in men[44].
BRANCHED CHAIN AMINO ACIDS AND COLORECTAL
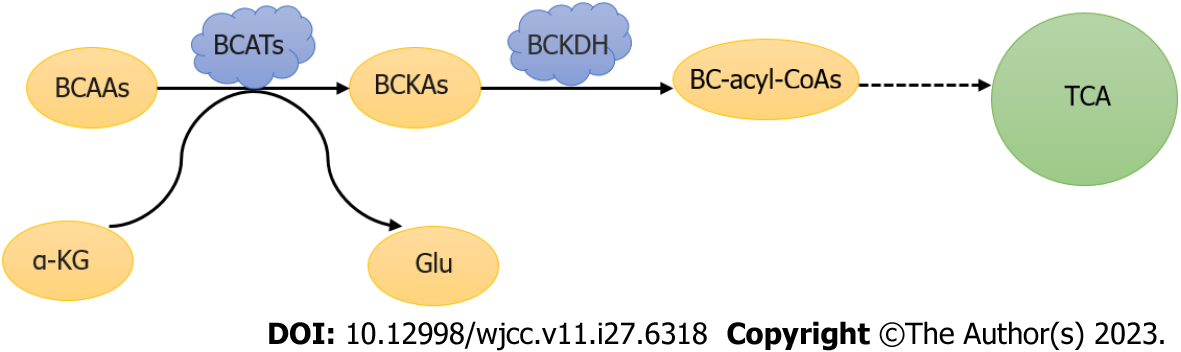
Branched chain amino acids (BCAAs) are the building blocks of all life forms, namely valine (Val), leucine (Leu), and isoleucine (Ile), and can be synthesized in bacteria, plants, and fungi, but not in animals. The branched amino acid group is first reversibly transaminated to alpha-ketoglutaric acid by branched aminotransaminases (BCATs) to produce glutamic acid and branched alpha-ketoic acids (BCKAs). BCKAs are then irreversely decarboxylated[45] and dehydrogenated by a branched chain alpha ketoate dehydrogenase (BCKDH) complex that is negatively regulated by BCKDH kinase (BCKDK) phosphorylation[46]. Catalyzed by BCKDH, BCKAs are further oxidized by a variety of enzymes into different substrates for biosynthesis or energy, and the three BCKAs can be further decomposed into acetyl and/or succinyl-CoA, which will enter the TCA cycle and contribute to energy production (Figure 6). Studies have shown that BCATs1 plays an important role in branched chain amino acid degradation and is considered a prognostic biomarker for CRC. Xue et al[47] found that in 117 patients with CRC, the expression level of BCKDK in cancer tissues was higher than that in neighboring tissues, which had a significant impact on survival time, indicating that BCKDK directly phosphorylated MEK at Ser221 to positively regulate MEK/ERK signaling. MAPK has a wide range of functions in cancer cell proliferation; thus, the BCKDK-MEK1 axis may contribute to the development of CRC. Phosphorylated BCKDK promotes EMT by regulating EMT genes, leading to CRC metastasis[48].
Figure 6 Metabolism of branched-chain amino acids.
BCAAs: Branched-chain amino acids; BCATs: Branched chain amino transaminase; α-KG: Alpha-ketoglutaric acid; Glu: Glutamate; BCKAs: Branched chain alpha keto acid; BCKDH: Branched chain alpha ketoate dehydrogenase; TCA: Tricarboxylic acid cycle.
As an important organ in the human body, the gut has the highest level of immune activity, and disruption of intestinal homeostasis is closely associated with the development of obesity, T2DM, IBD, atherosclerosis, and colon cancer[49]. BCAAs act as regulatory factors that promote intestinal development, nutrient transporters, and immune-related functions, thereby improving intestinal health[50]. However, most studies have focused on Leu function rather than Val or Ile in the gut. Leu supplementation maintains intestinal health by enhancing tight connections in fish, as well as improving intestinal epithelial cell proliferation, villus height, and small intestine growth in pigs. In addition, the high expression of BCAT and BCKDH enzymes in the intestinal tract indicates a certain correlation between BCAAs and intestinal function[51]. A recent experimental study of obesity-related CRC variant models reported that dietary BCAA supplements significantly inhibited the development of chemically induced CRC lesions[52]. All these findings indicate that there is a certain biological relationship between BCAAs and the occurrence and development of CRC.
CONCLUSION
Normally, the human body is in a state of equilibrium in relation to amino acids, but malignant tumors often rely on specific amino acids for survival and proliferation, resulting in an imbalance of amino acids in the blood, urine and body fluids. There is a strong relationship between colorectal cancer and amino acid metabolism, possibly for the following reasons: (1) Amino acid energy: Colorectal cancer cells are highly metabolically active and require a lot of energy to maintain their rapid proliferation and growth. Amino acids can produce energy through the amino acid metabolic pathway, providing cancer cells with the energy they need to survive. In colorectal cancer cells, the degradation of amino acids can be achieved through the involvement of enzymes such as amino acid transaminase and glutaminase, which convert amino acids into energy-producing substances such as pyruvate and lactic acid; (2) Amino acid synthesis: Rapid proliferation and growth of colorectal cancer cells require a large supply of amino acids. In some cases, cancer cells may meet their needs by increasing the synthesis of amino acids. For example, cancer cells can synthesize glutamate and derivatives of glutamate such as glutathione by increasing the glutamate synthesis pathway, thus providing antioxidants and anti-stress substances; (3) Amino acid transport: Amino acid transport also plays an important role in the development of colorectal cancer. Cancer cells increase the uptake and utilization of amino acids by increasing the expression and activity of amino acid transporters. This can provide the supply of amino acids that cancer cells need to promote their growth and proliferation; and (4) Regulation of amino acid metabolites: Amino acid metabolites can also affect the development of colorectal cancer. For example, some amino acid metabolites such as glutathione and serine can regulate biological processes such as oxidative stress and apoptosis of cells, thereby affecting the growth and metastasis of colorectal cancer. These characteristics may be closely related to the growth and invasion of tumor cells and the metabolic changes in the tumor microenvironment, and have important significance for the occurrence and development of colorectal cancer. Amino acid detection as a simple and convenient means of health diagnosis and tumor screening has a guiding role for the prevention of disease, improve the nutritional status of the body and provide reference for the treatment of tumors. There are currently many therapies for malignant tumors, and amino acid therapy is also crucial as an adjunct therapy. For example, by adjusting arginine in patients, the body can improve resistance and inhibit tumor cell growth, reduce treatment-related side effects, and improve survival. In the absence or inhibition of glutaminase, malignant tumors that rely on exogenous glutamine for energy supply will not be able to utilize this source of nutrients, thus blocking the uptake and utilization of amino acids by cancer cells and inhibiting tumor growth. A methionine-restricted diet resulted in reduced proliferation of colon cells and reduced precancerous abnormal crypt lesions, while overcoming 5-FU chemical resistance in CRC cells. Despite the potential of amino acids in the treatment of colorectal cancer, further studies are currently needed to verify their efficacy and safety. In addition, factors such as individual differences and tumor heterogeneity may also affect the effectiveness of amino acid therapy. Therefore, according to the specific conditions of different patients, it is very important to develop an individualized treatment plan. At present, we know that specific amino acids are associated with colorectal cancer, but whether these specific amino acid changes can be used as a reliable screening method for colorectal cancer needs further research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Amedei A, Italy; Osera S, Japan S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH