Published online Sep 6, 2023. doi: 10.12998/wjcc.v11.i25.5934
Peer-review started: April 12, 2023
First decision: July 3, 2023
Revised: July 14, 2023
Accepted: August 1, 2023
Article in press: August 1, 2023
Published online: September 6, 2023
Processing time: 142 Days and 8.8 Hours
Parathyroid carcinoma (PC) is a rare, slow-growing malignant tumor and a rare cause of primary hyperfunctioning of the parathyroid, with a highly variable clinical course, depending on the aggressiveness of the individual tumor and the degree of hypercalcemia.
The aim of this report is to summarize the diagnosis and treatment of three cases of PC and to review and conclude aspects regarding the three collected cases with reference to other relevant cases to explore the value of ultrasound in the diagnosis of PC. All three patients had hypercalcemia, consisting of a high serum calcium level and a high level of parathyroid hormone that was > 2-fold (even > 30-fold) of the normal upper limit. The ultrasonographic findings of the para
As clinical signs and laboratory results are nonspecific, it is difficult to diagnose PC preoperatively, so imaging examinations are often needed.
Core tip: Parathyroid carcinoma (PC) is a rare malignant tumor with a highly variable clinical course, depending on the aggressiveness of the individual tumor and the degree of hypercalcemia. As clinical signs and laboratory results are nonspecific, it is difficult to diagnose PC before surgery, so relevant imaging examinations are often needed. The aim of this report is to summarize the diagnosis and treatment of three cases of PC and to review and conclude aspects regarding the three collected cases with reference to other relevant cases to explore the value of ultrasound in the diagnosis of PC.
- Citation: Shi C, Lu N, Yong YJ, Chu HD, Xia AJ. Parathyroid carcinoma: Three case reports. World J Clin Cases 2023; 11(25): 5934-5940
- URL: https://www.wjgnet.com/2307-8960/full/v11/i25/5934.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i25.5934
Parathyroid carcinoma (PC) is a rare endocrine carcinoma that accounts for approximately 0.005% of all cancers and has an annual incidence of < 0.001%[1-4]. The main clinical features are hypersecretion of parathyroid hormone (PTH) and high serum calcium. PC has no clear diagnostic criteria, lacks specific manifestations, and is difficult to distinguish from parathyroid adenoma (PA) or hyperplasia. PC should be highly suspected if the serum calcium level exceeds 3.5 mmol/L, PTH exceeds 2-5 times the upper limit of normal, or the tumor touches the neck or is accompanied by vocal cord paralysis, or bone, kidney or other related manifestations[5]. In this study, ultrasound images of three patients with pathologically confirmed PC are summarized, and related literature was reviewed to explore the value of ultrasound in the diagnosis of PC.
Case 1: A 59-year-old man had a neck mass detected 10 d previously.
Case 2: A 28-year-old woman presented with weakness in the left lower limb.
Case 3: A 65-year-old woman presented with a neck mass that was noticed 6 mo previously and that had recently gradually increased in size.
Case 1: No tenderness was observed.
Case 1: Blood sugar control was satisfactory, and the patient had a history of diabetes for > 3 years.
Case 2: The patient had a 3-year history of kidney stones and presented with lumbago with pain.
Case 3: None.
Case 1: Serum calcium level was 2.78 mmol/L (normal range 2.11-2.52 mmol/L), and PTH was 207.6 pg/mL (normal range 12.3-88.3 pg/mL).
Case 2: Serum calcium was 3.47 mmol/L, and PTH was 2723 pg/mL.
Case 3: Serum calcium was 3.19 mmol/L, and PTH was 1388 pg/mL.
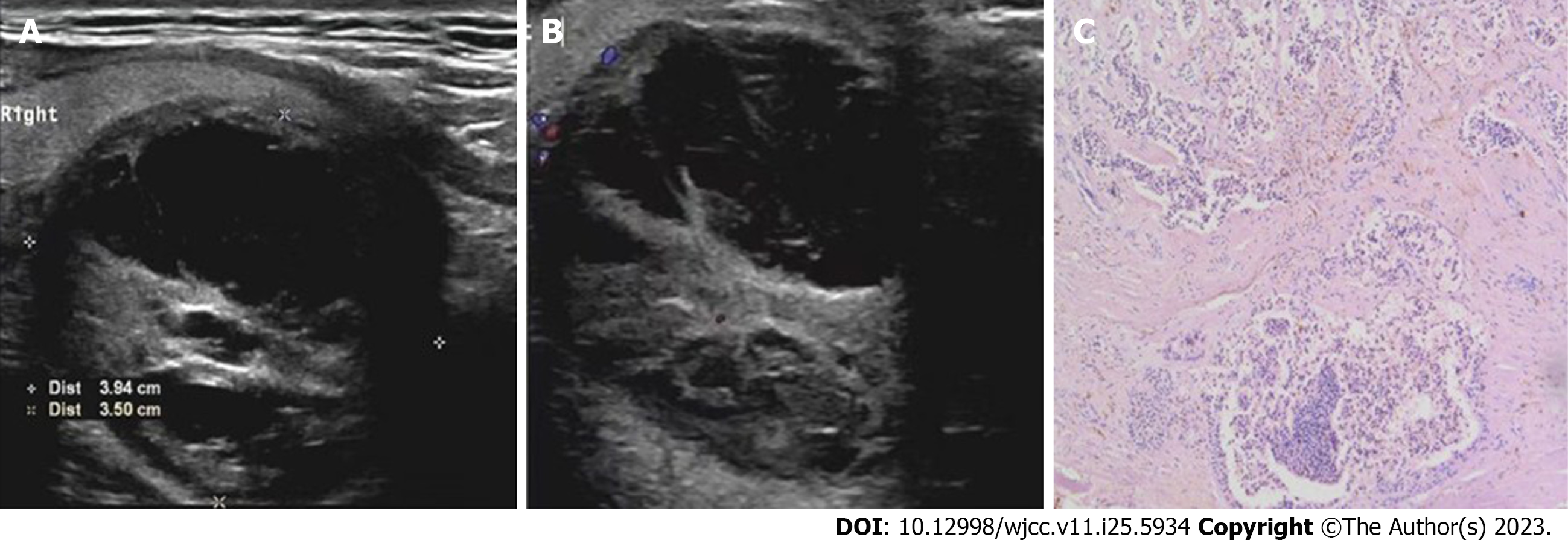
Case 1: Doppler ultrasound revealed a 3.9 cm × 3.5 cm × 2 cm regularly shaped complex nodule (with mixed echogenicity); that is, with solid and fluid components at the lower pole of the thyroid on the right side. The boundary between the nodule and the thyroid parenchyma was unclear (Figure 1A). Color doppler flow imaging (CDFI) indicated multiple blood flow signals in the internal solid part and around the nodule (Figure 1B). Computed tomography (CT) displayed circular shadow density at the lower pole of the right thyroid gland with a clear boundary. Mild-to-moderate enhancement was observed around the lesion, but no obvious enhancement was detected inside the lesion.
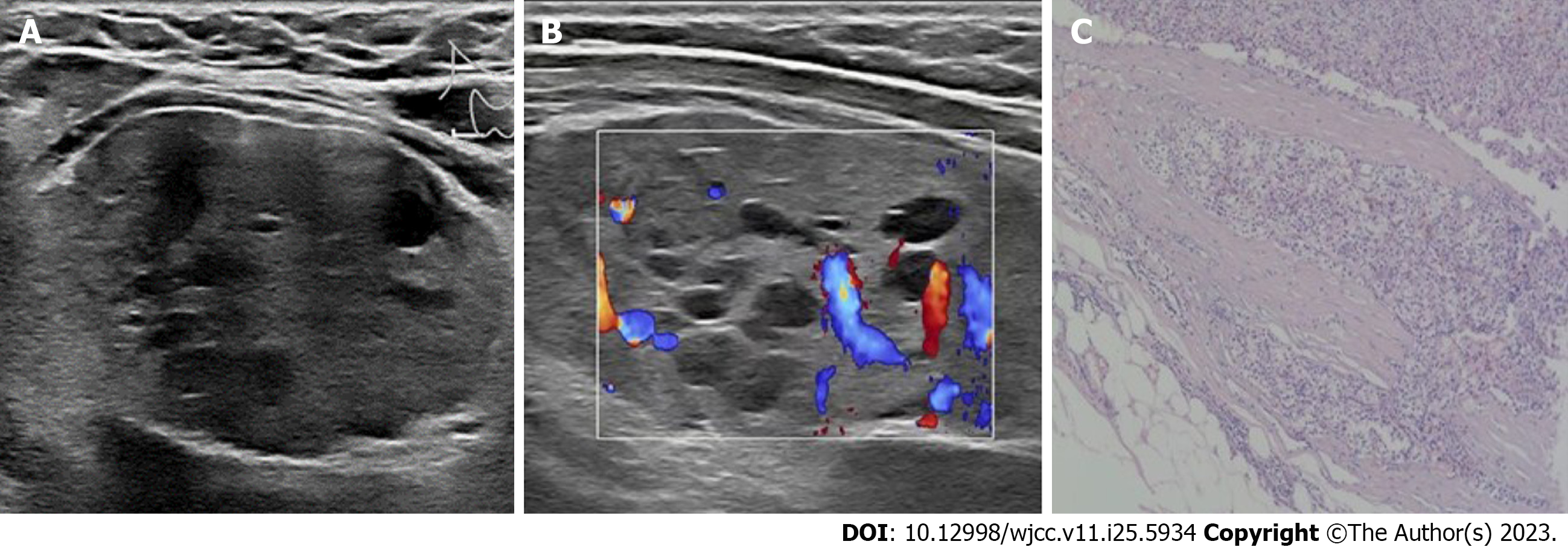
Case 2: Doppler ultrasound revealed a 5.4 cm × 4.4 cm × 2.8 cm regularly shaped solid nodule with small internal cystic foci in the lower right lobe of the thyroid gland. The nodule had a clear boundary (Figure 2A). CDFI revealed multiple blood flow signals in the solid part of the nodule (Figure 2B).
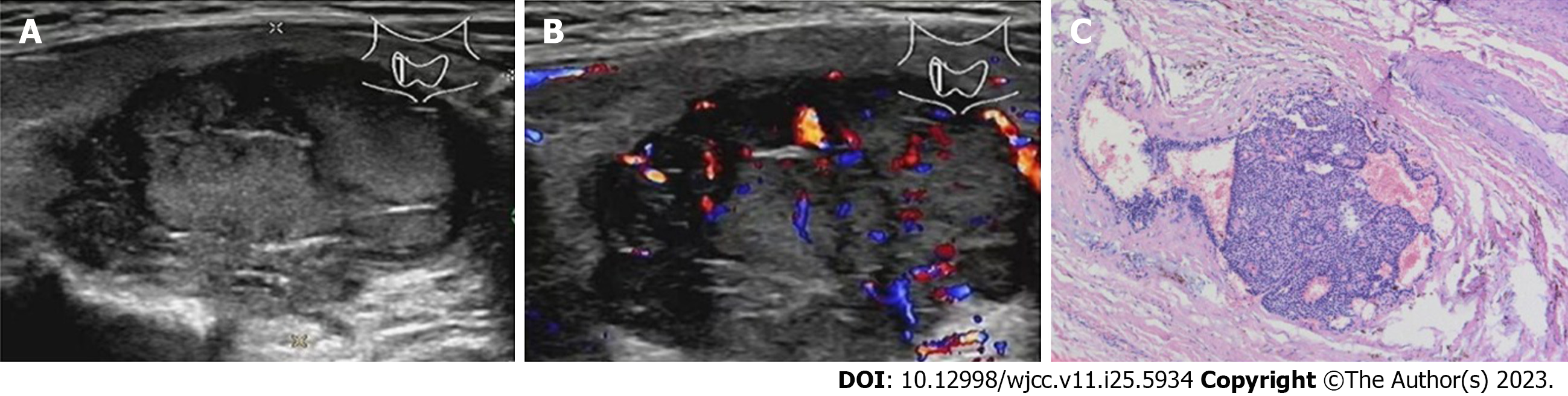
Case 3: Doppler ultrasound revealed a 3.2 cm × 2.6 cm × 2.4 cm, irregularly shaped, solid nodule with an inhomogeneous echo-structure at the lower right lobe of the thyroid gland. The boundary was unclear, and the internal echo was uneven (Figure 3A). CDFI indicated multiple blood flow signals within the nodule (Figure 3B). Several lymph nodes were detected on the right side of the neck; the larger lymph node was 1.3 cm × 0.5 cm, with poorly defined boundaries and no tenderness. A CT scan revealed an oval low-density lesion with a clear boundary in the middle and lower lobes of the right thyroid gland.
Case 1: A 3.5 cm × 3.0 cm × 2 cm mass was observed under the right thyroid gland, which was closely associated with the surrounding tissues. The mass surface was smooth, and the envelope was complete with a variable thickness. The section was a solid cyst. The cystic part was multilocular, the inner wall of the sac was smooth, and the solid part was grayish-red, soft and brittle. The parathyroid neoplastic master cells were arranged in a solid flaky-like, nest-like, acinar pattern with diffuse infiltrating growth that invaded a nerve and locally infiltrated but did not penetrate the capsule. A small amount of thyroid tissue was seen in the tumor margin focus (Figure 1C). Immunohistochemistry revealed PTH (+), calcitonin (-), carcinoembryonic antigen (-), chromogranin A (CgA) (+), synaptophysin (Syn) (-), thyroid transcription factor-1 (TTF-1) (-), thyroglobulin (Tg) (-) and Ki67 (1%).
Case 2: An enlarged parathyroid gland (6 cm × 4 cm × 3 cm) was observed below the right thyroid gland during the operation. The general surface of the nodule was smooth, the capsule was intact, and the section surface was dark red, multinodular and soft. The main cells of the parathyroid gland were neoplastic and hyperplastic, and coarse fiber segmentation was seen in the tumor. Some tumor cells were atypical, with mitotic images of approximately 2/50 per high-power field (HPF). The tumor envelope was of different thicknesses, and the tumor had invaded the envelope. The blood vessels had invaded the thyroid, and a tumor plug was seen in the blood vessels (Figure 2C). Immunohistochemistry revealed PTH (+) and Ki67 (4%).
Case 3: A 3.4 cm × 2.4 cm × 2.2 cm mass was found at the lower pole of the right thyroid gland, which had adhered to the surrounding esophageal and recurrent laryngeal nerves. The general surface was smooth, the envelope was relatively complete, and the section surface was grayish-brown, soft and slightly brittle. The local envelope of the tumor was not obvious, there were visible mitoses (< 2/10 HPF), and the tumor had invaded the thyroid tissue (Figure 3C). Immunohistochemistry revealed PTH (+), calcitonin (-), cytokeratin pan (+), cytokeratin19 (+), CgA (+), neural cell adhesion molecule (NCAM; CD56) (-), Syn (weak +), TTF-1 (-), Tg (-) and Ki67 (5%).
Case 1: The patient was pathologically diagnosed with highly differentiated PC (low grade).
Case 2: The pathological diagnosis was parathyroid adenocarcinoma.
Case 3: The lesion was pathologically diagnosed as parathyroid adenocarcinoma.
Case 1: The right inferior pole of the parathyroid and the right thyroid were resected.
Case 2: The right parathyroid lesion was resected.
Case 3: The right parathyroid gland was explored, the right parathyroid lesion, the right thyroid and the isthmus were resected, and a regional lymphadenectomy was performed.
Case 1: The 5-year follow-up did not show residual–recurrent disease.
Case 2: The 1-year follow-up did not show residual–recurrent disease.
Case 3: The 1-year follow-up did not show residual–recurrent disease.
PC is a rare, slow-growing malignant tumor and a rare cause of primary hyperfunctioning of the parathyroid (PHPT), with an incidence of 0.5%-7.0%[6,7]. Approximately 95% of PC tumors are functional and clinically manifested by various symptoms and signs caused by excessive secretion of PTH and hypercalcemia, including moderate to severe hypercalcemia (a parathyroid crisis may occur in some patients), kidney stones, renal calcinosis, osteoporosis, osteitis cystic fibrosis, brown tumors, fractures, constipation, abdominal pain, peptic ulcers and pancreatitis[8,9]. The PTH and serum calcium were increased in these three patients, and PTH was 2.35, 30.84 and 15.72 times the upper limit of normal, respectively. Case 2 presented with osteoporosis and recurrent kidney stones. Cases 1 and 3 presented at the clinic for painless anterior neck masses. It has been reported that 30%-76% of patients with PC have a palpable mass in the neck[10], which is uncommon in patients with PA. PC is considered a slow-growing malignant tumor with a highly variable clinical course, depending on the aggressiveness of the individual tumor and the degree of hypercalcemia. As clinical signs and laboratory results are nonspecific, it is difficult to diagnose PC before surgery, so relevant imaging examinations are often needed.
A variety of techniques can be used to image the parathyroid gland, including ultrasound, methoxy isobutyl isonitrile (MIBI) imaging, CT and magnetic resonance imaging (MRI). Ultrasound is the first choice to accurately assess the size of a tumor without radiation and at a low cost and has significant value for detecting parathyroid disease. Studies have shown that ultrasound has a sensitivity of 67%-96% and a positive predictive value of 82%-98% in patients undergoing PHPT surgery[11-14]. The ultrasonographic findings of normal parathyroid glands are oval hypoechoic or hyperechoic nodules with a maximum diameter of 4-7 mm, clear boundaries and a uniform echo. PC is usually larger, lobulated, hypoechoic, heterogeneous and ill-defined compared with PA. A high positive predictive value is obtained when the parathyroid lesion is > 30 mm, calcified, and has an unclear boundary with the surrounding tissue[15,16]. In this group of three patients, the tumors were > 30 mm, and the internal echo was uneven. In cases 1 and 2, the tumors were cystic and solid, and in cases 1 and 3, the tumors had unclear boundaries with the surrounding tissue, which was consistent with malignant signs. However, ultrasound has some limitations. Parathyroid diseases can be difficult to distinguish from thyroid nodules and lymph nodes, so the clinical manifestations and a laboratory examination should be combined for identification. In addition, when the parathyroid lesion is cystic, it should be distinguished from other cystic neck lesions. For cystic neck lesions, close attention should be paid to the age of presentation, lesion location, and association with surrounding structures[17,18]. If located on the midline, the differential diagnosis narrows to thyroglossal duct cysts, ranulas or dermoid cysts. Off-midline lesions can be, instead, branchial cleft cysts or lymphangiomas[17]. Due to interference by the sternum and clavicle, ultrasound examination of hypolocated parathyroid tumors or ectopic parathyroid tumors in the retropharyngeal space or mediastinum is limited, so other imaging examinations are needed. Tc-99 m-MIBI is widely used to localize parathyroid tumors, but there is no significant specificity between benign and malignant parathyroid diseases. CT and MRI have limited diagnostic value in patients with parathyroid disease but can be used to assess cervical lymph node status, detect ectopic parathyroid tumors and assess preoperative risk[19].
There are some difficulties when preoperatively and intraoperatively diagnosing PC. Fine-needle aspiration can be used to distinguish thyroid from parathyroid tissues, but it has limited value in the differential diagnosis of benign and malignant parathyroid tumors and may cause local dissemination and implantation of tumors, so it is not recommended[20,21]. Because the histopathological features of PC may overlap with PA, an intraoperative rapid frozen pathological examination has been demonstrated to lack reliability. Therefore, postoperative pathological examination of the lesion is the gold standard for diagnosing PC. PC is pathologically diagnosed if the tumor is invasive, such as in the capsule, blood vessels or nerves, or has local or distant metastasis[22].
Surgical excision is an effective method to treat PC, and the standard of treatment is total excision of the tumor and surrounding tissues[20]. Selective cervical lymph node dissection is also necessary when the cervical lymph nodes are involved. However, the necessity of prophylactic septal lymphadenectomy remains controversial[23]. In this study, all three patients underwent surgical resection of the diseased parathyroid gland combined with resection of the affected thyroid gland. PC is an indolent, slowly progressive cancer since the tumor has a low malignant potential. Few patients initially present with regional lymph node involvement (< 5%) or with distant metastatic disease (< 2%)[24]. PC can recur 2-5 years after the initial operation, and the local recurrence rate within 5 years is 33%-82%[25]. The prognosis mainly depends on whether the tumor is completely removed during the first operation. Most patients do not die from the tumor disease burden since it is slow growing. Instead, most patients with metastatic disease die from complications of hypercalcemia[26,27]. Consequently, postoperative serum calcium and PTH monitoring are also important to detect whether the tumor was completely removed and whether there is early recurrence and metastasis. This especially applies to serum calcium levels, since most of these patients are highly susceptible to hungry bone syndrome. They require regular monitoring of their calcium and PTH levels every 3 mo for up to 1 year to evaluate for recurrence[28]. If the levels remain stable at this point, the duration between laboratory assessments can gradually be increased.
PC is a rare head and neck malignant tumor. Significant increases in serum calcium and PTH should be warning signs of a mass in the neck. The best surgical method should be selected to improve the survival rate of patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cigrovski Berkovic M, Croatia; Corvino A, Italy; Pappachan JM, United Kingdom S-Editor: Qu XL L-Editor: Kerr C P-Editor: Zhang YL
| 1. | Duan K, Mete Ö. Parathyroid Carcinoma: Diagnosis and Clinical Implications. Turk Patoloji Derg. 2015;31 Suppl 1:80-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Ozolins A, Narbuts Z, Vanags A, Simtniece Z, Visnevska Z, Akca A, Wirowski D, Gardovskis J, Strumfa I, Goretzki PE. Evaluation of malignant parathyroid tumours in two European cohorts of patients with sporadic primary hyperparathyroidism. Langenbecks Arch Surg. 2016;401:943-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | James BC, Aschebrook-Kilfoy B, Cipriani N, Kaplan EL, Angelos P, Grogan RH. The Incidence and Survival of Rare Cancers of the Thyroid, Parathyroid, Adrenal, and Pancreas. Ann Surg Oncol. 2016;23:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Lee PK, Jarosek SL, Virnig BA, Evasovich M, Tuttle TM. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer. 2007;109:1736-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Agarwal S, Kumar T, Sharma MC, Damle NA, Gandhi AK. Parathyroid carcinoma with contralateral subcutaneous and breast recurrences: A rare presentation. Head Neck. 2016;38:E115-E118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Pelizzo MR, Piotto A, Bergamasco A, Rubello D, Casara D. [Parathyroid carcinoma. Therapeutic strategies derived from 20 years of experience]. Minerva Endocrinol. 2001;26:23-29. [PubMed] |
| 7. | Wermers RA, Khosla S, Atkinson EJ, Achenbach SJ, Oberg AL, Grant CS, Melton LJ 3rd. Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993-2001: an update on the changing epidemiology of the disease. J Bone Miner Res. 2006;21:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | Rodriguez C, Nadéri S, Hans C, Badoual C. Parathyroid carcinoma: a difficult histological diagnosis. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:157-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Kim HK, Oh YL, Kim SH, Lee DY, Kang HC, Lee JI, Jang HW, Hur KY, Kim JH, Min YK, Chung JH, Kim SW. Parafibromin immunohistochemical staining to differentiate parathyroid carcinoma from parathyroid adenoma. Head Neck. 2012;34:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Shane E. Clinical review 122: Parathyroid carcinoma. J Clin Endocrinol Metab. 2001;86:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 289] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Haber RS, Kim CK, Inabnet WB. Ultrasonography for preoperative localization of enlarged parathyroid glands in primary hyperparathyroidism: comparison with (99m)technetium sestamibi scintigraphy. Clin Endocrinol (Oxf). 2002;57:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Whitson BA, Broadie TA. Preoperative ultrasound and nuclear medicine studies improve the accuracy in localization of adenoma in hyperparathyroidism. Surg Today. 2008;38:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Ulanovski D, Feinmesser R, Cohen M, Sulkes J, Dudkiewicz M, Shpitzer T. Preoperative evaluation of patients with parathyroid adenoma: role of high-resolution ultrasonography. Head Neck. 2002;24:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Abboud B, Sleilaty G, Rabaa L, Daher R, Abou Zeid H, Jabbour H, Hachem K, Smayra T. Ultrasonography: highly accuracy technique for preoperative localization of parathyroid adenoma. Laryngoscope. 2008;118:1574-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Sidhu PS, Talat N, Patel P, Mulholland NJ, Schulte KM. Ultrasound features of malignancy in the preoperative diagnosis of parathyroid cancer: a retrospective analysis of parathyroid tumours larger than 15 mm. Eur Radiol. 2011;21:1865-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Cetani F, Pardi E, Marcocci C. Parathyroid Carcinoma. Front Horm Res. 2019;51:63-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Corvino A, Pignata S, Campanino MR, Corvino F, Giurazza F, Tafuri D, Pinto F, Catalano O. Thyroglossal duct cysts and site-specific differential diagnoses: imaging findings with emphasis on ultrasound assessment. J Ultrasound. 2020;23:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Wong KT, Lee YY, King AD, Ahuja AT. Imaging of cystic or cyst-like neck masses. Clin Radiol. 2008;63:613-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Mulla M, Schulte KM. Central cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the central compartment. Clin Endocrinol (Oxf). 2012;76:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Owen RP, Silver CE, Pellitteri PK, Shaha AR, Devaney KO, Werner JA, Rinaldo A, Ferlito A. Parathyroid carcinoma: a review. Head Neck. 2011;33:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Kassahun WT, Jonas S. Focus on parathyroid carcinoma. Int J Surg. 2011;9:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Schantz A, Castleman B. Parathyroid carcinoma. A study of 70 cases. Cancer. 1973;31:600-605. [PubMed] [DOI] [Full Text] |
| 23. | Mohebati A, Shaha AR. Anatomy of thyroid and parathyroid glands and neurovascular relations. Clin Anat. 2012;25:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Schulte KM, Talat N, Miell J, Moniz C, Sinha P, Diaz-Cano S. Lymph node involvement and surgical approach in parathyroid cancer. World J Surg. 2010;34:2611-2620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Kebebew E. Parathyroid carcinoma. Curr Treat Options Oncol. 2001;2:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Machado NN, Wilhelm SM. Parathyroid Cancer: A Review. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Salcuni AS, Cetani F, Guarnieri V, Nicastro V, Romagnoli E, de Martino D, Scillitani A, Cole DEC. Parathyroid carcinoma. Best Pract Res Clin Endocrinol Metab. 2018;32:877-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 28. | Givi B, Shah JP. Parathyroid carcinoma. Clin Oncol (R Coll Radiol). 2010;22:498-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |