Published online Sep 6, 2023. doi: 10.12998/wjcc.v11.i25.5947
Peer-review started: June 6, 2023
First decision: July 17, 2023
Revised: July 31, 2023
Accepted: August 8, 2023
Article in press: August 8, 2023
Published online: September 6, 2023
Processing time: 86 Days and 15.2 Hours
Alport syndrome (AS) is an inherited disease of the glomerular basement membrane caused by mutations in genes encoding α3, α4, or α5 chains of type IV collagen. It manifests with hematuria or proteinuria, which is often accompanied by hearing impairments and ocular abnormalities. Histopathologically, AS shows mesangial proliferation and sometimes incidental immunoglobulin A (IgA) deposition. Hematuria or proteinuria is also a common presentation in patients with IgA nephropathy that makes it difficult to differentially diagnose AS and IgA nephropathy solely based on these clinical and pathological features.
Herein, we present the case of a 59-year-old female patient who was admitted to our hospital with persistent microscopic hematuria and occasional proteinuria that had lasted for > 2 years. This patient had a familial history of renal disease and was diagnosed with autosomal dominant AS (ADAS) and IgA nephropathy based on the findings of renal biopsy as well as genetic testing performed using whole-exome sequencing, which suggested that the patient carried a novel heterozygous variation (c.888G>A:p.Gln296Gln) in the COL4A3 gene that enriches the mutation spectrum of ADAS. The proband received an angiotensin receptor blocker therapy after a definitive diagnosis was established. After one year of therapy, a significant reduction in proteinuria was observed. The number of microscopic red blood cells per high-power field decreased to one-quarter of the baseline levels. Renal function also maintained well during the follow-up.
Our case highlights the significance of performing kidney biopsy and genetic testing in the diagnosis of AS and familial IgA nephropathy.
Core Tip: It is challenging to distinguish between Alport syndrome (AS) and immunoglobulin A (IgA) nephropathy solely based on clinical and pathological findings. This report highlights the diagnostic value of whole-exome sequencing in the precise diagnosis of AS and emphasizes the significance of renal biopsy and genetic detection in the early diagnosis of AS and familial IgA nephropathy.
- Citation: Chen YT, Jiang WZ, Lu KD. Novel COL4A3 synonymous mutation causes Alport syndrome coexistent with immunoglobulin A nephropathy in a woman: A case report. World J Clin Cases 2023; 11(25): 5947-5953
- URL: https://www.wjgnet.com/2307-8960/full/v11/i25/5947.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i25.5947
Both Alport syndrome (AS) and immunoglobulin A (IgA) nephropathy (IgAN) present with persistent hematuria, proteinuria, and progressive renal dysfunction. The prevalence of AS is approximately 1 in 50000 newborns, with males exhibiting a higher propensity for symptomatic manifestations compared with females[1]. AS is a genetically heterogeneous nephropathy that results from the mutations of COL4A3, COL4A4, and COL4A5 genes encoding α3, α4, and α5 chains of type Ⅳ collagen[2]. AS comprises three hereditary forms: X-linked AS, autosomal recessive AS, and autosomal dominant AS (ADAS). Patients with ADAS typically harbor a heterozygous pathogenic variant in the COL4A3 or COL4A4 gene and have a wide spectrum of clinical features, ranging from lack of symptoms to renal failure[3]. The heterogeneity of ADAS phenotypes leads to its underdiagnosis clinically. As next-generation sequencing and whole-exome sequencing (WES) become ever more prevalent, the prevalence of ADAS has been reported increasingly and accounts for approximately 20% of AS[3]. Early diagnosis of AS is important because progression to renal failure can be slowed by renin-angiotensin-aldosterone system blockade[4]. Nephrologists recommend that the renin-angiotensin-aldosterone blockade should be administered to patients with a heterozygous pathogenic COL4A3 or COL4A4 mutation from the onset of microalbuminuria, hypertension, or kidney impairment[4-6].
IgAN is the most common primary glomerulonephritis, and patients with IgAN are characterized by the deposition of IgA1 immune complexes in the glomerular mesangium. The global prevalence of IgAN varies widely. IgAN is more common in Asian countries, including China (45%–58% of primary glomerulonephritis), than in European countries (10%–35% of primary glomerulonephritis) and has a higher prevalence among males than females[7]. IgAN usually occurs sporadically, but approximately 15% of cases have an inheritable component[8]. With advances in genetic medicine, pathogenic or likely pathogenic variants in COL4A3–5 were confirmed in the IgAN family members[9,10]. When hematuria is ascribed to familial IgAN, it is more likely owing to unknown disease-causing COL4A3–5 variants. In the present study, we performed sequencing on a 59-year-old female patient who was diagnosed with IgAN by renal biopsy. A novel heterozygous mutation (c.888G>A) in the COL4A3 gene was identified in the patient through WES. The same mutation was also found in the proband’s asymptomatic son. Based on the results of genetic testing, histopathological findings, and pedigree analysis, the proband was diagnosed with co-occurring ADAS and IgAN.
A 59-year-old female patient presented with persistent microscopic hematuria and occasional proteinuria for > 2 years.
The patient was admitted to our hospital on November 11, 2020, with persistent microscopic hematuria and occasional proteinuria, in addition to frequent complaints of waist soreness and thirst.
The patient had no prior past medical history.
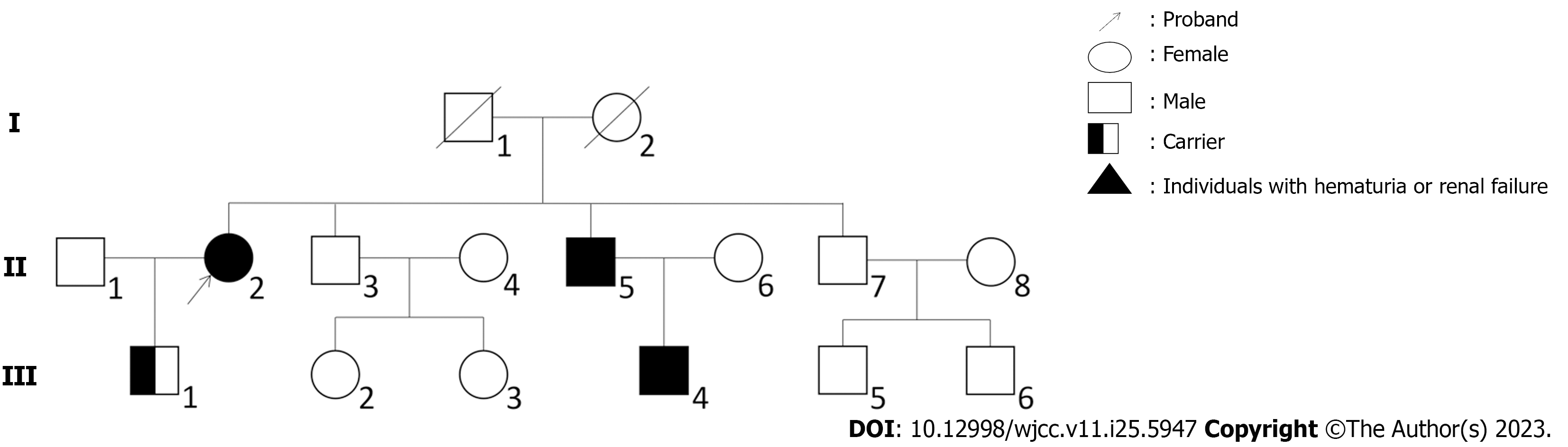
The patient had a family history of renal disease. Although her parents had died many years ago with no exact cause, one of her elder brothers was diagnosed with chronic renal failure in December 2021 whose 30-year-old son has been undergoing hemodialysis for many years (Figure 1).
The patient’s vital signs were normal, including a body temperature of 37.1 ℃, pulse of 91 beats/minute, respiratory rate of 18 breaths/minute, and blood pressure of 15.7/9.2 kPa. No abnormalities were detected in the eye and ear examinations. Physical examinations showed no abnormalities related to the abdomen, cardiopulmonary system, and lower extremities.
Laboratory tests showed the following results: 24-h urinary protein (223.2 mg), microhematuria (red blood cell count: 302.5/μL), poikilocyte (75%), albumin-to-creatinine ratio (181 mg/g), albumin (36.7 g/L), serum creatinine (0.52 mg/dL), potassium (13.65 mg/dL), total cholesterol (257.16 mg/dL), low-density lipoprotein (150.04 mg/dL), IgA (1.67 g/L), IgG (11.30 g/L), IgM (1.26 g/L), Complement 3 (1.03 g/L), and Complement 4 (0.32 g/L). The biochemical results are summarized in Table 1.
| Biochemical items | Results |
| Urinary albumin | ± |
| RBCs (/μL) | 302.5 |
| 24 h urinary protein (mg) | 223.2 |
| ACR (mg/g) | 181 |
| Poikilocyte | 75% |
| Total serum protein (g/L) | 62.8 |
| Albumin (g/L) | 36.7 |
| eGFR (mL/min per 1.73m2) | 149.20 |
| Serum creatinine (mg/dL) | 0.52 |
| Uric acid (mg/dL) | 5.23 |
| Potassium (mg/dL) | 13.65 |
| Total cholesterol (mg/dL) | 257.16 |
| Low density lipoprotein (mg/dL) | 150.04 |
| ANA | Negative |
| Anti-dsDNA antibody | Negative |
| IgA (g/L) | 1.67 |
| IgG (g/L) | 11.30 |
| IgM (g/L) | 1.26 |
| C3 (g/L) | 1.03 |
| C4 (g/L) | 0.32 |
| Anti-SSA antibody | Negative |
| Anti-SSB antibody | Negative |
| Hepatitis (A, B, or C) antibody | Negative |
No abnormalities were detected in the findings of the electrocardiogram and color Doppler echocardiography. The abdominal ultrasound revealed a left renal cyst and a 1.3 cm right upper ureteral calculus with expansion.
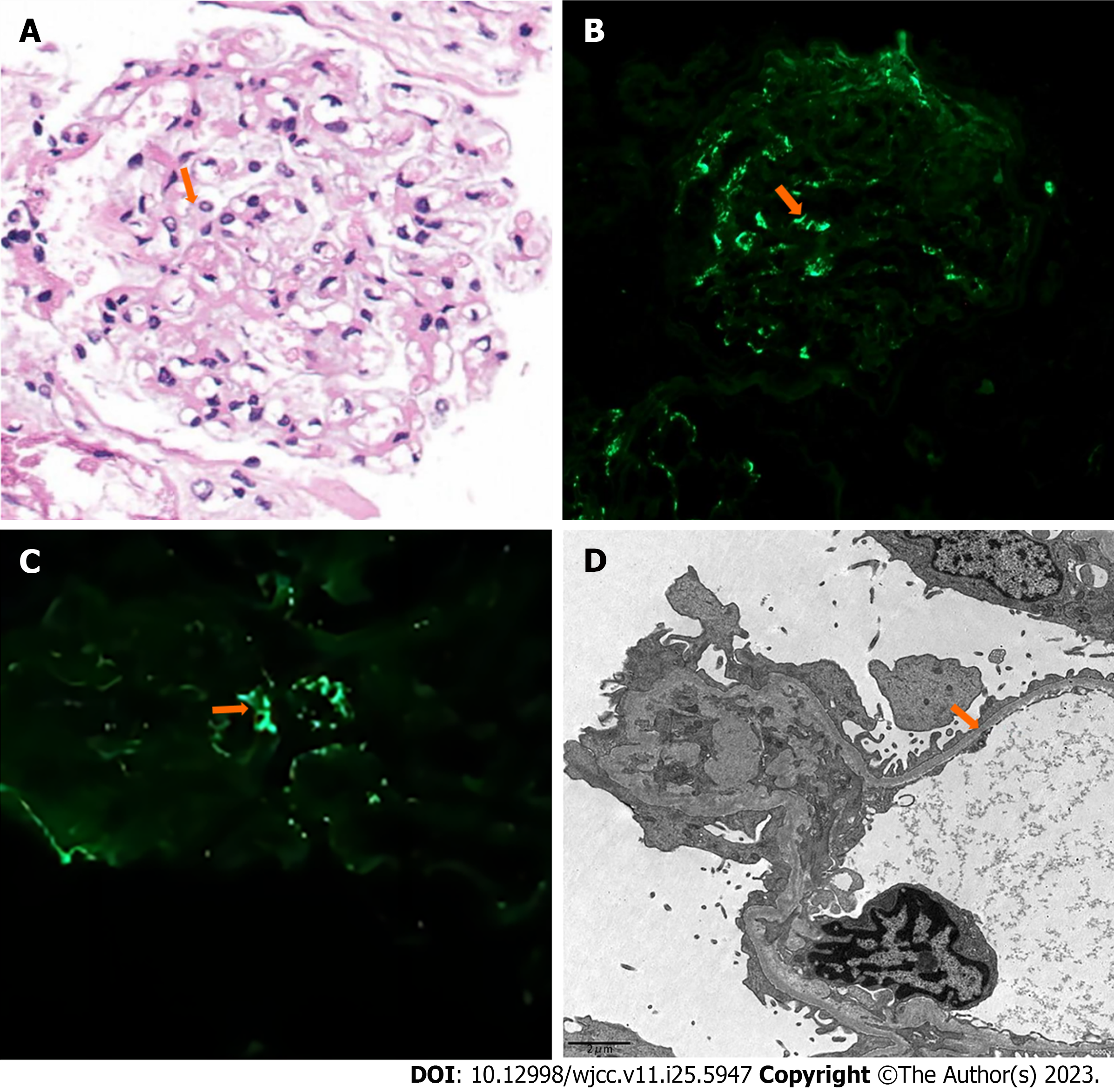
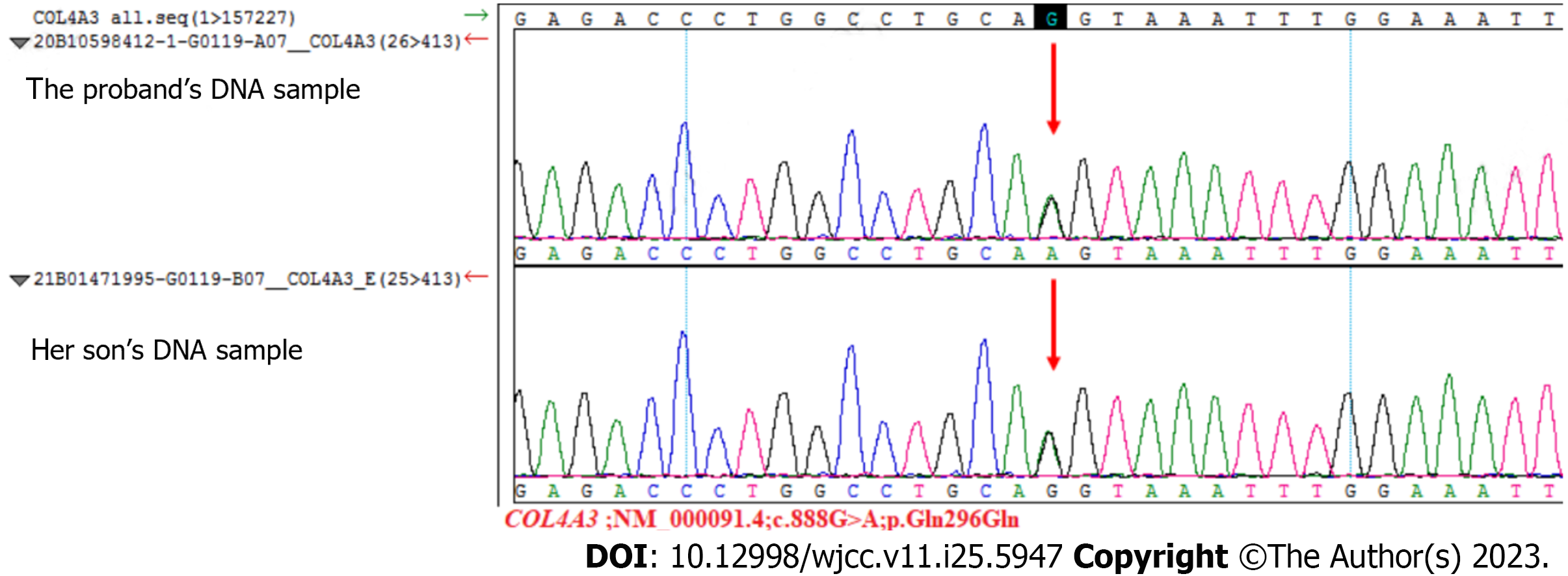
A holmium laser lithotripsy through ureteropyeloscopy was performed, and an indwelling double J stent was placed in the patient’s right ureter. One month later, the ureteral stent was removed, and a renal biopsy was performed the next day. We used hematoxylin and eosin, periodic acid-Schiff, periodic acid-silver methenamine, and Masson’s trichrome staining techniques for histopathological evaluation of tissue specimens. Twenty glomeruli were found in the biopsy specimen. Under light microscopy, the glomeruli showed a mild increase in mesangial cellularity and mesangial matrix. Congo red stain for amyloid gave negative results. Immunofluorescence showed a diffuse deposit of IgA (++) in the glomerular mesangial area. Furthermore, immunostaining for C3 and IgM was weakly positive, while immunostaining for C1q and IgG was negative. The electron microscopy image showed diffuse thinning of the glomerular basement membrane (GBM) and minor electron-dense depositions in a few mesangial areas (Figure 2). Based on the pathologic findings, the patient was diagnosed with IgAN. The MESTC score was M1E0S0T0-C0. We obtained the proband’s consent to carry out a genetic analysis to establish the diagnosis. Fresh venous blood was obtained from the patient for genomic DNA extraction. Firstly, the DNA was interrupted and prepared for library construction. Then, the exons of the target gene and the DNA adjacent to the shear region were captured and enriched by Roche KAPA HyperExome microarray. Finally, the mutation was detected by the MGISEQ-2000 sequencing platform. WES showed a unique heterozygous synonymous mutation in COL4A3. The mutation of c.888G>A:p.Gln296Gln locates in exon 15 of the COL4A3 gene (NM_000091.4) on chromosome 2, in which G in the 888th position was changed to A (c.888G>A), resulting in the CAG→CAA codon change without amino change. This mutation has not been reported so far in the literature. SpliceAI, dbscSNV_RF and dbscSNV_ADA indicated that it may have an effect on splicing. To widen the pedigree of patients, we also performed genetic testing of her son without clinical symptoms. Sequencing results demonstrated that the son harbored an identical mutation in the COL4A3 gene (Figure 3).
Based on the histopathological findings, family history, and genetic testing results, the diagnosis was established as ADAS with IgAN.
The patient was administered with atorvastatin calcium tablets (20 mg qd) and potassium chloride sustained-release tablets (0.5 g bid) when she was admitted. Subsequently, she received losartan potassium tablets (50 mg qd) after a definitive diagnosis.
One year after therapy, significant reduction in proteinuria was observed. Microscopic red blood cells per high-power field decreased to one quarter of the baseline level. Additionally, her renal function was maintained during the follow-up period.
Although the patient presented with minor microhematuria and proteinuria, her son had no clinical symptoms. Histologic findings included dominant IgA deposit in the mesangial area of the renal glomerulus and diffuse thinning of the GBM. The clinical manifestations and histologic findings were consistent with both IgAN and AS or thin basement membrane nephropathy (TBMN). The changes in the thickness of GBM as observed with electron microscopy suggested the possibility of TBMN, necessitating genetic testing. We sequenced the proband and her son with WES. WES showed that both the woman and her son had identical heterozygous mutation of c.888G>A:p.Gln296Gln in COL4A3 gene, which revealed the autosomal dominant pattern of genetic inheritance in the family. A comprehensive search of ESP6500, 1000 Genomes, ExAC, GnomAD and GnomAD-EAS databases showed that this mutation has not been previously reported associated with AS. Considering that her son had no renal symptoms, this hereditary condition showed incomplete penetrance in the family, which is consistent with a multifactorial disease model and confirms that other factors beyond a single causal factor leading to mutations are required to trigger disease onset[11].
The patient had no sensorineural deafness, characteristic retinopathy, or renal function impairment. Histologic findings showed a diffuse thin GBM with an average width of < 250 nm, without duplication, lamellation, or electron-dense deposits. These results suggested the diagnosis of TBMN rather than AS. Both AS and TBMN could result from heterozygous variations in COL4A3 or COL4A4 genes[5]. Generally, significant proteinuria, progressive kidney failure, and extrarenal diseases are not associated with TBMN, though some patients can progress to end-stage renal disease in later life. Inconsistent with the presentation of TBMN pedigree, the pedigree includes a brother diagnosed with chronic renal failure and his 30-year-old son has been undergoing hemodialysis for many years. One of the diagnostic criteria of AS is irregular thickening, thinning, and splitting of the GBM, whereas the electron microscopic findings in patients with ADAS commonly show diffuse thinning of the GBM. Therefore, it is difficult to make an accurate distinguishment between ADAS and TBMN. Clifford E. Kashtan[2] suggested establishing an early diagnosis and rendering timely treatment in patients with AS. Some patients with AS are undiagnosed with a label of “benign familial hematuria” or “TBMN” due to their relative lack of clinical signs. Nevertheless, some experts query the diagnosis of ADAS because the electron microscopy findings in many patients with ADAS include diffuse thinning rather than the typical basement membrane splitting, lamellation, and basket weaving[12]. They consider that the diagnosis of ADAS will add to excessive anxiety. However, the diagnostic term “TBMN” is inappropriate because the risk of end-stage renal disease will be underestimated and early treatment will be delayed. In the genomic era, there is growing agreement that patients with a heterozygous pathologic variation in the COL4A3 and/or COL4A4 genes should be considered to have ADAS[3].
IgAN may occur in a sporadic or familial form[13]. Intriguingly, clinical manifestations of IgAN can overlap considerably with AS, which results from mutations in the COL4A3, COL4A4, and COL4A5 genes[14,15]. Stapleton et al[10] conducted WES in 10 Irish families of which at least one member had biopsy-confirmed IgAN. They identified a likely disease-causing mutation in COL4A5 in one family and a variant of unknown significance in COL4A3 in another. Xu et al[16] confirmed a novel COL4A5 gene variation (chrX:107814698, c.438 +2->AAACCAATTATA-) in IgAN in a Chinese family and suggested that variable COL4A5 gene mutations may play different roles in IgAN. Li et al[9] discovered putatively disease-causing COL4A3–5 variations in 9 of the 46 familial IgAN cases (20%), of which 6 showed autosomal dominant inheritance, with 2 carrying heterozygous mutations in COL4A3 and 4 in COL4A4, and the remaining 3 demonstrated X-linked inheritance, with variations in the COL4A5 gene. These findings demonstrate that COL4A3–5 variations may be related to IgAN. Herein, our pedigree with biopsy-proven IgAN of the proband should be referred for assessment of familial IgAN. The case proposes a paradigm where AS and familial IgAN might represent two features of a single disease spectrum instead of two different pathologies.
Kamiyoshi et al[17] analyzed 25 patients with ADAS and their family members and revealed that the median age at detection of proteinuria was 17 years, the median renal survival time was 70 years, and only one patient developed hearing impairment and another had ocular abnormalities. Extrarenal manifestations in patients with ADAS are relatively rare compared with those in individuals with X-linked AS or autosomal recessive AS. The proband had a milder clinical course without ear and eye abnormalities which may be related to the autosomal dominant form of inheritance that has relatively minor impact on the renal function, and therefore the disturbance in the GBM architecture is milder. Besides, the synonymous variation in the gene loci may have reduced the severity of the disease.
Furlano et al[3] conducted a retrospective cohort study of 82 families (252 patients) with ADAS from Spanish hospitals, of which 216 of 252 patients underwent genetic testing. A heterozygous pathogenic mutation in COL4A3 was confirmed in 107 patients, and a pathogenic mutation in COL4A4 was confirmed in 133 patients. Digenic inheritance was confirmed in 12 patients. The most common type of pathogenic mutation was the missense variant (62.9%), the second was splicing variant (16.7%), and then in-frame indel > 5 amino acids (12.5%), indel truncating (11.6%), nonsense (6.5%), deletion (indel) < 5 amino acids (0.9%). We identified a heterozygous variation, c.888G>A, in exon 15 of COL4A3 gene in the proband and her son, which is predicted to lead to a synonymous amino acid change, and p.Gln296Gln. mutation results in illness by affecting the process of splicing primarily[18,19]. This novel mutation potentially caused abnormal exonic splicing and could be responsible for ADAS affecting the family.
In conclusion, the patient was diagnosed with ADAS with IgAN based on the findings of kidney biopsy and WES. The diagnosis of ADAS is difficult because of its wide phenotype spectrum. Renal biopsy is necessary to avoid misdiagnosis. Genetic testing is crucial for early diagnosis of patients and relatives at risk. In our case, the proband has a novel heterozygous synonymous mutation in the COL4A3 gene, which extends the gene mutational spectrum associated with ADAS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Weinstock BA, United States; Yari D, Iran S-Editor: Fan JR L-Editor: A P-Editor: Cai YX
| 1. | Pedrosa AL, Bitencourt L, Paranhos RM, Leitáo CA, Ferreira GC, Simões E Silva AC. Alport Syndrome: A Comprehensive Review on Genetics, Pathophysiology, Histology, Clinical and Therapeutic Perspectives. Curr Med Chem. 2021;28:5602-5624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Kashtan CE. Alport Syndrome: Achieving Early Diagnosis and Treatment. Am J Kidney Dis. 2021;77:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Furlano M, Martínez V, Pybus M, Arce Y, Crespí J, Venegas MDP, Bullich G, Domingo A, Ayasreh N, Benito S, Lorente L, Ruíz P, Gonzalez VL, Arlandis R, Cabello E, Torres F, Guirado L, Ars E, Torra R. Clinical and Genetic Features of Autosomal Dominant Alport Syndrome: A Cohort Study. Am J Kidney Dis. 2021;78:560-570.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Savige J, Lipska-Zietkiewicz BS, Watson E, Hertz JM, Deltas C, Mari F, Hilbert P, Plevova P, Byers P, Cerkauskaite A, Gregory M, Cerkauskiene R, Ljubanovic DG, Becherucci F, Errichiello C, Massella L, Aiello V, Lennon R, Hopkinson L, Koziell A, Lungu A, Rothe HM, Hoefele J, Zacchia M, Martic TN, Gupta A, van Eerde A, Gear S, Landini S, Palazzo V, Al-Rabadi L, Claes K, Corveleyn A, Van Hoof E, van Geel M, Williams M, Ashton E, Belge H, Ars E, Bierzynska A, Gangemi C, Renieri A, Storey H, Flinter F. Guidelines for Genetic Testing and Management of Alport Syndrome. Clin J Am Soc Nephrol. 2022;17:143-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 5. | Savige J, Gregory M, Gross O, Kashtan C, Ding J, Flinter F. Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J Am Soc Nephrol. 2013;24:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 6. | Kashtan CE, Ding J, Garosi G, Heidet L, Massella L, Nakanishi K, Nozu K, Renieri A, Rheault M, Wang F, Gross O. Alport syndrome: a unified classification of genetic disorders of collagen IV α345: a position paper of the Alport Syndrome Classification Working Group. Kidney Int. 2018;93:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 7. | Zhang Z, Zhang Y, Zhang H. IgA Nephropathy: A Chinese Perspective. Glomerular Dis. 2022;2:30-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 8. | Bhattacharyya A, Huang Y, Khan SH, Drachenberg CB, Malone LC. Tale of two nephropathies; co-occurring Alport syndrome and IgA nephropathy, a case report. BMC Nephrol. 2021;22:358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Li Y, Groopman EE, D'Agati V, Prakash S, Zhang J, Mizerska-Wasiak M, Caliskan Y, Fasel D, Karnib HH, Bono L, Omran SA, Sabban EA, Kiryluk K, Caridi G, Ghiggeri GM, Sanna-Cherchi S, Scolari F, Gharavi AG. Type IV Collagen Mutations in Familial IgA Nephropathy. Kidney Int Rep. 2020;5:1075-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Stapleton CP, Kennedy C, Fennelly NK, Murray SL, Connaughton DM, Dorman AM, Doyle B, Cavalleri GL, Conlon PJ. An Exome Sequencing Study of 10 Families with IgA Nephropathy. Nephron. 2020;144:72-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Kiryluk K, Julian BA, Wyatt RJ, Scolari F, Zhang H, Novak J, Gharavi AG. Genetic studies of IgA nephropathy: past, present, and future. Pediatr Nephrol. 2010;25:2257-2268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Imafuku A, Nozu K, Sawa N, Nakanishi K, Ubara Y. How to resolve confusion in the clinical setting for the diagnosis of heterozygous COL4A3 or COL4A4 gene variants? Discussion and suggestions from nephrologists. Clin Exp Nephrol. 2020;24:651-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Fennelly NK, Kennedy C, Jenkinson AC, Connaughton DM, Stapleton C, Dorman AM, Doyle B, Conlon PJ. Clinical Heterogeneity in Familial IgA Nephropathy. Nephron. 2018;139:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Gagliano Taliun SA, Sulem P, Sveinbjornsson G, Gudbjartsson DF, Stefansson K, Paterson AD, Barua M. GWAS of Hematuria. Clin J Am Soc Nephrol. 2022;17:672-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 15. | Gale DP. How benign is hematuria? Using genetics to predict prognosis. Pediatr Nephrol. 2013;28:1183-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Xu Z, Chen J, Yu W, Li X, Lin B, Lai D, Xu A, Tang Y. New COL4A5 mutation in IgA nephropathy. Postgrad Med J. 2022;98:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Kamiyoshi N, Nozu K, Fu XJ, Morisada N, Nozu Y, Ye MJ, Imafuku A, Miura K, Yamamura T, Minamikawa S, Shono A, Ninchoji T, Morioka I, Nakanishi K, Yoshikawa N, Kaito H, Iijima K. Genetic, Clinical, and Pathologic Backgrounds of Patients with Autosomal Dominant Alport Syndrome. Clin J Am Soc Nephrol. 2016;11:1441-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Pagani F, Baralle FE. Genomic variants in exons and introns: identifying the splicing spoilers. Nat Rev Genet. 2004;5:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 445] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 19. | Chamary JV, Parmley JL, Hurst LD. Hearing silence: non-neutral evolution at synonymous sites in mammals. Nat Rev Genet. 2006;7:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 609] [Article Influence: 32.1] [Reference Citation Analysis (0)] |