Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5083
Peer-review started: March 17, 2023
First decision: April 11, 2023
Revised: May 23, 2023
Accepted: June 16, 2023
Article in press: June 16, 2023
Published online: July 26, 2023
Processing time: 131 Days and 22.8 Hours
Progressive pancreatic β cell dysfunction is a fundamental aspect of the pathology underlying type 2 diabetes mellitus (T2DM). Recently, mesenchymal stem cell (MSC) transplantation has emerged as a new therapeutic method due to its ability to promote the regeneration of pancreatic β cells. However, current studies have focused on its efficacy, and there are few clinical studies on its safety.
To evaluate the safety of human umbilical cord (hUC)-MSC infusion in T2DM treatment.
An open-label and randomized phase 2 clinical trial was designed to evaluate the safety of hUC-MSC transplantation in T2DM in a Class A hospital. Ten patients in the placebo group received acellular saline intravenously once per week for 3 wk. Twenty-four patients in the hUC-MSC group received hUC-MSCs (1 × 106 cells/kg) intravenously once per week for 3 wk. Diabetic clinical symptoms and signs, laboratory findings, and imaging findings were evaluated weekly for the 1st mo and then at weeks 12 and 24 post-treatment.
No serious adverse events were observed during the 24-wk follow-up. Four patients (16.7%) in the hUC-MSC group experienced transient fever, which occurred within 24 h after the second or third infusion; this did not occur in any patients in the placebo group. One patient from the hUC-MSC group experienced hypoglycemic attacks within 1 mo after transplantation. Significantly lower lymphocyte levels (weeks 2 and 3) and thrombin coagulation time (week 2) were observed in the hUC-MSC group compared to those in the placebo group (all P < 0.05). Significantly higher platelet levels (week 3), immunoglobulin levels (weeks 1, 2, 3, and 4), fibrinogen levels (weeks 2 and 3), D-dimer levels (weeks 1, 2, 3, 4, 12, and 24), and neutrophil-to-lymphocyte ratios (weeks 2 and 3) were observed in the hUC-MSC group compared to those in the placebo group (all P < 0.05). There were no significant differences between the two groups for tumor markers (alpha-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 199) or blood fat. No liver damage or other side effects were observed on chest X-ray.
Our study suggested that hUC-MSC transplantation has good tolerance and high safety in the treatment of T2DM. It can improve human immunity and inhibit lymphocytes. Coagulation function should be monitored vigilantly for abnormalities.
Core Tip: Diabetes mellitus is a major public health problem worldwide. Type 2 diabetes mellitus is regarded as a chronic progressive disease that arises from an impairment in the insulin-sensing mechanisms culminating in insulin resistance. Our article focused on the safety of human umbilical cord mesenchymal stem cell infusion for treating type 2 diabetes mellitus. The results suggested that human umbilical cord mesenchymal stem cell treatment can impact human immunity and inhibit lymphocytes. We should pay attention to its influence on coagulation.
- Citation: Lian XF, Lu DH, Liu HL, Liu YJ, Yang Y, Lin Y, Xie F, Huang CH, Wu HM, Long AM, Hui CJ, Shi Y, Chen Y, Gao YF, Zhang F. Safety evaluation of human umbilical cord-mesenchymal stem cells in type 2 diabetes mellitus treatment: A phase 2 clinical trial. World J Clin Cases 2023; 11(21): 5083-5096
- URL: https://www.wjgnet.com/2307-8960/full/v11/i21/5083.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i21.5083
Diabetes mellitus (DM) is a major public health problem worldwide. Type 2 DM (T2DM) is the most common type of diabetes, with adults accounting for 90% of diagnoses[1]. T2DM is regarded as a chronic progressive disease that arises from an impairment in the insulin-sensing mechanisms culminating in insulin resistance. Long-term chronic hyperglycemia can cause multisystem complications, including cardiovascular and cerebrovascular diseases, retinopathy, nephropathy, diabetic foot, etc. Although novel medications and diet therapies continue to be developed, none have provided full protection against deterioration of β cell function[2,3]. Meanwhile, many side effects like hypoglycemia, gastrointestinal adverse reactions, heart failure, and atypical fracture have increased with drug treatment[4].
In recent years, mesenchymal stem cell (MSC) therapy has been studied extensively as a novel therapeutic option for diabetes[5,6]. Among the different types of MSCs, those from the human umbilical cord (hUC) have been widely applied in the treatment of different diseases[7]. The hUC-MSCs are a group of more primitive cells derived from neonates and express original stem cell-specific surface markers such as embryonic stem cell stage-specific surface antigen 4 and tumor rejection antigen 1-60. Compared with MSCs derived from other tissues such as bone marrow and fat, the hUC-MSCs have a more abundant content, stronger proliferation ability, and lower immunogenicity[8]. Moreover, hUC-MSCs can be sampled conveniently without damage to the health of the donor, and they do not present any ethical challenges. As such, they are attractive and preferred for clinical applications.
Studies have suggested that hUC-MSCs can promote pancreas regeneration by improving the microenvironment and restoring β cells[9,10]. With the development of hUC-MSC treatment for diabetes and its complications[11-13], the safety of hUC-MSCs is an important concern for clinicians. In practice, the clinical application of hUC-MSCs is well-tolerated (i.e., safe)[14,15]. Participants reportedly suffered from mild symptoms such as fever, dizziness, and vomiting, but no cases of tumor development or death were reported. However, the effect of hUC-MSCs on tumor development remains controversial. While some studies have shown that MSCs can promote tumor progression and metastasis[16], others have suggested that MSCs can suppress tumor proliferation and apoptosis[17]. Moreover, the safety data of hUC-MSCs in diabetes treatment are insufficient. To evaluate the safety and feasibility of hUC-MSC infusion in T2DM, we designed a phase 2 clinical trial. Importantly, this was the first clinical trial of hUC-MSC infusion for T2DM treatment approved by the China Medical Biotech Association.
The enrolled participants were patients admitted to Peking University Shenzhen Hospital (Shenzhen, China) for T2DM, and all provided signed informed consent. The study was conducted according to the Declaration of Helsinki and approved by the Institutional Review Board of Peking University Shenzhen Hospital [IRB Approval No. (2018) 29th]. The inclusion criteria and exclusion criteria, as previously reported[18], were applied thoroughly.
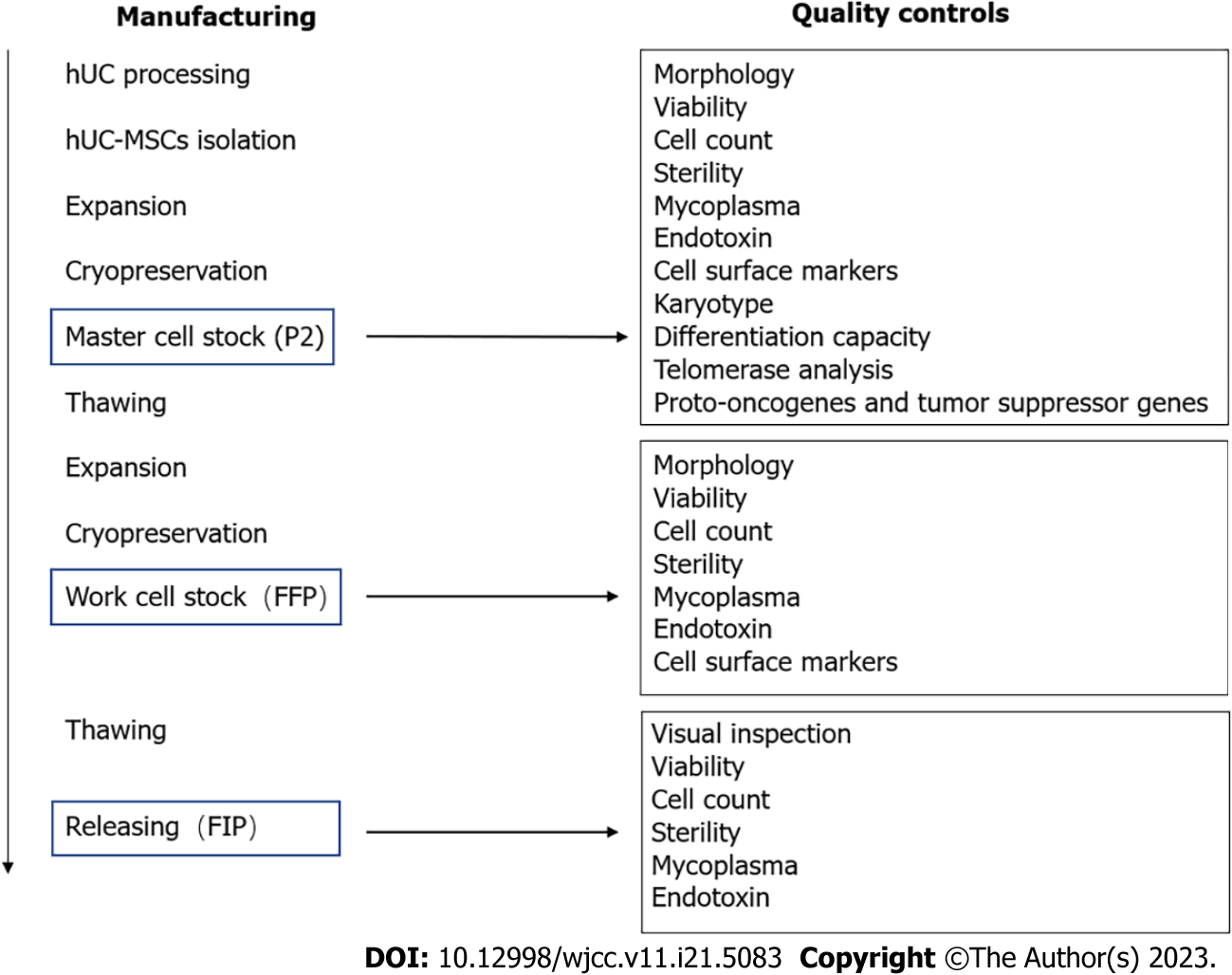
The hUC-MSCs were provided by Beike Biotechnology (Shenzhen, China)[19]. The isolation process involved Wharton’s jelly, a gelatinous tissue around umbilical vessels, from donated hUCs. First, the primary cells were obtained by tissue block adherent culture method, followed by inoculation with 5000 cells/cm2 and harvesting when the fusion degree reached 85%-90%. After continuous expansion, the fourth passage of hUC-MSCs was suspended in a 10% DMSO cryopreservation solution and stored in liquid nitrogen (-196 °C)[20] (Figure 1). In their future use as a cell stock material, the samples were thawed at 37 °C, washed to remove the DMSO cryopreservation solution, and resuspended in a com
| Test | Final frozen product | Final infusion product |
| Visual inspection | NA | Absence of visible, particle |
| Morphology | Fibroblastic | NA |
| Viability | ≥ 90% | ≥ 85% |
| Cell count | (4.5-6.0) × 107 | According to clinical needs |
| Pathogen tests | ||
| Sterility | Negative | Negative |
| Mycoplasma | Negative | Negative |
| Endotoxin | < 0.5 EU/mL | < 0.5 EU/mL |
| Cell surface markers | ||
| CD73 | ≥ 95% | - |
| CD90 | ≥ 95% | - |
| CD105 | ≥ 95% | - |
| CD29 | ≥ 95% | - |
| CD34 | ≤ 2% | - |
| CD45 | ≤ 2% | - |
| CD79a | ≤ 2% | - |
| CD14 | ≤ 2% | - |
| HLA-DR | ≤ 2% | - |
Once the samples passed the tests they were sent to the clinical department for intravenous infusion to the patient, which occurred within 2 h after sample receipt. The total time from the recovery of cell fluid to clinical application should be less than 12 h. The contents of the cell fluid quality test include the following: Integrity of the cell fluid package; appearance of the cell fluid (including coloration and whether there are floccules or any other precipitates or foreign matter); number of cells in the cell fluid; cell viability; endotoxin presence; and Gram stain for pathogenic bacteria.
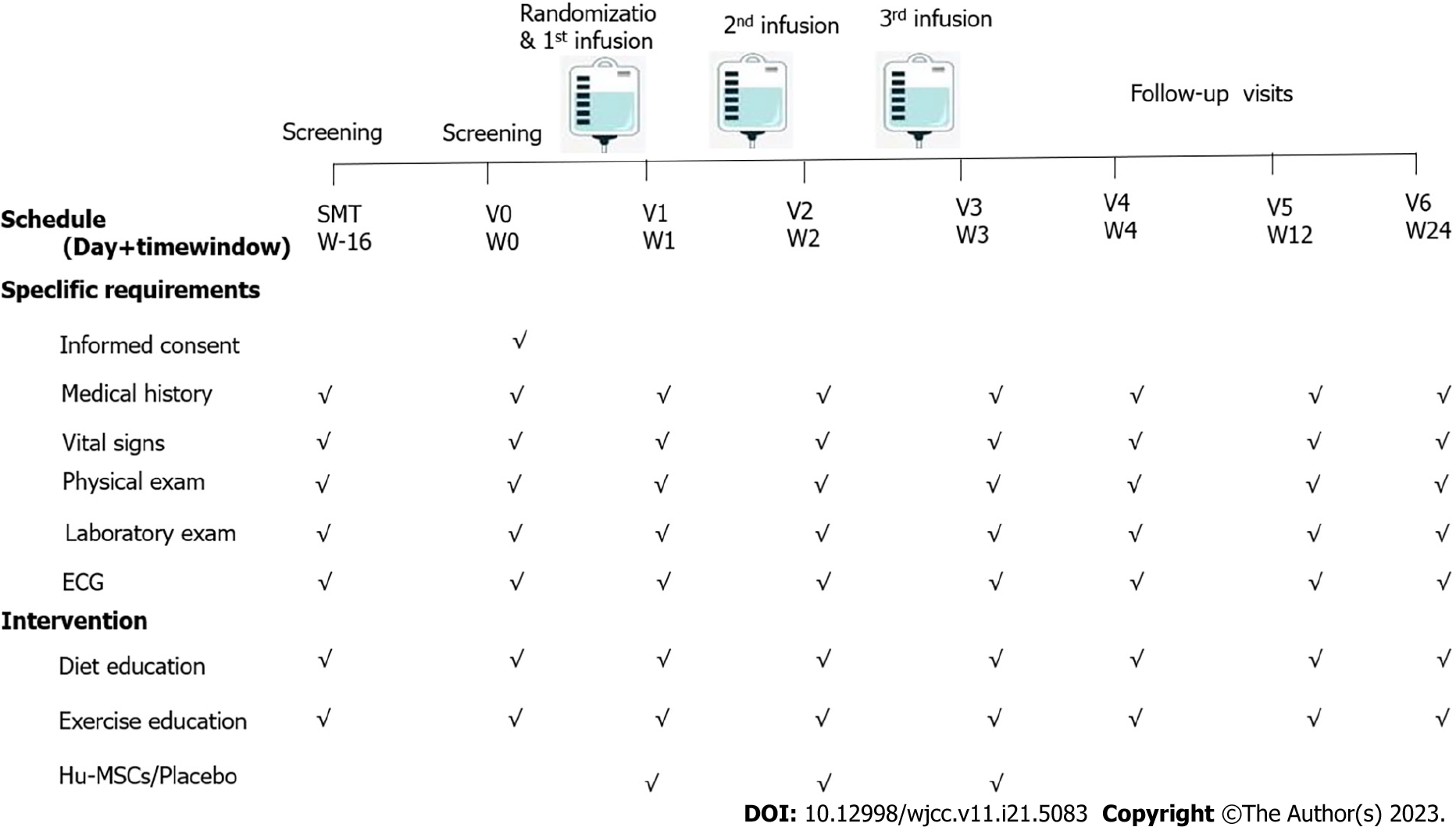
Treatment was given for a period of 16 wk, as previously reported[18], after which all patients were reassessed. The total of 34 patients who met the inclusion and exclusion criteria were randomized into two groups by random allocation software. The hUC-MSC group received an intravenous dosage of hUC-MSCs (1 × 106 cells/kg) once per week for 3 wk. The control group was given placebo, which consisted of an acellular injection of the compound electrolyte preservation solution (containing 5% albumin but lacking hUC-MSCs).
The follow-up visits were conducted at weeks 1, 2, 3, 4, 12, and 24 after the first infusion (Figure 2). Experience of fever, chest tightness, chest pain, dizziness, and any other clinical symptoms experienced during the treatment were recorded, along with any adverse reactions such as cardiocerebrovascular events and tumor occurrence.
All study participants were monitored by laboratory tests for routine blood parameters, liver function, renal function, blood lipids (e.g., total cholesterol and triglycerides), and coagulation indexes at weeks 1, 2, and 3 after the first infusion. We also conducted tests for diabetes antibody, specific infection indexes [hepatitis B surface antigen, antibodies against hepatitis C virus, combined detection of antigen and antibody of human immunodeficiency virus (HIV), and specific antibody against Treponema pallidum], and serum tumor markers [alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate antigen 199 (CA199)] as well as electrocardiogram, chest X-ray, and liver ultrasound at baseline and weeks 4, 12, and 24 after the first infusion.
All statistical analyses were carried out with SPSS® 25.0 software (IBM Corp, Armonk, NY, United States). Quantitative variables were summarized as median, and categorical variables were summarized numerically. Independent sample Wilcoxon test or χ2 test was used to assess between-group differences. Differences in proportions were analyzed by two-tailed test. P values < 0.05 were regarded as statistically significant.
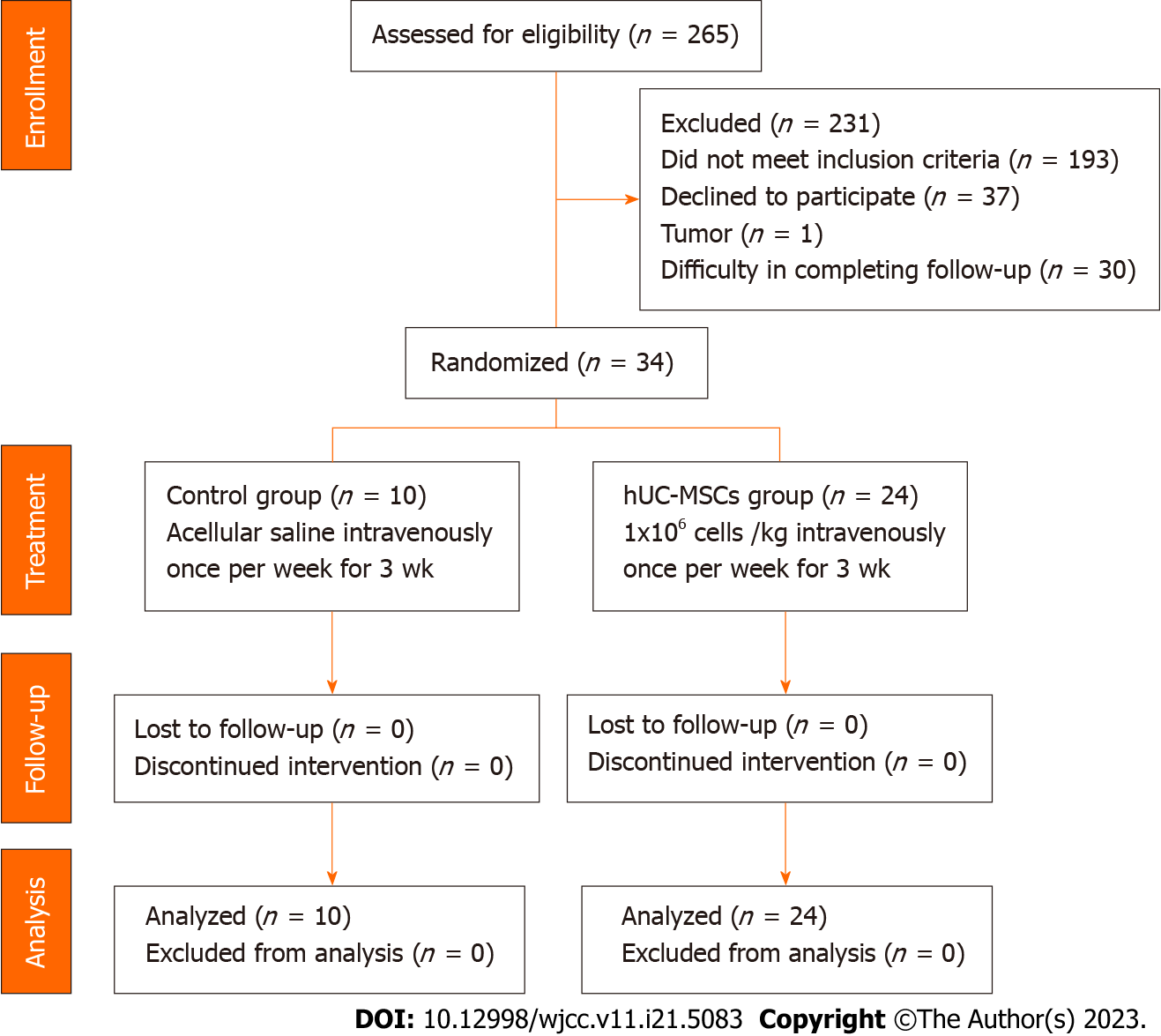
A total of 34 patients were included from September 2019 to September 2022 (Figure 3). After randomization, 24 of the patients were included in the treatment (“hUC-MSC”) group and 10 were included in the control (“placebo”) group. The clinical characteristics and baseline laboratory test findings are shown in Tables 2 and 3, respectively. No significant differences were observed between the two groups.
| Variable | All patients | Placebo group | hUC-MSC group | Z | P value |
| n = 34 | n = 10 | n = 24 | |||
| Sex | 0.45 | ||||
| Male | 28 (82.4) | 8 (80.0) | 20 (83.3) | ||
| Female | 6 (17.6) | 2 (20.0) | 4 (16.7) | ||
| Age in yr | 52 (45.5, 56.0) | 49.0 (44.0, 58.5) | 52.0 (46.0, 56.0) | -0.19 | 0.85 |
| Duration of T2DM in yr | 10.00 (4.00, 14.00) | 7.00 (3.75, 11.75) | 10.00 (4.50, 14.00) | -0.97 | 0.33 |
| BMI in kg/m2 | 24.21 (23.34, 26.25) | 23.72 (23.00, 26.25) | 24.61 (23.25, 26.50) | -0.42 | 0.68 |
| FPG in mmol/L | 8.95 (8.13, 10.14) | 8.82 (7.81, 9.97) | 9.13 (8.18, 11.91) | -0.42 | 0.68 |
| HbA1c as % | 7.95 (7.40, 8.50) | 8.00 (7.28, 8.35) | 7.95 (7.45, 8.50) | -0.49 | 0.70 |
| Variable | Placebo group | hUC-MSC group | Z | P value |
| n = 10 | n = 24 | |||
| Routine blood | ||||
| WBC | 6.48 (5.71, 8.17) | 6.87 (5.52, 8.13) | -0.227 | 0.821 |
| PLT | 232.0 (199.5, 253.8) | 214.0 (163.0, 258.3) | -0.718 | 0.473 |
| RBC | 5.15 (4.58, 5.46) | 4.94 (4.79, 5.45) | -0.076 | 0.94 |
| NEU | 3.80 (2.95, 4.89) | 4.20 (2.90, 5.15) | -0.189 | 0.850 |
| L | 2.21 (1.69, 2.44) | 2.23 (1.96, 2.61) | -0.454 | 0.65 |
| MONO | 0.45 (0.34, 0.53) | 0.36 (0.31, 0.44) | -1.192 | 0.233 |
| NLR | 1.89 (1.41, 2.36) | 1.64 (1.43, 2.13) | -0.227 | 0.821 |
| Liver function | ||||
| ALT | 21.00 (18.00, 31.25) | 25.50 (16.50, 48.25) | -0.606 | 0.545 |
| AST | 20.50 (17.00, 23.00) | 21.50 (18.00, 31.75) | -0.872 | 0.383 |
| γ-GT | 27.00 (20.75, 31.00) | 34.50 (23.75, 52.00) | -1.703 | 0.089 |
| TP | 73.70 (68.88, 76.93) | 71.70 (68.13, 76.78) | -0.529 | 0.597 |
| GLB | 29.25 (24.63, 30.98) | 25.75 (23.93, 28.33) | -1.966 | 0.049 |
| Renal function | ||||
| Urea | 6.51 (4.68, 7.23) | 5.87 (5.38, 6.61) | -0.227 | 0.821 |
| Cr | 65.00 (58.00, 73.00) | 67.00 (56.25, 81.00) | -0.227 | 0.82 |
| eGFR | 106.59 (97.44, 111.71) | 104.52 (84.42, 111.99) | -0.454 | 0.65 |
| Blood fat | ||||
| TC | 4.61 (4.06, 5.38) | 3.95 (3.55, 5.40) | -1.247 | 0.212 |
| TG | 1.67 (1.20, 3.04) | 1.87 (1.13, 2.79) | -0.094 | 0.925 |
| LDL-C | 2.91 (2.53, 3.29) | 2.48 (2.12, 3.72) | -1.077 | 0.281 |
| HDL-C | 1.14 (0.91, 1.33) | 1.12 (0.88, 1.46) | -0.019 | 0.985 |
| Coagulation function | ||||
| PT | 12.25 (12.00, 12.78) | 12.15 (12.00, 12.60) | -0.494 | 0.621 |
| APTT | 34.05 (32.68, 37.60) | 34.10 (32.58, 35.45) | -0.284 | 0.777 |
| TT | 17.95 (17.45, 19.00) | 18.00 (17.65, 18.90) | -0.265 | 0.791 |
| FIB | 2.95 (2.61, 3.53) | 3.07 (2.29, 3.22) | -0.302 | 0.762 |
| DD | 0.22 (0.22, 0.26) | 0.22 (0.22, 0.30) | -0.36 | 0.719 |
| Infection screening | ||||
| HBsAg | 0.00 (0.00, 0.00) | 0.00 (0.00, 20.31) | -1.081 | 0.28 |
| HCV-Ab | 0.05 (0.04, 0.06) | 0.09 (0.04, 0.14) | -1.679 | 0.093 |
| HIV-Ag/Ab | 0.07 (0.06, 0.08) | 0.06 (0.05, 0.08) | -0.731 | 0.465 |
| TP-Ab | 0.04 (0.02, 0.06) | 0.04 (0.04, 0.05) | -0.776 | 0.438 |
| Tumor marker | ||||
| AFP | 2.80 (2.20, 3.85) | 1.95 (1.65, 3.10) | -1.494 | 0.135 |
| CEA | 1.85 (1.40, 3.50) | 2.15 (1.58, 3.08) | -0.416 | 0.677 |
| CA199 | 8.45 (5.20, 17.33) | 5.55 (3.35, 15.90) | -0.643 | 0.52 |
Safety was evaluated through occurrence of adverse events (AEs) observed within 24 h after each infusion and each visit, including via clinical examinations and measurement of vital signs. No serious complications associated with the hUC-MSC infusion were observed.
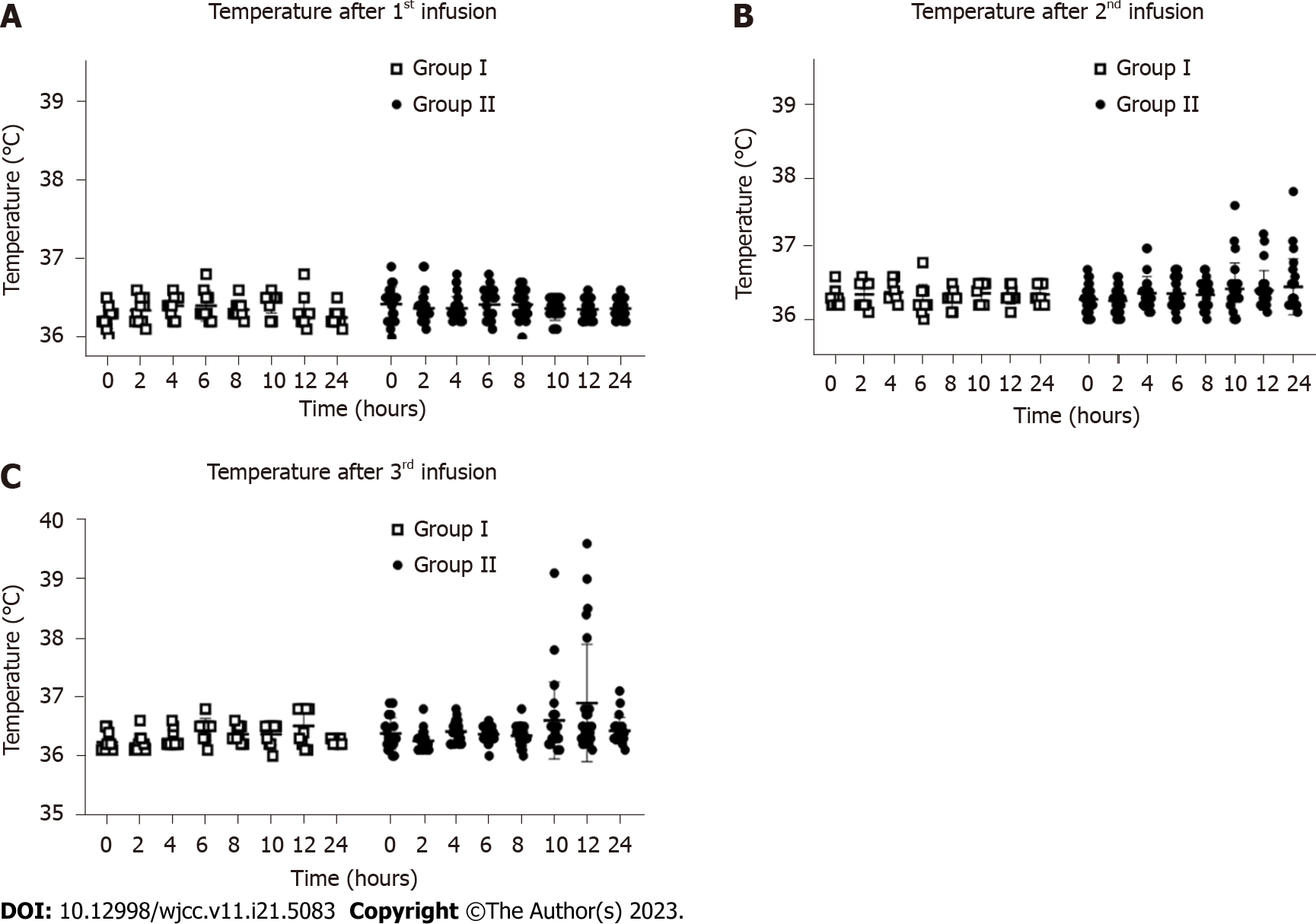
Four patients (16.67%) from the hUC-MSC group and none from the placebo group experienced transient fever, which primarily occurred within 24 h after the second and third infusions. There was no statistically significant difference between the two groups (P = 0.169) (Figure 4).
One patient (4.17%) from the hUC-MSC group and none from the placebo group experienced nocturnal hypoglycemia. The lowest blood glucose recorded for that single patient was 59.4 mg/dL, and the level returned to normal without intervention or food intake. During the follow-up period, that patient reduced their insulin dose and did not experience hypoglycemia again.
Four patients (16.67%) in the hUC-MSC group and none from the placebo group experienced fatigue within 3 d after the first infusion; in each case, it did not affect daily activities or work. In all, the AE was relieved gradually without any intervention.
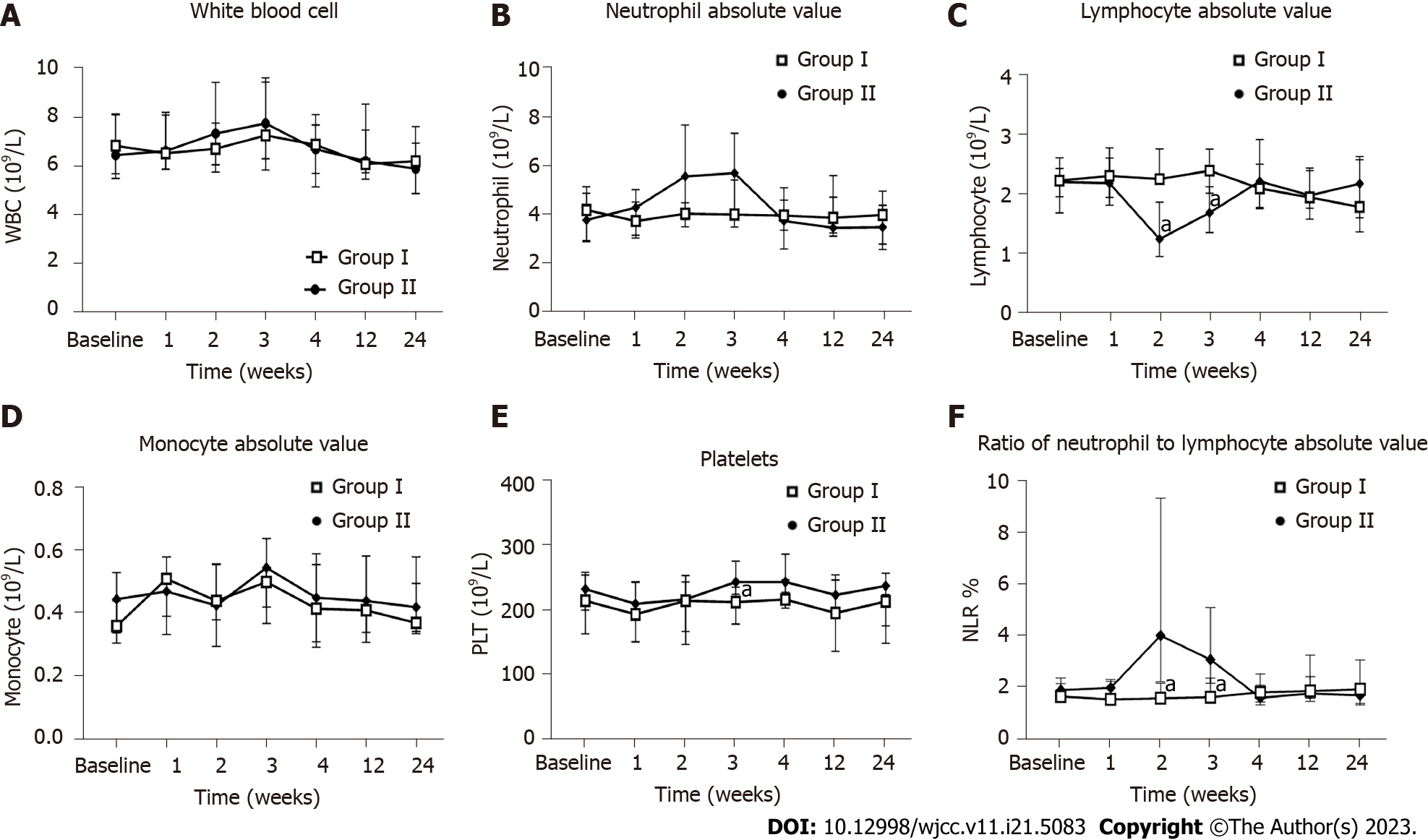
From the second week, patients in the hUC-MSC group showed a decrease in lymphocyte levels, with a return to the normal range after week 4. The lymphocyte levels in the hUC-MSC group were significantly lower than those in the placebo group at week 2 [1.26 (0.97, 1.87) vs 2.26 (2.20, 2.76); P < 0.05] and week 3 [1.70 (1.36, 2.13) vs 2.40 (2.02, 2.76); P < 0.05]. The neutrophil-to-lymphocyte ratio was significantly higher in the hUC-MSC group than in the placebo group at weeks 2 and 3 (P < 0.05 for both). The platelet levels were also significantly higher in the hUC-MSC group than in the placebo group at week 3 [243.00 (224.00, 275.25) vs 212.00 (178.25, 235.25); P < 0.05]. At the follow-up visits at weeks 4, 12, and 24, there were no statistically different findings between the two groups for the aforementioned indicators. There were also no significant differences between the two groups for white blood cell, neutrophil, and monocyte counts (Figure 5).
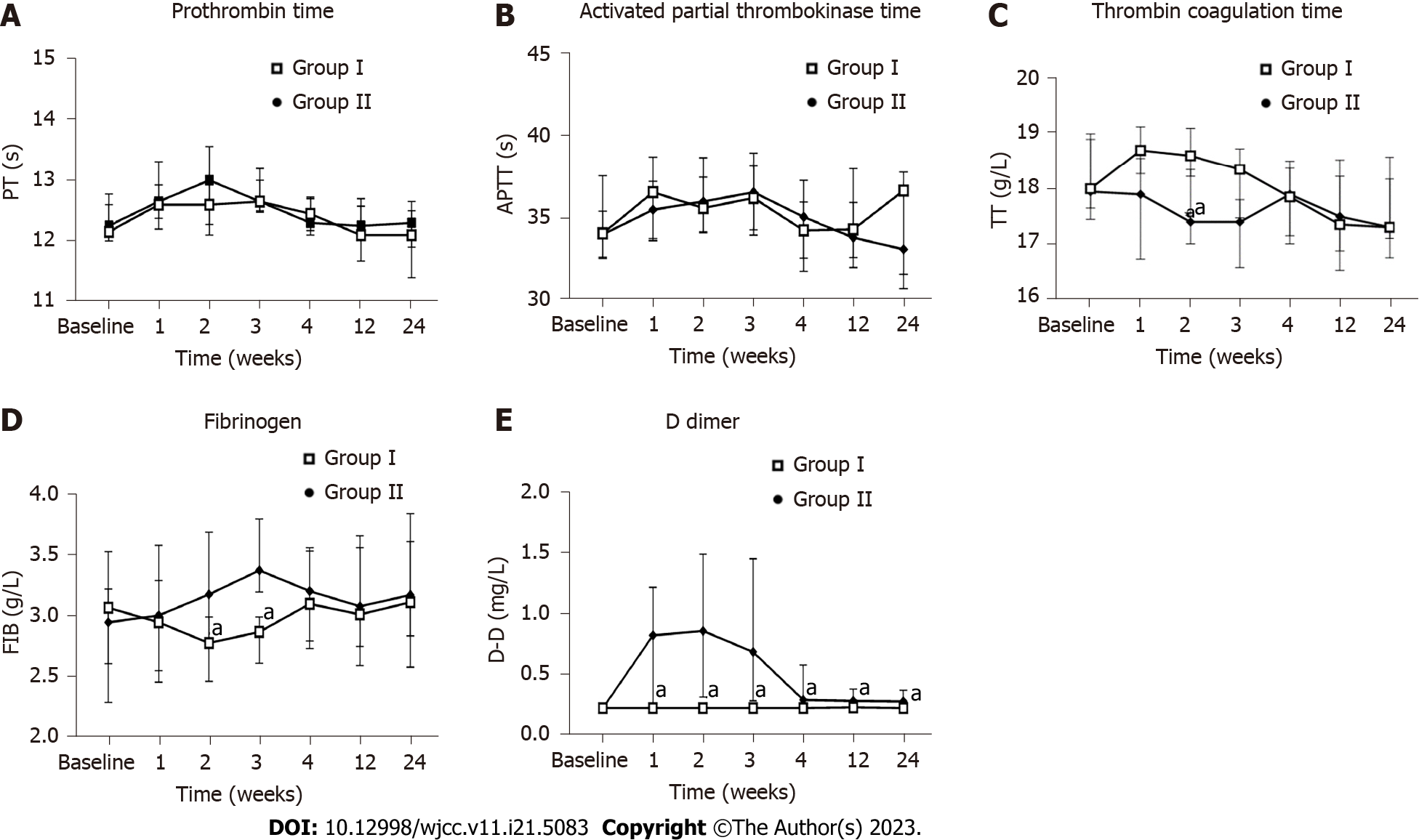
D-dimer values were significantly higher in the hUC-MSC group than in the placebo group at all timepoints (weeks 1, 2, 3, 4, 12, and 24; P < 0.05 for all). The fibrinogen levels were also significantly higher in the hUC-MSC group than in the placebo group at week 2 [3.18 (2.75, 3.69) vs 2.78 (2.46, 2.99); P < 0.05] and week 3 [3.37 (3.20, 3.79) vs 2.87 (2.61, 2.99); P < 0.05]. The thrombin coagulation time was significantly lower in the hUC-MSC group than in the placebo group at week 2 [17.40 (17.00, 18.35) vs 18.60 (18.23, 19.10); P < 0.01], decreasing and stabilizing to baseline at week 3. The prothrombin time (PT) and activated partial thrombokinase time (APTT) were also monitored during treatment, but there were no significant differences found between the two groups (P > 0.05) (Figure 6).
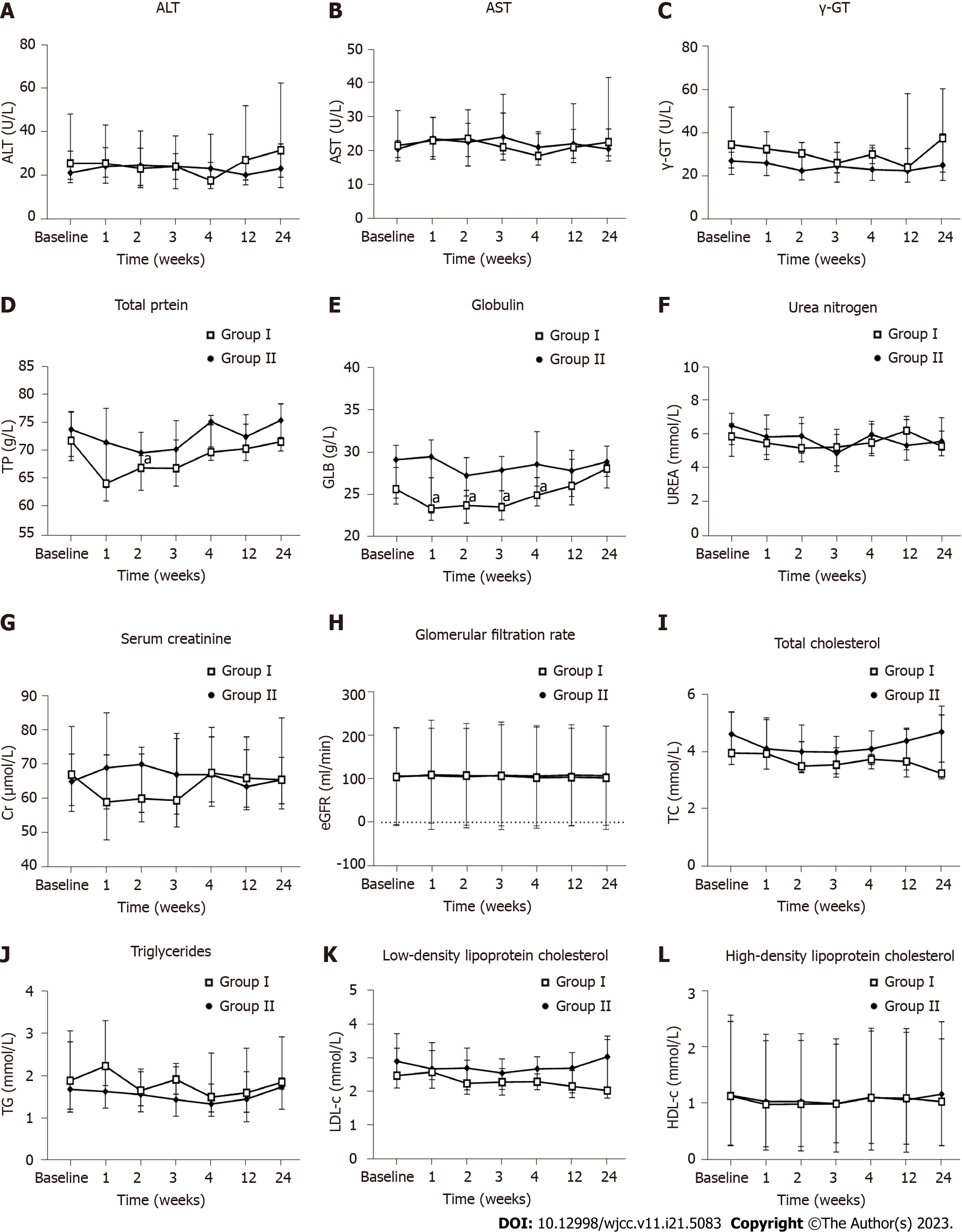
The immunoglobulin levels were significantly higher in the hUC-MSC group than in the placebo group at week 1 [29.60 (22.83, 31.60) vs 23.40 (21.98, 27.10); P < 0.05], week 2 [27.35 (24.93, 29.50) vs 23.75 (21.65, 25.60); P < 0.05], week 3 [28.00 (25.55, 29.65) vs 23.55 (22.03, 27.95); P < 0.05], and week 4 [28.70 (26.10, 32.60) vs 25.00 (23.70, 27.13); P < 0.05]. The liver function indexes (blood alanine transaminase, aspartate aminotransferase, gamma-glutamyl transferase, and total protein), renal function indexes (serum creatinine, estimated glomerular filtration rate, and urea nitrogen), and blood lipid indexes (triglycerides, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol) showed no significant differences between the two groups (P > 0.05) (Figure 7).
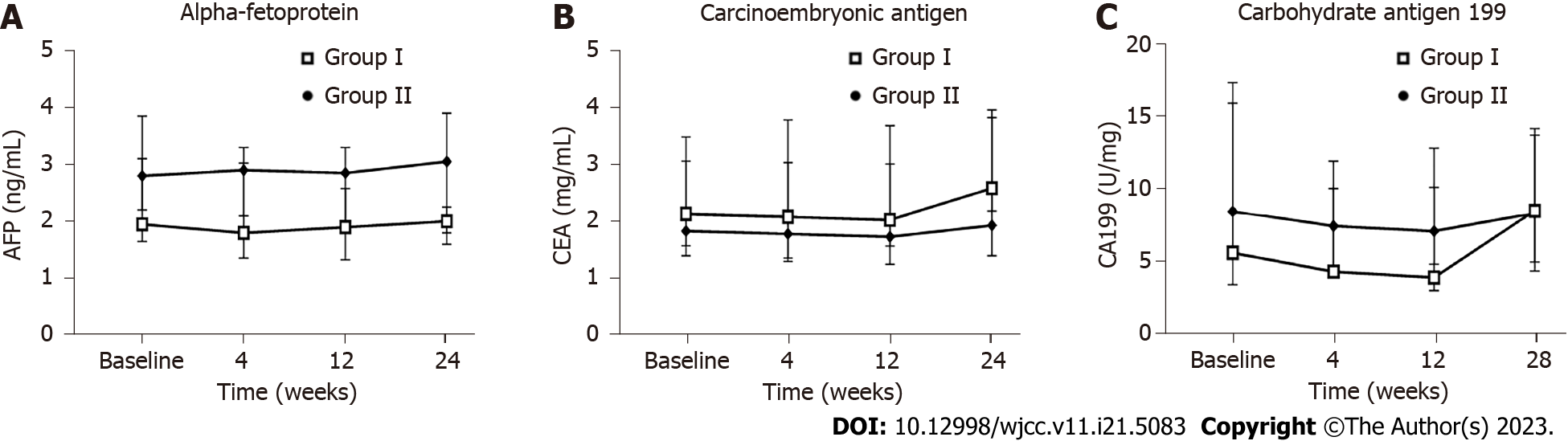
The AFP, CEA, and CA199 tumor markers, used to evaluate tumor trends, showed no differences between the two groups at any of the timepoints (Figure 8). At baseline we detected hepatitis B surface antigen in 2 patients in the placebo group and 1 patient in the hUC-MSC group. No patients in either group showed positivity for antibodies against hepatitis C virus, combined detection of HIV antigen and antibody, or specific antibody against Treponema pallidum. There were also no changes detected for these indicators during any of the follow-up visits after infusion.
Chest X-ray and liver color ultrasound examinations showed no changes after the infusion at any of the timepoints. In addition, no nodules or tumors were detected in any patient.
Currently, MSC-based cellular therapies are considered effective for many diseases, including DM and its complications[11,21,22]. Our previous study showed that intravenous infusion of hUC-MSCs could improve blood glucose, glycated hemoglobin, and islet function in T2DM[18]. However, clinical research on the safety of hUC-MSCs in treating T2DM has been insufficient. The purpose of this study was to evaluate the safety of hUC-MSC intravenous infusion for T2DM patients. The results indicated good tolerability and safety of hUC-MSC infusion in T2DM patients; the potential disadvantageous responses observed were decreased lymphocyte counts, which may relate to escape from immune attack, and an effect on coagulation function, which may lead to thrombotic disease.
After three hUC-MSC infusions, no patients developed a thromboembolic event. Only mild AEs that spontaneously resolved or with minimal intervention (see fever below), were detected in a small number of patients. Generally, this indicates safety (without any acute infusion-related issues, allergic reactions, delayed hypersensitivity, or secondary infections)[23]. Furthermore, no patients experienced a fever after the first infusion, although 4 patients (16.7%) experienced transient fever after the second and third hUC-MSC infusions. The highest temperature recorded was 39.5 °C. The patients’ body temperatures returned to normal naturally or gradually after administration of nonsteroidal anti-inflammatory drugs. Some patients also reported fatigue and hypoglycemia after the hUC-MSC infusions. Thus, post-intravenous hUC-MSC infusion immune responses were considered as mild symptoms. No novel acute cardiovascular and cerebrovascular events or tumors/nodules occurred during the follow-up.
Lymphocytes are an essential component of the immune system. Peripheral blood lymphocytes primarily include T and B cells, which constitute a significant component of the immune system and can produce specific immune responses. MSCs are reported to be closely related to immune cells, like lymphocytes. Di Nicola et al[24] found that bone marrow MSCs can inhibit T lymphocyte proliferation and apoptosis. Thus, reactivation stimulates efficient proliferation, which may be relevant to soluble factor production after MSC infusion. Our study revealed that patients experienced a reduction in peripheral blood lymphocytes after 2 wk of continuous intravenous hUC-MSC infusion and recovered spontaneously after discontinuation. The hUC-MSC therapy did not impact the absolute values of lymphocytes during long-term follow-up, consistent with the findings by Di Nicola et al[24].
It has also been reported that MSCs can induce T cell apoptosis via the FAS/FASL pathway. Moreover, MSCs are known to regulate immune response intensity and promote regulatory T cell production through various paracrine effects and cell-cell contact. This induced immune tolerance diminishes the adverse impacts of lymphocytes on MSCs[24-27].
The application of embryonic stem cells is currently limited because of related teratoma formation[28]. Although research has shown that MSCs do not induce malignant transformation[29,30] and some studies have even explored the efficacy of MSC therapy on tumors[30], concerns about possible tumorigenic actions remain undeniable. Guan et al[23] conducted a 3-year follow-up observational study on the efficacy of hUC-MSC treatment of T2DM, and no MSC-related malignancies occurred. Similarly, we did not observe an elevation in tumor-associated antigens (AFP, CEA, and CA199) in patients treated with MSCs. Additionally, during the follow-up period, no nodules/tumors occurred in the lungs, liver, gallbladder, spleen, nor pancreas. However, our follow-up duration was relatively short, and we plan to extend it out to 3 years to thoroughly investigate potential transplantation-related complications.
To date, the roles of MSCs in coagulation and inflammation have been inadequately explored. It has, however, been shown that MSC production may lead to the release of procoagulant tissue factors (TFs) at different levels during the treatment[31]. TFs are significant determinants of cell product blood compatibility and are crucial regulators for the extrinsic coagulation pathway of cytokines. TFs can trigger negative systemic inflammatory responses under some conditions. MSCs themselves can trigger platelet production and promote platelet activation, posing a risk of thrombosis[32]. In particular, an MSC dose of > 1 × 106 cells/kg has been shown to extend PT and APTT and to reduce concentrations of fibrinogen and factor VIII[33]. Small-dose MSC transfusion also affects PT but not APTT, indicating that MSCs mainly activate coagulation via TFs and extrinsic coagulation pathways.
Our study revealed that the D-dimer level was significantly elevated following hUC-MSC (1 × 106 cells/kg) intravenous infusion for three consecutive weeks and that elevation persisted to the 24th wk. Continuous transfusion can lead to transient fibrinogen elevation and thrombin coagulation time reduction. The levels recovered after discontinuation of the hUC-MSC treatment, without affecting PT and APTT. We, thus, considered this situation to be correlated with the hUC-MSC dosage and frequency. Previous studies have also found MSC dosage increases to be associated with acute AEs, including microvascular embolization[34,35].
For future clinical application of hUC-MSC treatment for T2DM, we will explore the interactive mechanism between hUC-MSCs and lymphocytes and the effect of cell dose, culturing and passaging techniques, and route of administration on the inflammatory mechanism; these findings may help to reduce and avoid related AEs in these patients. The sample size of the current study was small. At the same time, a small number of participants had delayed visits due to the coronavirus 2019 pandemic, which could have affected our results. We used a single-blind study design, and there may have been deviation during the follow-up. Reducing the error was a priority. Multiple patients were consulted, and follow-up registrants and examiners were single-blinded.
Our study demonstrated that hUC-MSCs were well tolerated during T2DM treatment and elicited no serious AEs. Transient fever, hypoglycemia, and fatigue may occur in the short-term, but no long-term AEs were detected. Lymphocyte levels decreased and inflammatory factors increased after the intravenous infusion of hUC-MSCs. The results of our study are expected to provide a more solid theoretical basis for the continued pursuit of clinical application of MSCs in the treatment of T2DM.
Cellular therapies represent a new opportunity for the treatment of type 2 diabetes mellitus (T2DM) and its complications. However, the safety of human umbilical cord-mesenchymal stem cells (hUC-MSCs) in clinical application has not been fully assessed.
We conducted a trial to evaluate the safety and tolerance of hUC-MSC infusion in T2DM treatment.
We hypothesized that hUC-MSC infusion may cause fevers or nodules, affect inflammatory mediators, and induce hypercoagulability. We conducted the present trial to treat T2DM patients with an hUC-MSC infusion and evaluated the safety of the hUC-MSC therapy.
T2DM patients were enrolled and received hUC-MSC (1 × 106 cells/kg) once per week for 3 wk. The safety was assessed by clinical symptoms and signs, laboratory tests, and imaging tests. The laboratory tests included routine blood parameters, coagulation indexes, and liver function, renal function, and tumor markers. Imaging tests included electrocardiogram, chest X-ray, and ultrasound of the liver, bile, spleen, and pancreas.
During the 24-wk follow-up period, there were no serious adverse events observed. However, a few patients experienced fever, fatigue, and hypoglycemia. The lymphocyte levels were significantly decreased in the hUC-MSC group compared to the placebo group. The D-dimer level, neutrophil-to-lymphocyte ratio, and immunoglobulin level were significantly increased in the hUC-MSC group compared to the placebo group.
Our study suggests that hUC-MSCs are safe for the treatment of T2DM, with only mild adverse events occurring. hUC-MSC treatment can affect immunity and inhibit lymphocytes. The influence of hUC-MSC on coagulation requires and warrants further research.
Lymphocyte subsets and the inflammatory mediators of the patients in this study will be extensively followed for further analysis to elucidate the mechanisms of the observed decrease in lymphocyte numbers and the influence on immune function.
The authors acknowledge the financial support from all the funders. The authors also want to thank all patients and their families who consented to participate in the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Popovic DS, Serbia; Triolo F, United States S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Yu HG
| 1. | International Diabetes Federation. IDF diabetes atlas ninth. Dunia: International Diabetes Federation, 2019. |
| 2. | Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR. Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet Med. 1998;15:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249-1258. [PubMed] |
| 4. | DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2013;36 Suppl 2:S127-S138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 5. | Zhang Y, Chen W, Feng B, Cao H. The Clinical Efficacy and Safety of Stem Cell Therapy for Diabetes Mellitus: A Systematic Review and Meta-Analysis. Aging Dis. 2020;11:141-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Kamal MM, Kassem DH. Therapeutic Potential of Wharton's Jelly Mesenchymal Stem Cells for Diabetes: Achievements and Challenges. Front Cell Dev Biol. 2020;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 7. | Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical Cord Mesenchymal Stem Cell Treatment for Crohn's Disease: A Randomized Controlled Clinical Trial. Gut Liver. 2018;12:73-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 8. | Mebarki M, Abadie C, Larghero J, Cras A. Human umbilical cord-derived mesenchymal stem/stromal cells: a promising candidate for the development of advanced therapy medicinal products. Stem Cell Res Ther. 2021;12:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 9. | Tsai PJ, Wang HS, Lin GJ, Chou SC, Chu TH, Chuan WT, Lu YJ, Weng ZC, Su CH, Hsieh PS, Sytwu HK, Lin CH, Chen TH, Shyu JF. Undifferentiated Wharton's Jelly Mesenchymal Stem Cell Transplantation Induces Insulin-Producing Cell Differentiation and Suppression of T-Cell-Mediated Autoimmunity in Nonobese Diabetic Mice. Cell Transplant. 2015;24:1555-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Khatri R, Mazurek S, Petry SF, Linn T. Mesenchymal stem cells promote pancreatic β-cell regeneration through downregulation of FoxO1 pathway. Stem Cell Res Ther. 2020;11:497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Cho J, D'Antuono M, Glicksman M, Wang J, Jonklaas J. A review of clinical trials: mesenchymal stem cell transplant therapy in type 1 and type 2 diabetes mellitus. Am J Stem Cells. 2018;7:82-93. [PubMed] |
| 12. | Fiori A, Terlizzi V, Kremer H, Gebauer J, Hammes HP, Harmsen MC, Bieback K. Mesenchymal stromal/stem cells as potential therapy in diabetic retinopathy. Immunobiology. 2018;223:729-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Cao Y, Gang X, Sun C, Wang G. Mesenchymal Stem Cells Improve Healing of Diabetic Foot Ulcer. J Diabetes Res. 2017;2017:9328347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Madani S, Larijani B, Keshtkar AA, Tootee A. Safety and efficacy of hematopoietic and mesanchymal stem cell therapy for treatment of T1DM: a systematic review and meta-analysis protocol. Syst Rev. 2018;7:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 848] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 16. | Wang W, Zhong W, Yuan J, Yan C, Hu S, Tong Y, Mao Y, Hu T, Zhang B, Song G. Involvement of Wnt/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget. 2015;6:42276-42289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Lin HD, Fong CY, Biswas A, Choolani M, Bongso A. Human Umbilical Cord Wharton's Jelly Stem Cell Conditioned Medium Induces Tumoricidal Effects on Lymphoma Cells Through Hydrogen Peroxide Mediation. J Cell Biochem. 2016;117:2045-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Lian XF, Lu DH, Liu HL, Liu YJ, Han XQ, Yang Y, Lin Y, Zeng QX, Huang ZJ, Xie F, Huang CH, Wu HM, Long AM, Deng LP, Zhang F. Effectiveness and safety of human umbilical cord-mesenchymal stem cells for treating type 2 diabetes mellitus. World J Diabetes. 2022;13:877-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Gu J, Huang L, Zhang C, Wang Y, Zhang R, Tu Z, Wang H, Zhou X, Xiao Z, Liu Z, Hu X, Ke Z, Wang D, Liu L. Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: a randomized, controlled trial. Stem Cell Res Ther. 2020;11:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (2)] |
| 20. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 21. | Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 959] [Article Influence: 159.8] [Reference Citation Analysis (35)] |
| 22. | Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Konari N, Fujimiya M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep. 2016;6:34842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 23. | Guan LX, Guan H, Li HB, Ren CA, Liu L, Chu JJ, Dai LJ. Therapeutic efficacy of umbilical cord-derived mesenchymal stem cells in patients with type 2 diabetes. Exp Ther Med. 2015;9:1623-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (2)] |
| 24. | Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2455] [Cited by in RCA: 2363] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 25. | Wang L, Zhao Y, Shi S. Interplay between mesenchymal stem cells and lymphocytes: implications for immunotherapy and tissue regeneration. J Dent Res. 2012;91:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 985] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 27. | Yu C, Tang W, Lu R, Tao Y, Ren T, Gao Y. Human Adipose-derived mesenchymal stem cells promote lymphocyte apoptosis and alleviate atherosclerosis via miR-125b-1-3p/BCL11B signal axis. Ann Palliat Med. 2021;10:2123-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11399] [Cited by in RCA: 10432] [Article Influence: 386.4] [Reference Citation Analysis (0)] |
| 29. | Zang L, Li Y, Hao H, Liu J, Cheng Y, Li B, Yin Y, Zhang Q, Gao F, Wang H, Gu S, Li J, Lin F, Zhu Y, Tian G, Chen Y, Gu W, Du J, Chen K, Guo Q, Yang G, Pei Y, Yan W, Wang X, Meng J, Zhang S, Ba J, Lyu Z, Dou J, Han W, Mu Y. Efficacy and safety of umbilical cord-derived mesenchymal stem cells in Chinese adults with type 2 diabetes: a single-center, double-blinded, randomized, placebo-controlled phase II trial. Stem Cell Res Ther. 2022;13:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 30. | Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 202] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 31. | Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringdén O, Volk HD, Geissler S, Reinke P. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol Med. 2019;25:149-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 292] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 32. | Guillamat-Prats R. Role of Mesenchymal Stem/Stromal Cells in Coagulation. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 33. | Liao L, Shi B, Chang H, Su X, Zhang L, Bi C, Shuai Y, Du X, Deng Z, Jin Y. Heparin improves BMSC cell therapy: Anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics. 2017;7:106-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 34. | Gleeson BM, Martin K, Ali MT, Kumar AH, Pillai MG, Kumar SP, O'Sullivan JF, Whelan D, Stocca A, Khider W, Barry FP, O'Brien T, Caplice NM. Bone Marrow-Derived Mesenchymal Stem Cells Have Innate Procoagulant Activity and Cause Microvascular Obstruction Following Intracoronary Delivery: Amelioration by Antithrombin Therapy. Stem Cells. 2015;33:2726-2737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 35. | Toma C, Wagner WR, Bowry S, Schwartz A, Villanueva F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res. 2009;104:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |