Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5129
Peer-review started: April 14, 2023
First decision: May 19, 2023
Revised: June 8, 2023
Accepted: June 30, 2023
Article in press: June 30, 2023
Published online: July 26, 2023
Processing time: 103 Days and 16 Hours
Simultaneous bilineage hematologic malignancies are rare; however, several cases of acute myeloid leukemia (AML) and T-lymphoblastic lymphoma (T-LBL) co-occurrence have been reported. A standard treatment for simultaneous AML and T-LBL has not yet been established, and its prognosis is very poor. Further studies to develop standard treatments are required to increase patient survival rates.
A 69-year-old man complaining of pleuritic chest pain visited the emergency room. Computed tomography revealed multiple enlarged lymph nodes (LNs) in the neck and groin and pulmonary thromboembolism with pulmonary infarction. Furthermore, a peripheral blood smear performed due to leukocytosis revealed circulating blasts. Acute myelomonocytic leukemia (AMML) was diagnosed after bone marrow examination, and T-LBL positivity for terminal deoxynucleotidyl transferase, cluster of differentiation (CD)34, and CD4 was confirmed by cervical LN biopsy. Decitabine and dexamethasone were administered because he could not receive intensive chemotherapy due to poor performance status. Complete remission of AMML and T-LBL was achieved after 4 cycles of decitabine plus dexamethasone.
We report the therapeutic effect of decitabine, a hypomethylating agent (HMA), in patients with concurrent bilineage hematologic malignancies and suggest that further studies are required to evaluate the therapeutic effect of HMAs on both lymphoid and bilineage hematologic malignancies.
Core Tip: Simultaneous bilineage hematologic malignancies are rare. Here, we report an unusual case of concurrent acute myelomonocytic leukemia (AMML) and T-lymphoblastic lymphoma (T-LBL), treated with decitabine. After the fourth cycle of decitabine and dexamethasone administration, a patient achieved complete remission of AMML and T-LBL. Hypomethylating agents (HMAs) have been approved for the treatment of myelodysplastic syndrome and intensive induction-ineligible acute myeloid leukemia; however, their therapeutic effect on lymphoid malignancy has not yet been sufficiently investigated. This case suggests the need of further studies to evaluate the therapeutic effects of HMAs on lymphoid and bilineage hematologic malignancies.
- Citation: Jeon SY, Lee NR, Cha S, Yhim HY, Kwak JY, Jang KY, Kim N, Cho YG, Lee CH. Acute myelomonocytic leukemia and T-lymphoblastic lymphoma as simultaneous bilineage hematologic malignancy treated with decitabine: A case report. World J Clin Cases 2023; 11(21): 5129-5135
- URL: https://www.wjgnet.com/2307-8960/full/v11/i21/5129.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i21.5129
Simultaneous bilineage hematologic malignancies are extremely rare and their pathogenesis remains unclear. This disease has a poor prognosis, and a standard chemotherapy regimen has not yet been established. According to an analysis of several case reports, intensive chemotherapy followed by hematopoietic stem cell transplantation has shown better outcomes; however, treatment remains challenging[1].
DNA methylation is a mechanism of DNA modification that suppresses gene transcription, and methylation of tumor suppressor genes contributes to cancer cell growth and survival. Hypomethylating agents (HMAs) have been approved for the treatment of myelodysplastic syndrome and acute myeloid leukemia (AML), and a combination of HMAs and venetoclax, a B-cell lymphoma 2 (Bcl-2) inhibitor has been reported to improve survival outcome in patients with intensive chemotherapy-ineligible AML.
Here, we report a case of concurrent acute myelomonocytic leukemia (AMML) and T-lymphoblastic lymphoma (T-LBL), treated with decitabine.
A 69-year-old man with chest wall pain visited the emergency room.
The patient presented with pain in the right chest wall that worsened with breathing. The pain suddenly occurred the day before the visit and gradually worsened thereafter. He was examined at a primary medical center before visiting our hospital and was suspected to have pneumonia.
The patient had taken clopidogrel after being diagnosed with cerebral infarction.
The patient had no relevant family history.
Physical examination revealed multiple enlarged lymph nodes (LNs) in the neck and groin with a body temperature of 38.5 °C.
Initial testing showed leukocytosis (white blood cell: 156900/μL, normal range: 4000-10000/μL), anemia (Hb: 8.3 g/dL, normal range: 13-16.5 g/dL), and thrombocytopenia (platelet: 67000/μL, normal range: 150000-450000/μL). Peripheral blood smear revealed leukocytosis with blast cells (95%). C-reactive protein (142.29 mg/L, normal range: 0-5.0 mg/L), lactate dehydrogenase (1193 U/L, normal range: 0-250 U/L), and d-dimer (2.2 mg/L FEU, normal range: 0-0.49 mg/L FEU) levels were elevated.
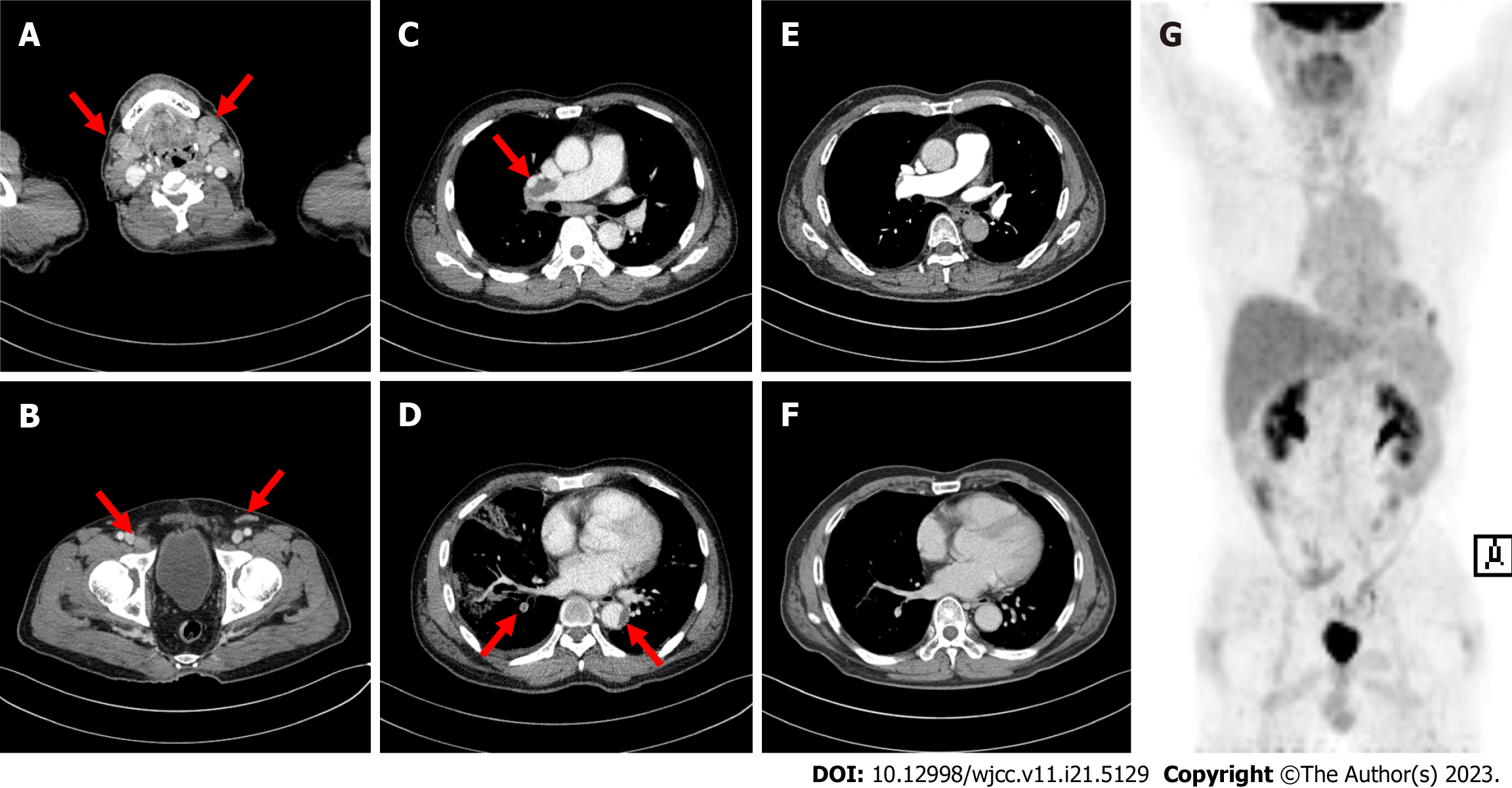
Computed tomography (CT) revealed multiple enlarged LNs on both sides of the diaphragm (cervical, axillary, mediastinal, inguinal, and external iliac LNs) (Figure 1A and B). Acute pulmonary thromboembolism (PTE) with pul
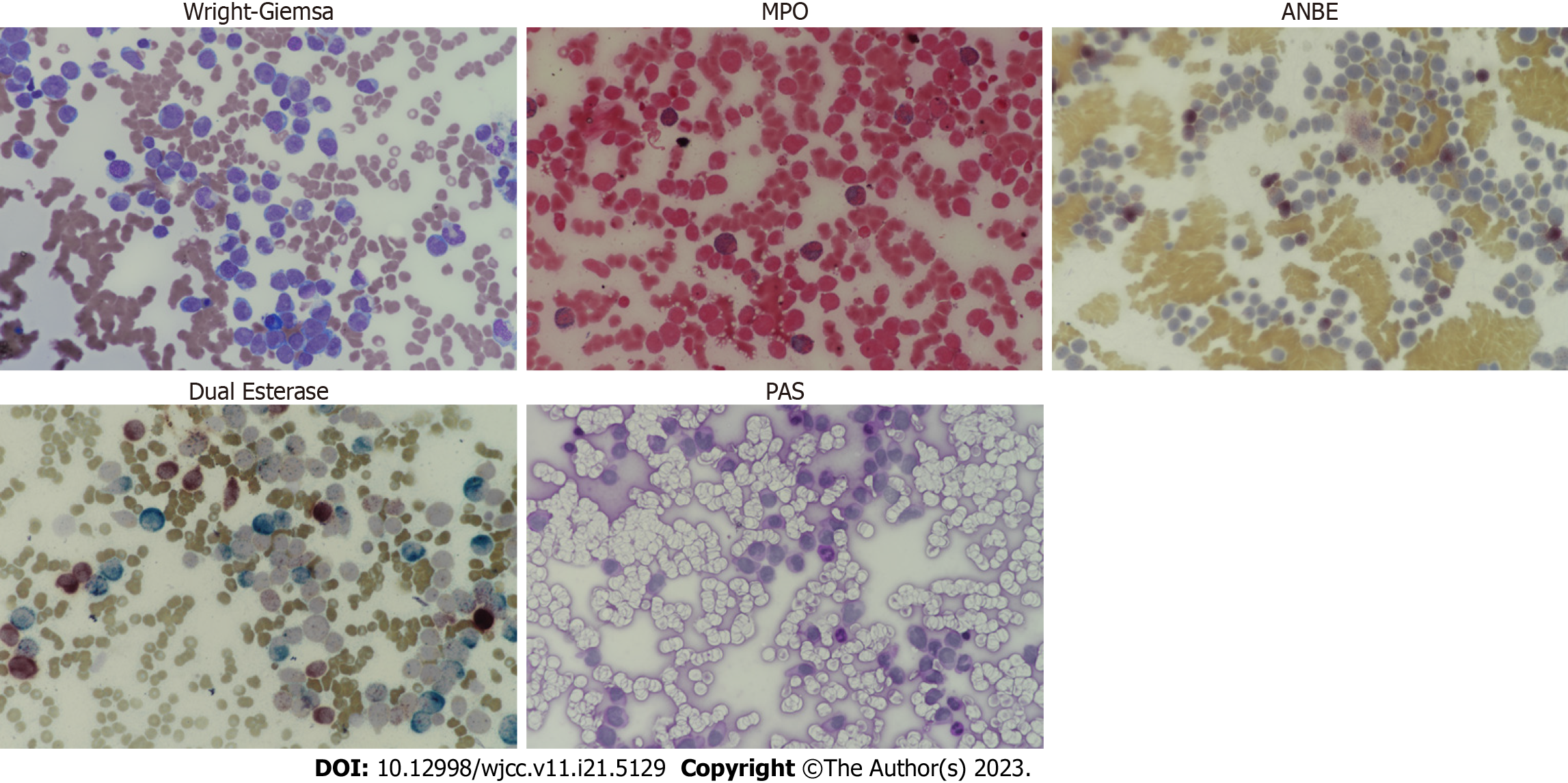
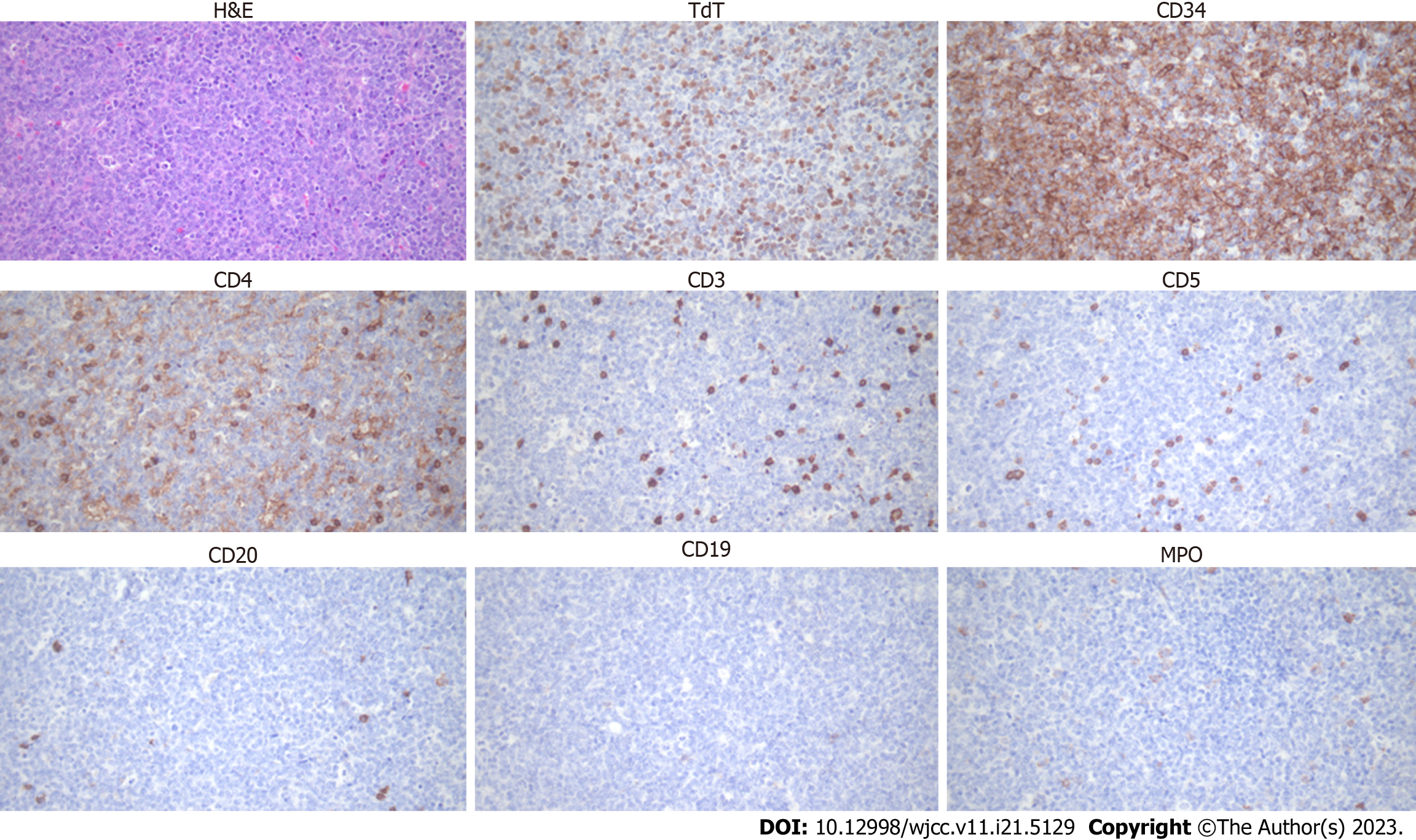
Bone marrow examination after leukapheresis revealed AMML; positive for immunophenotypes of cluster of differentiation (CD)13, CD33, CD34, CD64, human leukocyte antigen-DR, and myeloperoxidase (MPO) and cytochemical staining of MPO, alpha-naphthyl butyrate esterase (ANBE), chloroacetate esterase, and acid phosphatase (Figure 2). Cervical lymph node biopsy confirmed T-LBL diagnosis; positive for CD4, CD34, and terminal deoxynucleotidyl transferase (TdT) and negative for CD5, CD7, CD8, CD19, CD20, CD56, and MPO (Figure 3). It was negative for T-cell markers such as CD5, CD7, and CD8; however, positive for immunophenotypes of T-LBL, TdT, and CD34 and another T-cell marker, CD4. Additionally, B-cell, natural killer cell, and myeloid markers were negative, so malignant cell infiltration caused by other lineages could be excluded. We performed next-generation sequencing (NGS) with bone marrow sample, and a RUNX1 (M466I) and TET2 (E135K) mutations were detected. We also performed fluorescence in situ hybridization analysis to evaluate cytogenetic abnormalities, including platelet-derived growth factor receptor alpha (PDGFRA) and platelet-derived growth factor receptor beta (PDGFRB) rearrangements; however, no abnormal cytogenetic abnormalities were detected. Chromosome analysis was performed with a bone marrow sample and revealed normal male karyotype; 46, XY.
Due to the poor performance status and comorbidities, the patient could not undergo intensive chemotherapy; instead, decitabine (20 mg/d for 5 d) and dexamethasone (40 mg for 4 d) were administered. He underwent anticoagulant therapy with dalteparin (200 IU/kg) followed by apixaban (10 mg twice daily for 7 d and then 5 mg twice daily) for PTE and thrombus treatment. Bone marrow examination was performed after four cycles, and complete remission (CR) was achieved. CT confirmed complete resolution of the PTE (Figure 1E and F), and positron emission tomography/CT revealed no remnants of lymphoma (Figure 1G).
The patient received 13 cycles of decitabine without any complications; however, AMML recurred 14 mo after treatment initiation. He received chemotherapy with idarubicin (12 mg/m2 for 2 d) and cytarabine (200 mg/m2 for 5 d); however, he failed to respond and died due to disease progression and pneumonia. The progression-free survival and overall survival rates were 14 and 19 mo, respectively.
Simultaneous bilineage hematologic malignancies have a poor prognosis, with a median survival time of 15 mo[1]. Only 24 cases have been reported since 1976, and concurrent AML and T-LBL and chronic myeloid leukemia and T-LBL were the most common[1]. Its pathogenesis remains unclear; however, Tsukasaki et al[2] reported two possible mechanisms: First, AML develops primarily due to compromised immunity in patients with T-cell lymphoma. Moreover, increased levels of growth factors, such as granulocyte-colony stimulating factor, macrophage-colony stimulating factor, and granulocyte-macrophage colony-stimulating factor, produced by lymphoma cells result in AML[2]. PDGFRA and PDGFRB rearrangements are associated with myeloid/lymphoid neoplasms. Chang et al[3] reported a case of concurrent AML and T-LBL with PDGFRB rearrangement. We performed a fluorescence in situ hybridization analysis; however, PDGFRA and PDGFRB rearrangements were not detected. In contrast, RUNX1 and TET2 mutations were detected by NGS conducted with bone marrow samples. Since RUNX1 plays a role in hematopoietic stem cell differentiation, somatic mutations and chromosomal rearrangements involving this gene contribute to hematologic malignancy development[4,5]. RUNX1 mutations promote leukemogenesis by offering growth advantages and differentiation defects to hema
Since T-LBL cells usually invade the bone marrow, accurate lineage analysis in bone marrow specimen is required. Moreover, differential diagnosis of mixed-phenotype acute leukemia should be considered as well. Cytochemical staining results showed strong positivity for MPO, and monocyte marker, ANBE, while Periodic acid-Schiff stain, a marker of lymphoblast, was negative. Furthermore, immunofluorescence assay revealed that 78.06%, 99.68%, and 99.34% of bone marrow cells showed positivity for MPO, CD13, and CD33, respectively; 28% of cells were positive for CD64, a monocyte marker as well. These results imply that leukemic cells infiltrated in the bone marrow are derived from myeloid lineage, and the possibility that malignant T-cells invade the bone marrow was expected to be low.
Standard chemotherapy for simultaneous bilineage hematologic malignancies has not yet been established. However, a preliminary study showed that chemotherapy followed by transplantation was superior to chemotherapy without transplantation[1]. Methylation of gene promoters results in gene silencing, and aberrant methylation of tumor suppressor genes is associated with malignancy development such as AML. HMAs, including azacitidine and decitabine, have been approved for AML and myelodysplastic syndrome treatment; however, genetic methylation also plays an important role in lymphoid malignancy development[9,10]. Furthermore, decitabine and azacitidine have been reported to exert antileukemic effects on ALL[11-13]. The patient achieved CR after four cycles of decitabine and dexamethasone. Dexamethasone was administered in combination with decitabine to suppress T-lymphoma cells and was discontinued after the fourth cycle. The patient maintained the CR until the 13th cycle. Considering the therapeutic effects of HMA on both myeloid and lymphoid malignancies[9,10], it may be used to treat patients with concurrent AML and T-lymphoid malignancy as well as those with concurrent AML and B-lymphoid malignancy who cannot receive intensive che
Here, we present an unusual case of concurrent AMML and T-LBL with thromboembolism. Due to the poor performance status and comorbidities, the patient received decitabine and dexamethasone instead of intensive chemotherapy, and strikingly, a CR was achieved. We suggest that decitabine may be a good treatment option for unfit patients diagnosed with bilineage hematologic malignancies. Further research is required to confirm the therapeutic efficacy of HMAs in both lymphoid and bilineage hematologic malignancies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fagioli F, Italy; Nakamura Y, Japan S-Editor: Lin C L-Editor: A P-Editor: Zhao S
| 1. | Fu X, Shang Y, Zhang L, Li L, Li X, Wang X, Sun Z, Zhang M. Analyses and treatment of simultaneous bi-lineage malignancies of myeloid leukemia and lymphoma: Two case reports and a literature review. Oncol Lett. 2018;16:6624-6632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Tsukasaki K, Koba T, Iwanaga M, Murata K, Maeda T, Atogami S, Nakamura H, Yamada Y, Kamihira S, Tomonaga M. Possible association between adult T-cell leukemia/lymphoma and acute myeloid leukemia. Cancer. 1998;82:488-494. [PubMed] [DOI] [Full Text] |
| 3. | Chang H, Chuang WY, Sun CF, Barnard MR. Concurrent acute myeloid leukemia and T lymphoblastic lymphoma in a patient with rearranged PDGFRB genes. Diagn Pathol. 2012;7:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Sood R, Kamikubo Y, Liu P. Role of RUNX1 in hematological malignancies. Blood. 2017;129:2070-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 354] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 5. | Sanda T. RUNX1 in T-ALL: tumor suppressive or oncogenic? Blood. 2017;130:1686-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Grossmann V, Kern W, Harbich S, Alpermann T, Jeromin S, Schnittger S, Haferlach C, Haferlach T, Kohlmann A. Prognostic relevance of RUNX1 mutations in T-cell acute lymphoblastic leukemia. Haematologica. 2011;96:1874-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Della Gatta G, Palomero T, Perez-Garcia A, Ambesi-Impiombato A, Bansal M, Carpenter ZW, De Keersmaecker K, Sole X, Xu L, Paietta E, Racevskis J, Wiernik PH, Rowe JM, Meijerink JP, Califano A, Ferrando AA. Reverse engineering of TLX oncogenic transcriptional networks identifies RUNX1 as tumor suppressor in T-ALL. Nat Med. 2012;18:436-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Chiba S. Dysregulation of TET2 in hematologic malignancies. Int J Hematol. 2017;105:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Roman-Gomez J, Jimenez-Velasco A, Barrios M, Prosper F, Heiniger A, Torres A, Agirre X. Poor prognosis in acute lymphoblastic leukemia may relate to promoter hypermethylation of cancer-related genes. Leuk Lymphoma. 2007;48:1269-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Garcia-Manero G, Jeha S, Daniel J, Williamson J, Albitar M, Kantarjian HM, Issa JP. Aberrant DNA methylation in pediatric patients with acute lymphocytic leukemia. Cancer. 2003;97:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Zhang G, Gao X, Zhao X, Wu H, Yan M, Li Y, Zeng H, Ji Z, Guo X. Decitabine inhibits the proliferation of human T-cell acute lymphoblastic leukemia molt4 cells and promotes apoptosis partly by regulating the PI3K/AKT/mTOR pathway. Oncol Lett. 2021;21:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Roolf C, Richter A, Konkolefski C, Knuebel G, Sekora A, Krohn S, Stenzel J, Krause BJ, Vollmar B, Murua Escobar H, Junghanss C. Decitabine demonstrates antileukemic activity in B cell precursor acute lymphoblastic leukemia with MLL rearrangements. J Hematol Oncol. 2018;11:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Paulson K, Kumar R, Ahsanuddin A, Seftel MD. Azacytidine as a novel agent in the treatment of acute lymphoblastic leukemia. Leuk Lymphoma. 2011;52:134-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH, Kantarjian HM, Xu T, Hong WJ, Chyla B, Potluri J, Pollyea DA, Letai A. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 1326] [Article Influence: 189.4] [Reference Citation Analysis (0)] |