Published online Sep 6, 2023. doi: 10.12998/wjcc.v11.i25.6019
Peer-review started: July 6, 2023
First decision: July 18, 2023
Revised: July 20, 2023
Accepted: August 1, 2023
Article in press: August 1, 2023
Published online: September 6, 2023
Processing time: 56 Days and 20.5 Hours
Kidney transplantation is the standard treatment for end-stage renal disease. Particularly, rare and specific pathogenic infections which are asymptomatic are often difficult to diagnose, causing delayed and ineffective treatment and thus seriously affecting prognosis. Tropheryma whipplei (T. whipplei) is a Gram-positive actinomycete widely found in soil, sewage, and other external environments and is present in the population as an asymptomatic pathogen. There is relatively little documented research on T. whipplei in renal transplant patients, and there are no uniform criteria for treating this group of post-transplant patients. This article describes the treatment of a 42-year-old individual with post-transplant T. whipplei infection following kidney transplantation.
To analyze clinical features of Whipple’s disease and summarize its diagnosis and treatment effects after renal transplantation. Clinical data of a Whipple’s disease patient treated in the affiliated hospital of Guizhou Medical University were collected and assessed retrospectively. The treatment outcomes and clinical experience were then summarized via literature review. The patient was admitted to the hospital due to recurrent diarrhea for 1 mo, shortness of breath, and 1 wk of fever, after 3 years of renal transplantation. The symptoms of the digestive and respiratory systems were not significantly improved after adjusting immunosuppressive regimen and anti-diarrheal, empirical antibiotic treatments. Bronchoscopic alveolar fluid was collected for meta-genomic next-generation sequencing (mNGS). The deoxyribonucleic acid sequence of Tropheryma whipplei was detected, and Whipple’s disease was diagnosed. Meropenem, ceftriaxone, and other symp
Whipple’s disease is rare, with no specific symptoms, which makes diagnosis difficult. Polymerase chain reaction or mNGS should be immediately performed when the disease is suspected to confirm the diagnosis.
Core Tip: Whipple disease is rare and has no specific symptoms, which makes diagnosis difficult. When the disease is suspected, polymerase chain reaction or meta-genomic next-generation sequencing should be performed immediately to confirm the diagnosis.
- Citation: Chen Q, Niu YL, Zhang T. Diagnosis and treatment of Whipple disease after kidney transplantation: A case report. World J Clin Cases 2023; 11(25): 6019-6024
- URL: https://www.wjgnet.com/2307-8960/full/v11/i25/6019.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i25.6019
Kidney transplantation is standard treatment for end-stage renal disease[1,2]. Human and kidney survival rates have improved significantly after kidney transplantation with the development of immunosuppressive drugs, advancements in surgical techniques, and improved perioperative management protocols[3,4]. However, infectious diseases are significantly more frequent in post-transplant recipients than in general population due to prolonged immunosuppressive medication, and infection has become one of the main causes of death after kidney transplantation[5]. Particularly, rare and specific pathogenic infections which are asymptomatic are often difficult to diagnose, causing delayed and ineffective treatment and thus seriously affecting prognosis[6]. Tropheryma whipplei (T. whipplei), a Gram-positive actinomycete, is widely found in soil, sewage, and other external environments and is present in the population as an asymptomatic pathogen[7,8]. Historically, the symptoms of Whipple’s disease included intermittent and recurrent arthralgia or arthritis with chronic diarrhea, abdominal pain, weight loss, and central nervous and cardiovascular systems effects[9,10]. The occurrence of Whipple’s disease after renal transplantation has not been reported yet. A patient with Whipple’s disease was admitted with respiratory and digestive systems infections after renal transplantation and the treatment outcomes were good. This case aimed to report clinical data and treatment outcomes. Furthermore, the relevant domestic and international literature was also included to provide a reference for the timely diagnosis and treatment of this rare disease.
Diarrhea for 1 mo, shortness of breath with fever for 1 wk.
A 46 years old male patient was enrolled at the affiliated hospital of Guizhou Medical University for the uremic stage of chronic renal failure (primary nephropathy unknown) and underwent a living donor kidney transplant in July 2019. The patient received left kidney of his father. The donor-recipient blood type is A. The human leukocyte antigen mismatch was 2. The recipient had a negative preoperative penal reactive antibody and a complement-dependent cytotoxicity test of 3%. The donor underwent laparoscopic kidney resection, and the recipient underwent conventional kidney transplantation at the right iliac fossa. The rabbit anti-human thymocyte immunoglobulin (r-ATG) was administered intra- and post-operatively at 50 and 25 mg from D1 to D4, respectively. There were no postoperative complications, and the patient was discharged 14 days after the surgery. With regular follow-up after discharge, a triple immunosuppressive regimen of tacrolimus + morte-tacrolimus + glucocorticoids was used to prolong anti-rejection and tacrolimus trough maintained between 5 and 10 ng/mL was prescribed. The patient with 1 mo of diarrhea visited the hospital; he had 5-6 stools per day, diluted and watery, without mucus, pus, and blood. Outpatient treatment with symptomatic anti-diarrhea and intestinal flora regulation did not significantly relieve diarrhea symptoms. Subsequently, the immunosuppressive regimen was adjusted, and diarrhea frequency decreased to 2 to 3 times daily. After one week, the patient developed shortness of breath with fever after activity with no apparent cause, with a maximum temperature of 38.5 °C. He was diagnosed with “diarrhea and fever to be investigated” and was admitted to the hospital without cough, sputum, nausea, vomiting, and frequent and urgent urination. On the day of admission, he relieved loose stools twice and urinated 1200 mL. He had lost about 2.5 kg of weight in the past month.
Denied unclean diet, cold and close contact with febrile patients before the disease onset, hypertension, diabetes, coronary heart disease, tuberculosis, hepatitis B, etc. There was no history of trauma, blood transfusion, drug or food allergy, living in infected areas, contact with infected water or sources, or exposure to radiation, toxins, or drugs. Furthermore, there was no residence history in areas with medium to high risk of neoconiosis or close contact.
Born and raised in Guizhou Province, no history of extended travel outside the country, no smoking or drinking. Parents, children and spouse are all healthy.
The initial checkup indicated a temperature of 37.8 °C, pulse 106/min, respiration 28/min, blood pressure 123/85 mmHg, and finger pulse oxygen 91%. After a physical examination, he was clear and had shortness of breath, with a medium body shape, general nutrition, and cooperation. The pupils were equally large and rounded bilaterally, and the light reflex was sensitive. There was no yellowing of the skin and sclera, and no petechiae were observed on the skin mucosa. No enlargement of superficial lymph nodes was detected throughout the body. Breath sounds were coarse in both lungs, and a few wet rales could be heard in the middle and lower lungs. He had a uniform heart rhythm with no murmur in the valves. Abdominal and neurological investigations were negative; no abnormal external genitalia development or external urethral discharge was identified. Furthermore, there was no abnormal joint movement of the limbs.
The blood profile revealed the following: Leukocytes = 8.94 × 109/L, lymphocyte = 4.3%, absolute lymphocyte value = 0.38 × 109/L, neutrophil = 90.5%, absolute neutrophil value = 8.09 × 109/L, calcitoninogen = 0.45 μg/L, creatinine = 131.0 μmol/L, blood sodium = 132.1 mmol/L, blood chloride = 79.9, blood glucose = 8.43 mmol/L, albumin = 32.5 g/L, and ultrasensitive C-reactive protein = 24.94 mg/L. The routine stool test and Vibrio cholera, Salmonella, and Shigella culture were negative. Tacrolimus blood concentration was 4.76 ng/mL.
Chest computed tomography (CT) showed multiple exudates in both lungs, and infection was considered.
Whipple disease.
After the admission, the cardiac profile was monitored, nasal catheter oxygenation was administered, the empirical anti-infection regime of moxifloxacin was initiated, mortification with mortification, symptomatic anti-diarrhea medication was started, the water-electrolyte acid-base balance was carefully maintained for active infection improvement and pathogenesis-related tests were performed.
Blood and urine BK and human cytomegalovirus virus deoxyribonucleic acid were negative, fungal (1,3)-β-D glucan ≤ 37.5 pg/mL, Aspergillus galactomannan = 0.205 S/CO, and antibodies to 12 common respiratory pathogens were also negative. After 3 d of empirical anti-infective treatment, the patient’s shortness of breath and fever did not improve; the fever peak rose to 38.9 °C and was mainly experienced from 2 pm to 10 pm daily. Therefore, the anti-infective regimen was adjusted to moxifloxacin + cefoperazone-sulbactam sodium with micafungin (antifungal). Furthermore, blood bacterial and fungal cultures and fiberoptic bronchoscopy were performed, which were negative. Fibronectomy indicated abnormal trachea and bronchi. On brush examination of the posterior segment of the right lung’s upper lobe, ciliated columnar cells, a few erythrocytes, and phagocytes were seen microscopically, and no acid-resistant bacilli were found on antacid staining. Bronchoalveolar lavage fluid (BALF) was obtained for metagenomics next-generation sequencing (mNGS), which Whipple’s nutrient barrier sequence was detected after 2 days. Since this was a rare pathogen and our department has no previous experience with this disease, literatures weas reviewed, and multidisciplinary consultation (MDT) with the Department of Infection, Clinical Pharmacy, Respiratory Medicine, and Gastroenterology was requested to change the anti-infective regimen to meropenem alone. After 48 h of this new regime, the patient’s temperature dropped to 36.8 °C and fluctuated within the normal range after that. After 9 d, the regimen was changed to ceftriaxone; post- 5 days of ceftriaxone, it was changed to oral cotrimoxazole.
The patient was discharged after 20 d of hospitalization and continued on oral cotrimoxazole for 3 mo after discharge. At 6 mo post-discharge follow-up, the patient’s transplanted renal function was stable, no significant abnormalities were observed in the relevant biochemical indices, and no extended symptoms such as diarrhea or fever occurred.
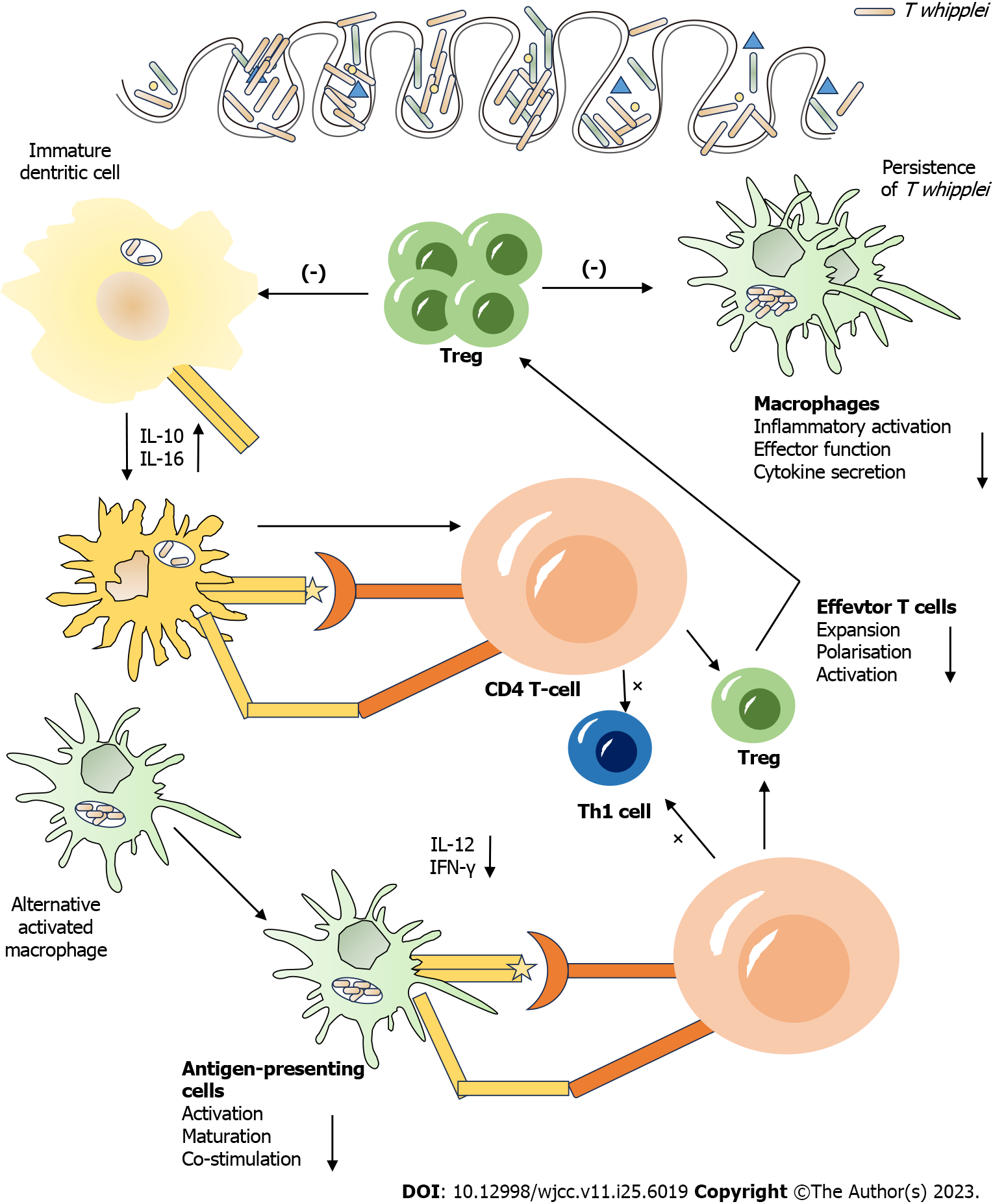
Whipple’s disease is a rare systemic infection caused by T. whipplei, most frequently observed in patients with diabetes, chronic kidney disease, liver disease, and immunodeficiency[11,12]. Its Pathogenesis of Whipple’s disease is shown in Figure 1. It is also detected in the general population as an asymptomatic pathogen. A study reported its incidence to be 3 in a million, and no relevant epidemiological report from China exists[13]. It is primarily manifested with intermittent and recurrent arthralgia or arthritis with chronic diarrhea, abdominal pain, and weight loss. It can affect multiple systems such as cardiovascular, central nervous, respiratory, and skin systems. If left untreated, many serious complications can occur, with poor prognosis[14]. Because of the rarity, non-specific clinical manifestations, and absence of classical diagnostic techniques (smear microscopy, microbial culture, antigenic antibody testing, etc.) to identify T. whipplei nurturing bodies, the disease is difficult to diagnose and is mainly confirmed using tissue biopsy and pathogenetic genetic testing[15]. Upon suspicion, despite gastrointestinal symptoms, small intestinal mucosal specimens should be obtained via microscopy for periodic acid Schiff staining, polymerase chain reaction (PCR), and mNGS testing[16]. If the results of small intestinal tissue examination are not diagnostic, the above tests should be performed by sampling the corresponding lesion sites (synovial membrane, lymph nodes, alveolar lavage fluid, cerebrospinal fluid, blood, etc.) according to the patient’s clinical manifestations. With the rapid development of molecular biology techniques, the application of mNGS for pathogenic detection has been increasing, especially for clinically rare, atypical, or caustic microorganisms with important diagnostic value[17,18]. mNGS also allow the detection of multiple pathogens simultaneously in a single specimen, dismissing repeated sampling, and multiple testing[19].
In review, the patient received long-term maintenance therapy with immunosuppressive drugs after renal trans
Currently, there are no guidelines or expert consensus on treating Whipple’s disease. The primary treatment includes anti-infective therapy against the pathogen and supportive symptomatic therapy against the corresponding systemic symptoms. Effective anti-infective drugs reported in the literature include meropenem, ceftriaxone, cotrimoxazole, doxycycline, and hydroxychloroquine[20,21]. In this case, meropenem was selected as an anti-infection after MDT consultation because of severe respiratory symptoms, which quickly improved the patient’s fever and was subsequently stepped down to ceftriaxone. Furthermore, A research reported that a recurrence rate of this disease was 30% to 40%[22]; therefore, an oral combination of sulfamethoxazole or doxycycline + hydroxychloroquine for 3 mo to 1 year was proposed. The patient continued using sulfamethoxazole for 3 mo after discharge. The followed up of > 6 mo reported no further symptoms of diarrhea and fever, as well as had stable transplant renal function[23].
In summary, prolonged immunosuppressive medication in organ transplant patients can cause opportunistic infections, especially some relatively rare pathogens in the normal population, and often causes difficulties in clinical management. Whipple’s disease after renal transplantation is rarely reported. Therefore, if a patient enrolls with diarrhea and symptoms of motor, respiratory, cardiovascular, or other systems after renal transplantation and indicates poor outcomes after conventional management, Whipple’s disease should be considered. PCR or mNGS testing can be performed on the appropriate specimens for early detection of pathogens for targeted anti-infective treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Susak YM, Ukraine; Teng X, China S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY
| 1. | Chitty DW, Hartley-Brown MA, Abate M, Thakur R, Wanchoo R, Jhaveri KD, Nair V. Kidney transplantation in patients with multiple myeloma: narrative analysis and review of the last two decades. Nephrol Dial Transplant. 2022;37:1616-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Tingle SJ, Figueiredo RS, Moir JA, Goodfellow M, Talbot D, Wilson CH. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst Rev. 2019;3:CD011671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Mason P, Robb ML. Improving Access to Renal Transplantation for Highly Sensitized Patients. Transplantation. 2022;106:2299-2300. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Slagter JS, Outmani L, Tran KTCK, Ijzermans JNM, Minnee RC. Robot-assisted kidney transplantation as a minimally invasive approach for kidney transplant recipients: A systematic review and meta-analyses. Int J Surg. 2022;99:106264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Sigera LSM, Denning DW. Invasive Aspergillosis after Renal Transplantation. J Fungi (Basel). 2023;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Danger R, Le Berre L, Cadoux M, Kerleau C, Papuchon E, Mai HL, Nguyen TV, Guérif P, Morelon E, Thaunat O, Legendre C, Anglicheau D, Lefaucheur C, Couzi L, Del Bello A, Kamar N, Le Quintrec M, Goutaudier V, Renaudin K, Giral M, Brouard S; DIVAT Consortium. Subclinical rejection-free diagnostic after kidney transplantation using blood gene expression. Kidney Int. 2023;103:1167-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Boumaza A, Ben Azzouz E, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22:e280-e291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Wiech T, Reinhard L, Wulf S, Giuffrida AE, Longhitano E, Caruso R, Gröne HJ, Stahl RAK, Zipfel PF, Kikhney J, Moter A, Hoxha E, Santoro D. Bacterial infection possibly causing autoimmunity: Tropheryma whipplei and membranous nephropathy. Lancet. 2022;400:1882-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Yoon S, Choi YJ, Lim YK, Kweon OJ, Kim HR, Kim TH, Lee MK. Prevalence and detection of Tropheryma whipplei in the stools of Korean patients with diarrhea using real-time PCRs. Ann Clin Microbiol Antimicrob. 2022;21:55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Meyer S, Puéchal X, Quesne G, Marques I, Jamet A, Ferroni A. Contribution of PCR to Differential Diagnosis between Patients with Whipple Disease and Tropheryma whipplei Carriers. J Clin Microbiol. 2023;61:e0145722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 11. | Ioannou P, Kourtidis M, Mytilinis DO, Psyllaki A, Baliou S, Kofteridis D. Whipple’s disease-associated infective endocarditis: a systematic review. Infect Dis (Lond). 2023;55:447-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 12. | Manini A, Querzola G, Lovati C, Pantoni L. Rapidly progressive dementia and intractable diarrhea: a teaching case report and a systematic review of cognitive impairment in Whipple’s disease. Neurol Sci. 2022;43:907-926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 13. | Hajduczenia MM, Klefisch FR, Hopf AGM, Grubitzsch H, Stegemann MS, Pfäfflin F, Puhlmann B, Ocken M, Kretzler L, von Schöning D, Falk V, Moter A, Kikhney J. New Perspectives for Prosthetic Valve Endocarditis: Impact of Molecular Imaging by FISHseq Diagnostics. Clin Infect Dis. 2023;76:1050-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Makka S, Papadogiannaki I, Voulgari-Kokota A, Georgakopoulou T, Koutantou M, Angelakis E. Tropheryma whipplei Intestinal Colonization in Migrant Children, Greece. Emerg Infect Dis. 2022;28:1926-1928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Wang L, Su P, Song L, Sai L. Subcutaneous Nodules Caused by Tropheryma whipplei Infection. Emerg Infect Dis. 2022;28:761-763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Lin M, Wang K, Qiu L, Liang Y, Tu C, Chen M, Wang Z, Wu J, Huang Y, Tan C, Chen Q, Zheng X, Liu J. Tropheryma whipplei detection by metagenomic next-generation sequencing in bronchoalveolar lavage fluid: A cross-sectional study. Front Cell Infect Microbiol. 2022;12:961297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Diao Z, Han D, Zhang R, Li J. Metagenomics next-generation sequencing tests take the stage in the diagnosis of lower respiratory tract infections. J Adv Res. 2022;38:201-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 18. | Liu H, Zhang Y, Yang J, Liu Y, Chen J. Application of mNGS in the Etiological Analysis of Lower Respiratory Tract Infections and the Prediction of Drug Resistance. Microbiol Spectr. 2022;10:e0250221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Yang A, Chen C, Hu Y, Zheng G, Chen P, Xie Z, Fan H, Sun Y, Wu P, Jiang W, Wang C, Zhang J, Zhang D, Wang J, Hu X, Xia H, Yin G, Guo Y. Application of Metagenomic Next-Generation Sequencing (mNGS) Using Bronchoalveolar Lavage Fluid (BALF) in Diagnosing Pneumonia of Children. Microbiol Spectr. 2022;10:e0148822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 20. | Feurle GE, Junga NS, Marth T. Efficacy of ceftriaxone or meropenem as initial therapies in Whipple’s disease. Gastroenterology. 2010;138:478-86; quiz 11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Lagier JC, Fenollar F, Lepidi H, Giorgi R, Million M, Raoult D. Treatment of classic Whipple’s disease: from in vitro results to clinical outcome. J Antimicrob Chemother. 2014;69:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Marth T, Moos V, Müller C, Biagi F, Schneider T. Tropheryma whipplei infection and Whipple’s disease. Lancet Infect Dis. 2016;16:e13-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 23. | Feurle GE, Moos V, Bläker H, Loddenkemper C, Moter A, Stroux A, Marth T, Schneider T. Intravenous ceftriaxone, followed by 12 or three months of oral treatment with trimethoprim-sulfamethoxazole in Whipple’s disease. J Infect. 2013;66:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |