Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5700
Peer-review started: May 29, 2023
First decision: June 19, 2023
Revised: July 13, 2023
Accepted: July 28, 2023
Article in press: July 28, 2023
Published online: August 26, 2023
Processing time: 87 Days and 17.5 Hours
Diabetic ketoacidosis (DKA) manifests as hyperglycemia, metabolic acidosis, and ketosis. However, euglycemic DKA (eu-DKA) conceals severe DKA with glucose levels below 200 mg/dL. Sodium-glucose cotransporter-2 (SGLT2) inhibitors can induce eu-DKA in diabetic patients. Notably, coronavirus disease 2019 (COVID-19) -infected individuals with diabetes using SGLT2 inhibitors face an augmented risk of eu-DKA due to the direct toxic impact of the virus on pancreatic islets. This study aims to comprehensively investigate the association between SGLT2 inhibitors and eu-DKA in COVID-19 patients through meticulous case report analysis. Additionally, we endeavor to examine the outcomes and treatment approaches for COVID-19-infected diabetics receiving SGLT2 inhibitors, providing indispensable insights for healthcare professionals managing this specific patient population.
To investigate the connection between SGLT2 inhibitors and euglycemic DKA in COVID-19 patients through a meticulous analysis of case reports.
We conducted an exhaustive search across prominent electronic databases, including PubMed, SCOPUS, Web of Science, and Google Scholar. This search encompassed the period from December 2019 to May 2022, incorporating published studies and pre-prints. The search terms employed encompassed “SGLT2 inhibitors”, “euglycemic DKA”, “COVID-19”, and related variations. By incorporating these diverse sources, our objective was to ensure a thorough exploration of the existing literature on this subject, thereby augmenting the validity and robustness of our findings.
Our search yielded a total of seven case reports and one case series, collectively comprising a cohort of twelve patients. These reports detailed instances of eu-DKA in individuals with COVID-19. Crucially, all twelve patients were utilizing SGLT2 as their primary anti-diabetic medication. Upon admission, all oral medications were promptly discontinued, and the patients were initiated on intravenous insulin therapy to effectively manage the DKA. Encouragingly, eleven patients demonstrated a favorable outcome, while regrettably, one patient succumbed to the condition. Subsequently, SGLT2 were discontinued for all patients upon their discharge from the hospital. These findings provide valuable insights into the clinical management and outcomes of eu-DKA cases associated with COVID-19 and SGLT2, underscoring the critical importance of prompt intervention and vigilant medication adjustments.
Our study sheds light on the possibility of diabetic patients developing both drug-related and unrelated DKA, as well as encountering adverse outcomes in the context of COVID-19, despite maintaining satisfactory glycemic control. The relationship between glycemic control and clinical outcomes in COVID-19 remains ambiguous. Consequently, this systematic review proposes that COVID-19-infected diabetic patients using SGLT2 should contemplate alternative treatment protocols until their recovery from the disease.
Core Tip: This systematic review provides a comprehensive analysis of the relationship between sodium-glucose cotransporter-2 (SGLT2) inhibitors, euglycemic diabetic ketoacidosis (eu-DKA), and coronavirus disease 2019 (COVID-19) in patients with diabetes. Despite maintaining optimal glycemic control, individuals using SGLT2 inhibitors are still susceptible to both drug-induced and unrelated diabetic ketoacidosis (DKA), with potential adverse consequences during COVID-19 infection. Clinicians should exercise caution when prescribing SGLT2 inhibitors to diabetic patients affected by COVID-19 and carefully consider alternative treatment strategies. A thorough understanding of the intricate interplay between SGLT2 inhibitors, euglycemic DKA, and COVID-19 is crucial for optimizing patient management and achieving favorable clinical outcomes.
- Citation: Khedr A, Hennawi HA, Khan MK, Eissa A, Mir M, Rauf I, Nitesh J, Surani S, Khan SA. Sodium-glucose cotransporter-2 inhibitor-associated euglycemic diabetic ketoacidosis in COVID-19-infected patients: A systematic review of case reports. World J Clin Cases 2023; 11(24): 5700-5709
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5700.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5700
Understanding the signs, symptoms, risk factors, and outcomes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection remains an ongoing endeavor for clinicians and scientists. While coronavirus disease 2019 (COVID-19) research progresses rapidly, recent discoveries call for rigorous investigation to establish new hypotheses and evidence-based conclusions. Patients with diabetes mellitus face a higher likelihood of developing severe COVID-19 and experiencing adverse outcomes[1]. Notably, SGLT2 inhibitors exhibit cardiovascular benefits that extend beyond glycemic control[2]. Diabetic ketoacidosis (DKA) represents a life-threatening complication of diabetes mellitus (DM) characterized by elevated serum glucose (> 250 mg/dL), high anion gap metabolic acidosis, and plasma ketone levels. However, DKA can occur even with mild to moderate serum glucose elevation, known as euglycemic DKA (eu-DKA)[3].
The Food and Drug Administration has issued warnings about the increased risk of eu-DKA associated with sodium-glucose cotransporter-2 (SGLT2) inhibitors[4]. However, findings from the CANVAS trial suggest a low incidence of eu-DKA in patients using SGLT2 inhibitors (0.6 events per 1000 patient years)[5]. It is important to note that COVID-19 infection may amplify this risk due to the potential pancreato-toxic effects of the virus. Although three studies have examined the relationship between SGLT2 inhibitors and eu-DKA, methodological variations exist[6-8]. The prevalence of eu-DKA among type 2 diabetes mellitus (T2DM) patients treated with SGLT2 inhibitors is less than 0.1%[9]. Eu-DKA is a critical crisis that often goes unnoticed due to the absence of evident hyperglycemic symptoms. Pathophysiological studies have elucidated the mechanisms underlying this effect[10]. SGLT2 inhibitors induce glucosuria by inhibiting the sodium-glucose cotransporter in the convoluted proximal tubule, leading to decreased serum glucose levels and subsequent insulinopenia. Insulinopenia triggers an increase in counter-regulatory hormones (catecholamines and glucagon), which stimulates lipolysis and enhances fatty acid production, resulting in ketosis in the euglycemic state given the prior reduction in blood glucose by SGLT2 inhibitors.
The combined effect of COVID-19’s pancreatic toxicity and SGLT2 inhibitors’ glucosuria induces severe insulinopenia, potentially elevating the risk of eu-DKA in affected patients. However, the understanding of this mechanism and the management of eu-DKA in individuals with concurrent COVID-19 infection remains limited in the existing literature. In this systematic review, we have included a comprehensive analysis of relevant case report studies that provide important insights into the association between SGLT2 inhibitors, eu-DKA, and COVID-19 in patients with diabetes mellitus. These case reports shed light on the clinical manifestations, treatment approaches, and outcomes of eu-DKA in individuals with COVID-19 who are receiving SGLT2 inhibitor therapy.
This systematic review adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[11]. A comprehensive electronic search was conducted from December 2019 to May 2022 using the SCOPUS, Web of Science, MEDLINE, and Google Scholar databases. The search was limited to English language publications, and the detailed search string employed is provided in the Supplementary material. In addition, we included the pharmaceutical, generic, and trade names of SGLT2 inhibitors in our search. To ensure thoroughness, we manually examined the reference lists of the included studies to identify any potentially relevant articles that might have been overlooked. Detailed search strategies used for all databases are available in the Supplementary material. The research protocol for this review was registered in the International Prospective Register of Systematic Reviews under registration number CRD42022341562.
We included published case reports, case series, and pre-print articles. The selection of studies was based on specific eligibility criteria, which included the following: (1) Reports describing patients with euglycemic diabetic ketoacidosis (eu-DKA) who were admitted and treated following the guidelines set by the Association of British Clinical Diabetologists[12]; (2) inclusion of adult patients with either type 1 diabetes mellitus (T1DM) or T2DM; (3) utilization of SGLT2 inhibitors as the primary intervention for glucose control; and (4) confirmation of eu-DKA cases. Exclusion criteria were as follows: (1) Studies that did not focus on the association between SGLT2 inhibitors and eu-DKA in COVID-19 patients; (2) studies involving pediatric patients; (3) non-English language publications; (4) studies without clear documentation of eu-DKA cases or treatment approaches; and (5) review articles, editorials, and conference abstracts. The study selection process followed specific eligibility criteria based on the PICO framework:
Participants (P): Adult patients with either T1DM or T2DM diagnosed with eu-DKA.
Intervention (I): Utilization of SGLT2 inhibitors as the primary intervention for glucose control.
Comparator (C): Not applicable as this review focused on the association between SGLT2 inhibitors and eu-DKA in COVID-19 patients.
Outcomes (O): Clinical manifestations, treatment approaches, and outcomes of eu-DKA in patients with concurrent COVID-19 infection. The favorable outcome was defined as survival and hospital discharge.
The systematic search yielded articles that were imported into the Endnote Reference Library software, where duplicates were identified and removed. Initially, two independent reviewers (Khedr A and Hennawi HA) screened the articles based on titles and abstracts. Subsequently, two additional independent reviewers (Mir M and Rauf I) performed full-text screening of the filtered articles. The entire content of each article was thoroughly reviewed, with two authors (Khan MK and Eissa A) ensuring its relevance and piloting the process with one report. In case of any discrepancies, a third investigator (Nitesh J) was consulted for resolution. Information extracted from the articles included demographic background, comorbidities, disease onset, initial symptoms, laboratory tests, diabetes mellitus type, SGLT2 inhibitor type, study date, study design, treatment intervention, and case outcomes. The quality of the included studies was assessed using the CARE (case report) guidelines[13].
The extracted information was qualitatively synthesized using a narrative approach due to the limitations of the included case reports, which presented small effect sizes and precluded quantitative analysis for calculating effect estimates.
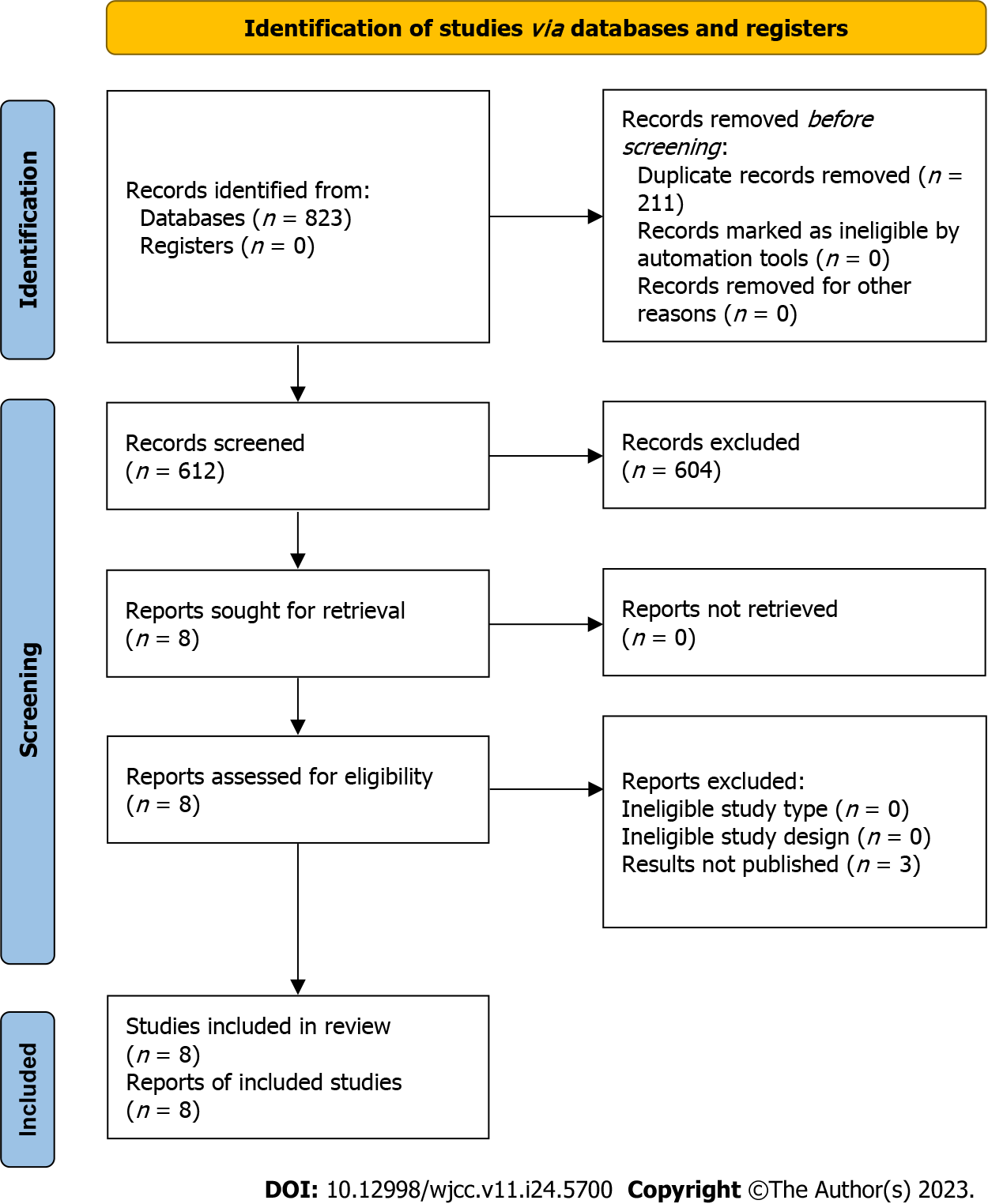
The study selection process was summarized using the PRISMA flow chart (Figure 1), which illustrates the search and selection of studies. Initially, a total of 3370 studies were identified from the databases, and after removing duplicates (n = 1823), eight studies met the inclusion criteria and were included in this review. These studies consisted of seven case reports and one case series that investigated the incidence of eu-DKA in COVID-19 patients receiving SGLT2 inhibitors[14-21]. The combined study population across these studies comprised 12 patients.
All the included case reports adhered to the CARE guidelines, ensuring a standardized reporting of case details. A comprehensive quality assessment table can be found in Supplementary Table 1, providing a detailed evaluation of the methodological quality of each included study.
The patients included in the studies were from various countries, including the United States (n = 3)[14,18,19], United Kingdom (n = 5)[15], Brazil (n = 1)[16], Malaysia (n = 1)[20], and Belgium (n = 2)[17,21]. The reported cases of type 1 diabetes mellitus (T1DM) were observed in the studies conducted in the United Kingdom and Belgium[17,21].
The age range of the patients included in the studies varied from 40 to 79 years, with a higher proportion of male patients (75%) compared to female patients. Among the patients, T2DM was the most prevalent form, accounting for 83.33% of the cases. The most commonly observed comorbidities among the patients were hypertension (50%), hypothyroidism (16.66%), obstructive sleep apnea (16.7%), and hyperlipidemia (8.3%). Empagliflozin was the predo
Common symptoms reported by the patients included tachypnea, dyspnea, and tachycardia. The diagnostic criteria for eu-DKA frequently involved a combination of arterial blood gas (ABG) analysis, serum tests, and urine analysis. Serum glucose levels ranged from 113 to 286 mg/dL. ABG analysis revealed deviations from normal levels, with pCO2 ranging from 13 to 43 mmHg, bicarbonate ranging from 3 to 20 mEq/L, and pH ranging from 6.94 to 7.48.
COVID-19 was primarily diagnosed using reverse transcription-polymerase chain reaction, and the symptoms of eu-DKA appeared 2 to 9 d after the identification of COVID-19. Treatment for COVID-19 included intubation and oxygen therapy for the majority of patients, except for one patient with T1DM who also required invasive mechanical ventilation. Another patient received High Flow Nasal Cannula. Empagliflozin use was associated with a shorter duration between COVID-19 infection and the onset of eu-DKA symptoms. All patients received intravenous fluids and intravenous insulin as part of the eu-DKA treatment protocol.
Out of the 12 patients included in the studies, 11 patients successfully survived, recovered, and were discharged from the hospital, while unfortunately, one patient died. A favorable outcome was defined as not requiring oxygen or life support and having blood pH within the normal range of 7.35-7.45. The patients with T1DM also experienced successful recoveries. SGLT2 inhibitors were discontinued until the resolution of COVID-19, and subcutaneous insulin was initiated after recovery from eu-DKA. A summary of the results can be found in Table 1.
| Ref. | Study Origin | Number of patients | Sex | Age | Type of DM | Previous co-morbidities | SGLT2 inhibitors used before | COVID-19 symptoms onset | Eu-DKA diagnosis | Method of COVID-19 diagnosis | Autonomic symptoms | ABG count | Serum Analysis | Urinalysis | Eu-DKA management | COVID-19 Management | Outcome | Treatment intervention after resolution |
| Dass et al[14] | United States | 1 | Female | 59 | T2DM | COVID-19, Community acquired pneumonia | Empagliflozin, sitagliptin | 9 d before eu-DKA onset | ABG analysis, serum analysis, urine analysis | Not reported | Tachypnea and tachycardia | pH: 6.94; PaCO2: 13; | Lactate: 0.9; glucose: 154; confirmed bicarb: < 10; serum osmolality: 346; anion gap: 30 | 3+ glucose and 2+ ketones; negative UDS and normal salicylate levels | insulin drip and IV fluid | Not reported | Discharged after complete recovery | Sitagliptin and metformin continued; empagliflozin discontinued; started with 20 units of insulin glargine |
| Vitale et al[15] | United Kingdom | 5 | 3 males, 2 females | 52-79 | T2DM | Covid-19, Hypertension | Empagliflozin, Canagliflozin | 3-8 d before eu-DKA onset | ABG analysis, serum analysis | RT-PCR | Tachypnea, dyspnea, nausea, anorexia, abdominal pain | pH: 7.09-7.31; PaCO2: 19-43; HCO3: 5-20 | Lactate: 1.1-2.4; glucose: 146-286; anion gap: 20-40 | Not analyzed | IV insulin, intubation | Not reported | 1 male died; rest of the patients recovered | Not reported |
| Batista et al[16] | Brazil | 1 | Male | 56 | T2DM | COVID-19 | Empagliflozin | 5 d before eu-DKA onset | ABG analysis, serum analysis, urine analysis | RT-PCR | Tachypnea, tachycardia | pH: 7.28; pCO2: 19 mmHg; HCO3: 8.9; base excess: 15.7 | Sodium: 132; potassium: 5.6; chloride: 99; glucose: 118; hemoglobin A1c (HbA1c) 7.2% | ketones | Glucose, insulin, and KCl | Oxygen therapy, Azithromycin 500 mg | Discharged after complete recovery | Not reported |
| Philippe et al[17] | Belgium | 1 | Male | 60 | T1DM | COVID-19, hypothyroidism, obstructive sleep apnea | Empagliflozin | 2 d before eu-DKA onset | ABG analysis, serum analysis, urine analysis | RT-PCR | Polypnea, myalgia, diarrhea. And fever | pH: 7.48; pO2: 17, pCO2: 27; HCO3: 19; anion gap: 17 | Lactate: 1.4; glucose: 234; HbA1c: 7.4 | 3+ ketones | IV fluids, IV insulin | Oxygen therapy, invasive mechanical ventilation | Discharged after complete recovery | Empagliflozin was discontinued and subcutaneous glargine was started |
| Morrison et al[18] | United States | 1 | Male | 40 | T2DM | COVID-19 | Empagliflozin | 3 d before eu-DKA onset | ABG analysis, serum analysis, urine analysis | RT-PCR | Tachypnea, tachycardia, diaphoretic | pH: 7.06; pCO2: 37; pO2: 31; HCO3: 10.0; lactate: 2.3 | Sodium level: 133; carbon dioxide: 11; glucose:177; anion gap: 25; HbA1c: 10.6% | Glucose (> 1000 mg/dL) and ketones (> 80 mg/dL) | IV fluids, IV insulin | No intervention due to mild symptoms | Discharged after complete recovery | Subcutaneous glargine started |
| Fang et al[19] | United States | 1 | Male | 52 | T2DM | COVID-19, hypertension, hyperlipidemia | Empagliflozin | 2 d before eu-DKA onset | ABG analysis, serum analysis, urine analysis | RT-PCR | Hypoxia, dyspnea. Fever, anorexia | pH: 7.30; pCO2: 37 | glucose:113; anion gap: 18 | Glucose (> 500 mg/dL) and ketones (> 80 mg/dL) | IV fluids, IV insulin | Intubation, oxygen therapy | Discharged after complete recovery | Not reported |
| Yii ESS et al[28] | Malaysia | 1 | Male | 37 | T2DM | COVID-19 | Empagliflozin | 3 d before eu-DKA onset | ABG analysis, serum analysis, urine analysis | Not reported | Dyspnea, Tachypnea, Stable heart rate | pH: 6.87; pCO2: 17; pO2: 37; HCO3: 3.1; lactate: 1.7 | Glucose: 11.9; urea 11.4; sodium 136; potassium 4.5; chloride 106; creatinine 105 | Ketone: 3.0 | 18 h of renal replacement therapy, noradrenaline infusion | Intubation, ICU | Discharged after complete recovery | Not reported |
| Oriot et al[29] | Belgium | 1 | Male | 52 | T1DM | COVID-19, hypothyroidism, obstructive sleep apnea syndrome | Empagliflozin | 2 d before | ABG analysis, serum analysis | RT-PCR | Polypnea, myalgia, diarrhea, and fever | pH: 7.48; pCO2: 27; pO2: 47; HCO3: 19; lactate: 1.4; anion gap: 17 | HbA1c: 7.4 | Ketone: 3+ | IC fluids, IV insulin | Not reported | Discharged after complete recovery | Empagliflozin was discontinued |
The use of SGLT2 inhibitors in adults with COVID-19 infection remains a controversial topic, and this systematic review represents the first attempt to define the outcomes of this particular patient cohort. However, the availability of data is unfortunately limited. Nonetheless, the available data suggest that SGLT2 inhibitors may contribute to the development of eu-DKA in COVID-19-infected patients.
We included 8 studies comprising a total of 12 patients. The survival rate among these patients exceeded 90%. Most patients presented with symptoms of COVID-19 within 2-3 d before the onset of eu-DKA. Significant deviations in arterial blood pH levels were observed, with the lowest reported pH being 6.87. In response, all patients had their SGLT2 inhibitors immediately discontinued and received treatment with fluids and intravenous insulin.
The term “eu-DKA” was first defined by Munro et al[22] in 1973. It is distinct from classic DKA in that it is characterized by severe metabolic acidosis despite normal blood glucose levels. Diagnosis of eu-DKA is confirmed through direct measurement of beta-hydroxybutyrate levels in the blood and assessment of arterial blood pH levels[23].
The occurrence of eu-DKA due to SGLT2 inhibitors involves decreased insulin production (insulinopenia) and increased glucagon secretion. Multiple factors influence increased glucagon secretion, which occurs through both direct and indirect mechanisms. The inhibitory effects of SGLT2 inhibitors on the SGLT2 transporters in the glucagon-secreting pancreatic alpha cells of the Langerhans islets directly contribute to increased glucagon secretion. Indirectly, increased glucose excretion leads to lower insulin levels and decreased insulin to glucagon ratio. The resultant decrease in insulin stimulates the synthesis of free fatty acids and ketone bodies, leading to excessive catabolism of fatty acids and subsequent ketosis[24]. Ketosis is further exacerbated in the presence of SGLT2 inhibitors, as these medications inhibit the reabsorption of glucose in the proximal renal tubules, leading to glucosuria and promoting a state of starvation[25].
Eu-DKA has emerged as a prevalent condition observed in diabetic patients with COVID-19 who are also using SGLT2 inhibitors. The higher incidence of eu-DKA in male diabetic patients with COVID-19 aligns with reports indicating a greater propensity for presentation in this subgroup. The clinical manifestation of eu-DKA in COVID-19-infected diabetic patients taking SGLT2 inhibitors is diverse, including tachypnea and tachycardia during clinical examination, along with an anion gap metabolic acidosis and normal serum glucose levels observed in laboratory investigations.
In patients infected with COVID-19, eu-DKA differs from the non-infected population due to the pancreatic toxicity induced by the virus, leading to severe insulinopenia. Consequently, infected patients require insulin therapy in addition to the discontinuation of SGLT2 inhibitors to prevent the recurrence of eu-DKA. Conversely, in patients without COVID-19 infection, resolution and prevention of eu-DKA can typically be achieved by discontinuing SGLT2 inhibitors, allowing insulin levels to return to normal[18]. Unlike individual case reports, the accumulation of evidence from multiple cases supports the occurrence of eu-DKA in diabetic patients following the onset of COVID-19 and concurrent use of SGLT2 inhibitors.
The severe toxic effects of COVID-19 on the pancreas have been well-documented. When these toxic effects combine with the dehydrating and glycosuric effects of SGLT2 inhibitors, it can lead to the development of eu-DKA. Recent studies have discussed the occurrence of pancreatic injury in patients with COVID-19, highlighting the link between COVID-19-related pancreatic toxicity, enzyme elevation, and insulinopenia[26,27]. The angiotensin converting enzyme 2 (ACE2) receptor, which is expressed in the beta cells of Langerhans islets, plays a role in both COVID-19 and SARS-CoV-1 infections. These viruses utilize the ACE2 receptor to enter the cell, triggering immune system activation and the release of cytokines and chemokines that lead to cell death[28,29]. The destructive effect on beta cells results in insulinopenia and subsequent ketoacidosis. Moreover, the acidic environment of eu-DKA is known to facilitate the growth of COVID-19.
Given the widespread use of SGLT2 inhibitors for their cardiovascular and renal benefits, clinicians must familiarize themselves with eu-DKA to enable timely diagnosis and treatment, particularly in the context of the ongoing COVID-19 pandemic.
This study has limitations, including a small number of studies with small effect sizes, all of which were case reports or case series. The non-randomized allocation of interventions and the absence of a standardized protocol for diagnosing eu-DKA in COVID-19 patients introduce selection and bias risks. The diverse treatment settings and limited data availability further hinder comprehensive analysis. Further research is needed to address these limitations and provide more robust evidence on the topic.
Our systematic review suggests that SGLT2 inhibitors may increase the risk of euglycemic diabetic ketoacidosis in COVID-19-infected diabetic patients. The pancreatic toxicity associated with SARS-CoV-2 infection may contribute to this effect. While this narrative synthesis of case reports provides valuable insights, further research with larger sample sizes and rigorous designs, such as retrospective cohorts, is needed to investigate the association between eu-DKA development in COVID-19 patients and SGLT2 inhibitors. Studies with larger effect sizes and randomization would help elucidate clinically relevant endpoints and enable more effective management of individuals at higher risk.
The coexistence of coronavirus disease 2019 (COVID-19) infection and the use of sodium-glucose cotransporter-2 (SGLT2) inhibitors have generated debate due to the potential risk of euglycemic diabetic ketoacidosis (eu-DKA) development. Limited information regarding this specific patient population is available, necessitating a systematic review to investigate the outcomes and characteristics associated with eu-DKA in COVID-19-infected diabetic patients treated with SGLT2 inhibitors.
Given the controversy and limited data surrounding the association between SGLT2 inhibitors, COVID-19 infection, and eu-DKA, there is a pressing need to investigate this topic to enhance our understanding of the potential risks and outcomes.
The primary objectives of this study are to examine the association between SGLT2 inhibitors and the development of eu-DKA in COVID-19-infected diabetic patients, explore the potential mechanisms underlying this relationship, and assess the clinical outcomes and management strategies for this patient population.
We conducted a comprehensive search of relevant databases to identify studies reporting on the association between SGLT2 inhibitors and eu-DKA in COVID-19-infected diabetic patients. We followed the PRISMA guidelines for study selection and data extraction. The extracted data were qualitatively synthesized to provide a narrative overview of the findings.
The systematic review included eight studies comprising 12 patients, investigating the association between SGLT2 inhibitors and eu-DKA in COVID-19-infected diabetic patients. The majority of patients presented with eu-DKA symptoms 2-3 d after the onset of COVID-19 symptoms. The survival rate was over 90%, with one reported fatality. Significant pH deviations were observed, with the lowest reported pH being 6.87. All patients discontinued SGLT2 inhibitors and received treatment with fluids and IV insulin. The results highlight the potential risk of developing eu-DKA in this patient population.
This systematic review concludes that the use of SGLT2 inhibitors in COVID-19-infected diabetic patients may increase the risk of eu-DKA. The pancreatic toxicity induced by the severe acute respiratory syndrome coronavirus 2 virus is believed to contribute to this phenomenon. The analysis of case reports provides evidence supporting the association between SGLT2 inhibitors and eu-DKA in this patient population. Further studies with larger sample sizes and robust designs are necessary to enhance our understanding and inform clinical decision-making for high-risk individuals.
Further research is needed to investigate the mechanisms of eu-DKA in COVID-19 patients on SGLT2 inhibitors. Larger randomized studies are necessary to establish a causal relationship and identify risk factors. Standardized protocols for diagnosis and management should be developed to improve patient outcomes. These research perspectives will enhance understanding and guide evidence-based approaches in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Chest Physician; American College of Physician; Society of Critical Care Medicine.
Specialty type: Medicine, general and internal
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Feizi A, Iran; Martinez-Castelaoa A, Spain; Shao JQ, China; Su G, China S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY
| 1. | Peña JE, Rascón-Pacheco RA, Ascencio-Montiel IJ, González-Figueroa E, Fernández-Gárate JE, Medina-Gómez OS, Borja-Bustamante P, Santillán-Oropeza JA, Borja-Aburto VH. Hypertension, Diabetes and Obesity, Major Risk Factors for Death in Patients with COVID-19 in Mexico. Arch Med Res. 2021;52:443-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 2. | Usman MS, Siddiqi TJ, Memon MM, Khan MS, Rawasia WF, Talha Ayub M, Sreenivasan J, Golzar Y. Sodium-glucose co-transporter 2 inhibitors and cardiovascular outcomes: A systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Modi A, Agrawal A, Morgan F. Euglycemic Diabetic Ketoacidosis: A Review. Curr Diabetes Rev. 2017;13:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (5)] |
| 4. | Food and Drug Administration. The FDA warns that SGLT2 inhibitors for diabetes can cause a serious condition of too much acid in the blood. FDA gov, 2015. [DOI] [Full Text] |
| 5. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5370] [Article Influence: 671.3] [Reference Citation Analysis (0)] |
| 6. | Barski L, Eshkoli T, Brandstaetter E, Jotkowitz A. Euglycemic diabetic ketoacidosis. Eur J Intern Med. 2019;63:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care. 2015;38:1687-1693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 518] [Cited by in RCA: 557] [Article Influence: 55.7] [Reference Citation Analysis (2)] |
| 8. | Meyer EJ, Gabb G, Jesudason D. SGLT2 Inhibitor-Associated Euglycemic Diabetic Ketoacidosis: A South Australian Clinical Case Series and Australian Spontaneous Adverse Event Notifications. Diabetes Care. 2018;41:e47-e49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Tang H, Li D, Wang T, Zhai S, Song Y. Effect of Sodium-Glucose Cotransporter 2 Inhibitors on Diabetic Ketoacidosis Among Patients With Type 2 Diabetes: A Meta-analysis of Randomized Controlled Trials. Diabetes Care. 2016;39:e123-e124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7:135-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 11. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 46994] [Article Influence: 2937.1] [Reference Citation Analysis (0)] |
| 12. | Winocour PH, Ford M, Ainsworth A; Association of British Clinical Diabetologists. Association of British Clinical Diabetologists (ABCD): survey of specialist diabetes care services in the UK, 2000. 2. Workforce issues, roles and responsibilities of diabetes specialist nurses. Diabet Med. 2002;19 Suppl 4:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D; CARE Group. The CARE guidelines: consensus-based clinical case report guideline development. J Diet Suppl. 2013;10:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Dass B, Beck A, Holmes C, Morton G. Euglycemic DKA (euDKA) as a presentation of COVID-19. Clin Case Rep. 2021;9:395-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Vitale RJ, Valtis YK, McDonnell ME, Palermo NE, Fisher NDL. Euglycemic Diabetic Ketoacidosis With COVID-19 Infection in Patients With Type 2 Diabetes Taking SGLT2 Inhibitors. AACE Clin Case Rep. 2021;7:10-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Batista DV, Vieira CAFA, Costa TA, Lima EG. COVID-19-associated euglycemic diabetic ketoacidosis in a patient with type 2 diabetes on SGLT2 inhibitor: a case report. Diabetol Int. 2021;12:313-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Oriot P, Hermans MP. Euglycemic diabetic ketoacidosis in a patient with type 1 diabetes and SARS-CoV-2 pneumonia: case-report and review of the literature. Acta Clin Belg. 2022;77:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Morrison N, Barnett K, Tantum J, Morrison HK, Whalen M. A Case of Euglycemic Diabetic Ketoacidosis in a Patient With Type 2 Diabetes Mellitus and COVID-19. Cureus. 2020;12:e12029. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Fang J, Genco M, Caskey RN. COVID-19 Precipitating Euglycaemic Diabetic Ketoacidosis with SGLT2 Inhibitor Use. Eur J Case Rep Intern Med. 2020;7:001943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: Basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev. 2017;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 21. | Yii ESS, Azli AW, Sitaram PN. Sodium-glucose cotransporter 2 inhibitor-induced euglycemic diabetic ketoacidosis in a patient with coronavirus disease 2019: a case report. J Med Case Rep. 2022;16:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Munro JF, Campbell IW, McCuish AC, Duncan LJ. Euglycaemic diabetic ketoacidosis. Br Med J. 1973;2:578-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 186] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 23. | Vanelli M, Chiari G, Capuano C, Iovane B, Bernardini A, Giacalone T. The direct measurement of 3-beta-hydroxy butyrate enhances the management of diabetic ketoacidosis in children and reduces time and costs of treatment. Diabetes Nutr Metab. 2003;16:312-316. [PubMed] |
| 24. | Taylor SI, Blau JE, Rother KI. SGLT2 Inhibitors May Predispose to Ketoacidosis. J Clin Endocrinol Metab. 2015;100:2849-2852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 364] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 25. | Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F, Zhang T, Kim TW, Harschnitz O, Redmond D, Houghton S, Liu C, Naji A, Ciceri G, Guttikonda S, Bram Y, Nguyen DT, Cioffi M, Chandar V, Hoagland DA, Huang Y, Xiang J, Wang H, Lyden D, Borczuk A, Chen HJ, Studer L, Pan FC, Ho DD, tenOever BR, Evans T, Schwartz RE, Chen S. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell. 2020;27:125-136.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 510] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 26. | Wang F, Wang H, Fan J, Zhang Y, Zhao Q. Pancreatic Injury Patterns in Patients With Coronavirus Disease 19 Pneumonia. Gastroenterology. 2020;159:367-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 325] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 27. | Ashok A, Faghih M, Singh VK. Mild Pancreatic Enzyme Elevations in COVID-19 Pneumonia: Synonymous With Injury or Noise? Gastroenterology. 2021;160:1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 779] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 29. | Fukuda M, Nabeta M, Muta T, Fukami K, Takasu O. Euglycemic diabetic ketoacidosis caused by canagliflozin: a case report. Int J Emerg Med. 2020;13:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |