Published online Jul 16, 2023. doi: 10.12998/wjcc.v11.i20.4944
Peer-review started: April 9, 2023
First decision: May 8, 2023
Revised: May 23, 2023
Accepted: June 21, 2023
Article in press: June 21, 2023
Published online: July 16, 2023
Processing time: 93 Days and 21.9 Hours
Eosinophilic granuloma (EG) is a proliferative condition that affects the cells of bone tissue. There are no specific clinical signs or imaging manifestations in the early stages of the disease, making it simple to overlook and misdiagnose. Because of the disease's rarity, there is presently no standardized treatment principle. There are few accounts of such occurrences affecting the axis among children. We discovered a case of a child whose EG resulted in atlantoaxial joint dislocation and destruction of the axial bone.
After having pharyngeal discomfort for more than six months without a clear explanation, a 6-year-old boy was brought to our hospital. Following a careful evaluation, the pathology indicated a strong likelihood of an axial EG. Ultimately, we decided to treat the boy with posterior pedicle screw fixation and local steroid injections.
EGs of the upper cervical spine are quite uncommon in children, and they are exceedingly easy to overlook or misdiagnose. Posterior pedicle screw fixation and local steroid injections are effective treatments for patients with axial EGs affecting the atlantoaxial junction.
Core Tip: One rare cause of neck pain is axial eosinophilic granuloma. Patients with abrupt neck discomfort should be evaluated with a radiological investigation. This study illustrates that axial eosinophilic granuloma with atlantoaxial joint dislocation can be treated with posterior pedicle screw fixation combined with local steroid injections and that histopathological biopsy is an excellent diagnostic tool for verifying the condition.
- Citation: Tu CQ, Chen ZD, Yao XT, Jiang YJ, Zhang BF, Lin B. Posterior pedicle screw fixation combined with local steroid injections for treating axial eosinophilic granulomas and atlantoaxial dislocation: A case report. World J Clin Cases 2023; 11(20): 4944-4955
- URL: https://www.wjgnet.com/2307-8960/full/v11/i20/4944.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i20.4944
Eosinophilic granulomas (EGs) are osteolytic tumor-like masses that usually develop slowly without presenting with any clinical symptoms when they are in their early stages[1,2]. Most EG lesions are isolated, and they can affect the whole skeletal system[1,3]. Numerous Langerhans cells proliferate, and eosinophils and lymphocytes invade the diseased tissue. Bone EG has a modest occurrence, at approximately 1/1500000[1,4]. With a male-to-female ratio of approximately 2 to 5:1 and a spinal prevalence of 6.5% to 25%, the majority of cases affect children between the ages of 5 and 14[4-6].
According to previous reports, cervical EG is a rare disease among children[1,3]. There is not yet a standard treatment philosophy; therefore, each patient requires an individual plan of care that is tailored to their specific needs.
A boy presenting axial EG with dislocation of the atlantoaxial joint was treated by posterior pedicle screw fixation combined with local steroid injections at the hospital's spine surgery department.
For the past five days, the patient had experienced neck pain and restricted neck motion.
A 6-year-old boy who had been experiencing pharyngeal discomfort for more than six months with an unspecified cause was brought to our hospital.
The boy had endured 6 mo of conservative treatment at the neighborhood clinic after receiving a false diagnosis of chronic tonsillitis. However, the pain did not improve.
The patient had no family or personal history related to the symptom.
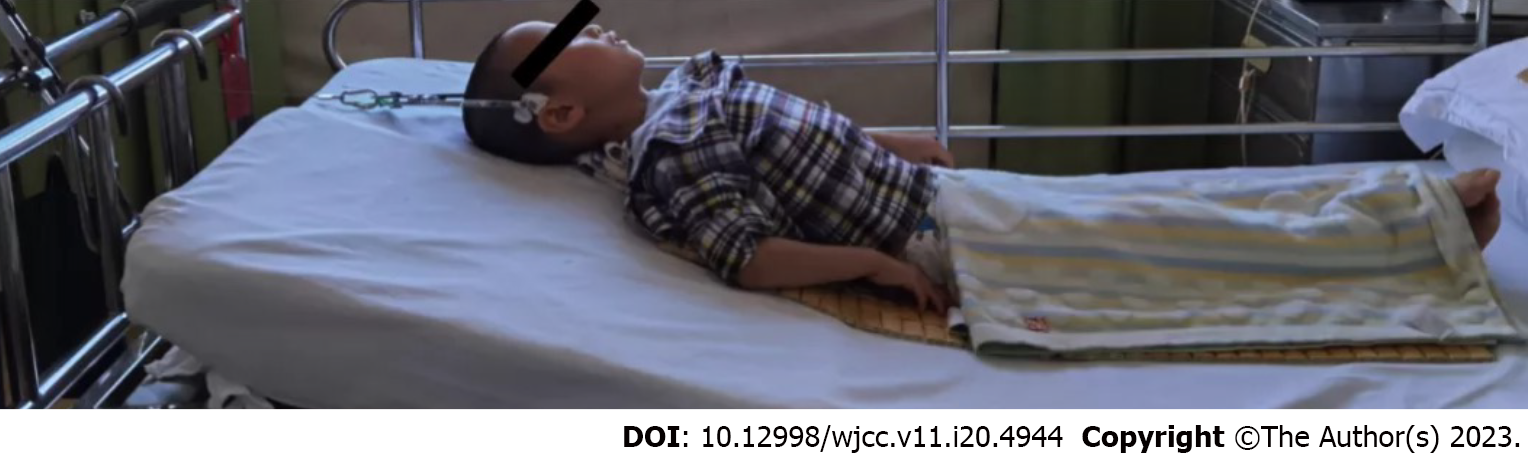
There was no association with any cranial or occipital discomfort, pain or numbness in the limbs, dyspnea, nausea, or vomiting. A professional examination revealed straightening of the cervical spine, no skin ulcerations, and slight neck swelling. There was equal length of the lower and upper limbs, evident neck mobility restriction, and upper neck discomfort without aberrant bone rubbing; limb sensations - including touch, pain, warmth, and deep proprioception - were all normal.
According to laboratory test results, the regular blood and procalcitonin levels were normal. The hypersensitive C-reactive protein (CRP) level was 21.78 mg/L (normal CRP values for adults and children are 0.068-8.2 mg/L), and the erythrocyte sedimentation rate (ESR) was 78 mm/h (normal ESR values are 0 mm/h to 15 mm/h for men and 0 mm/h to 20 mm/h for women).
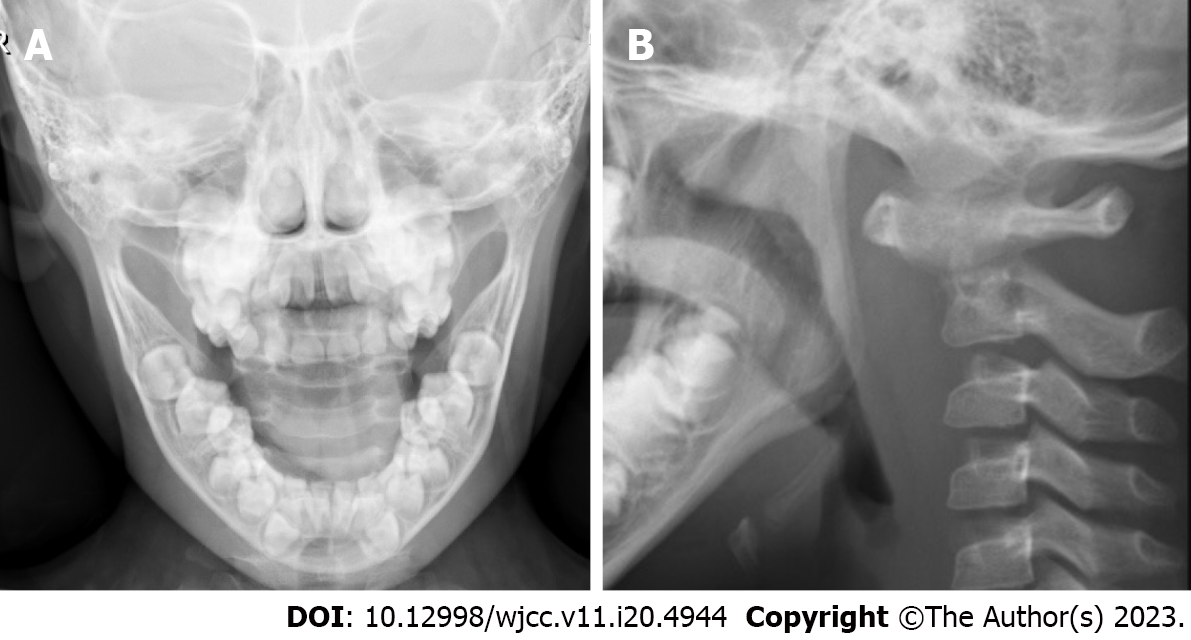
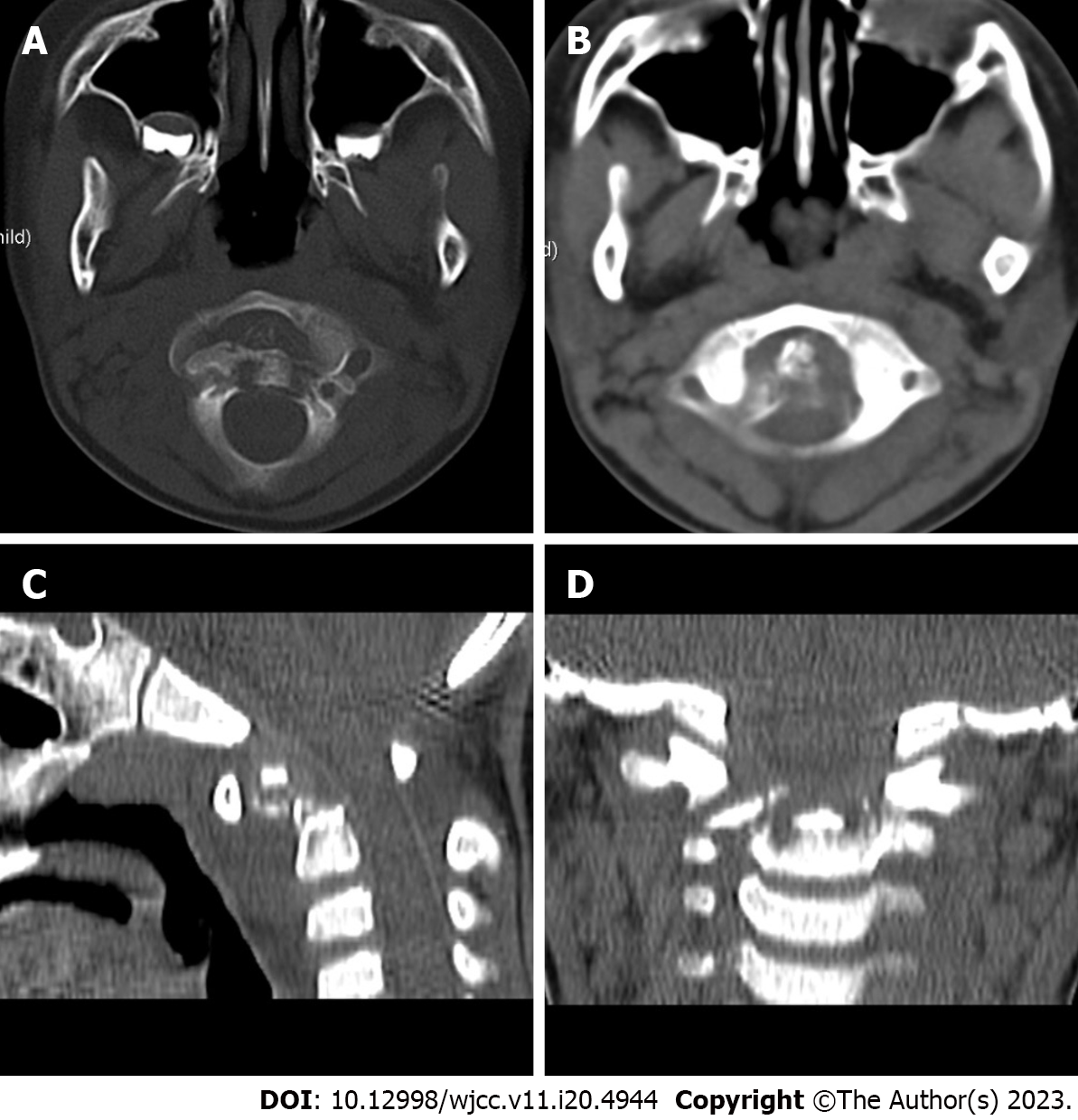
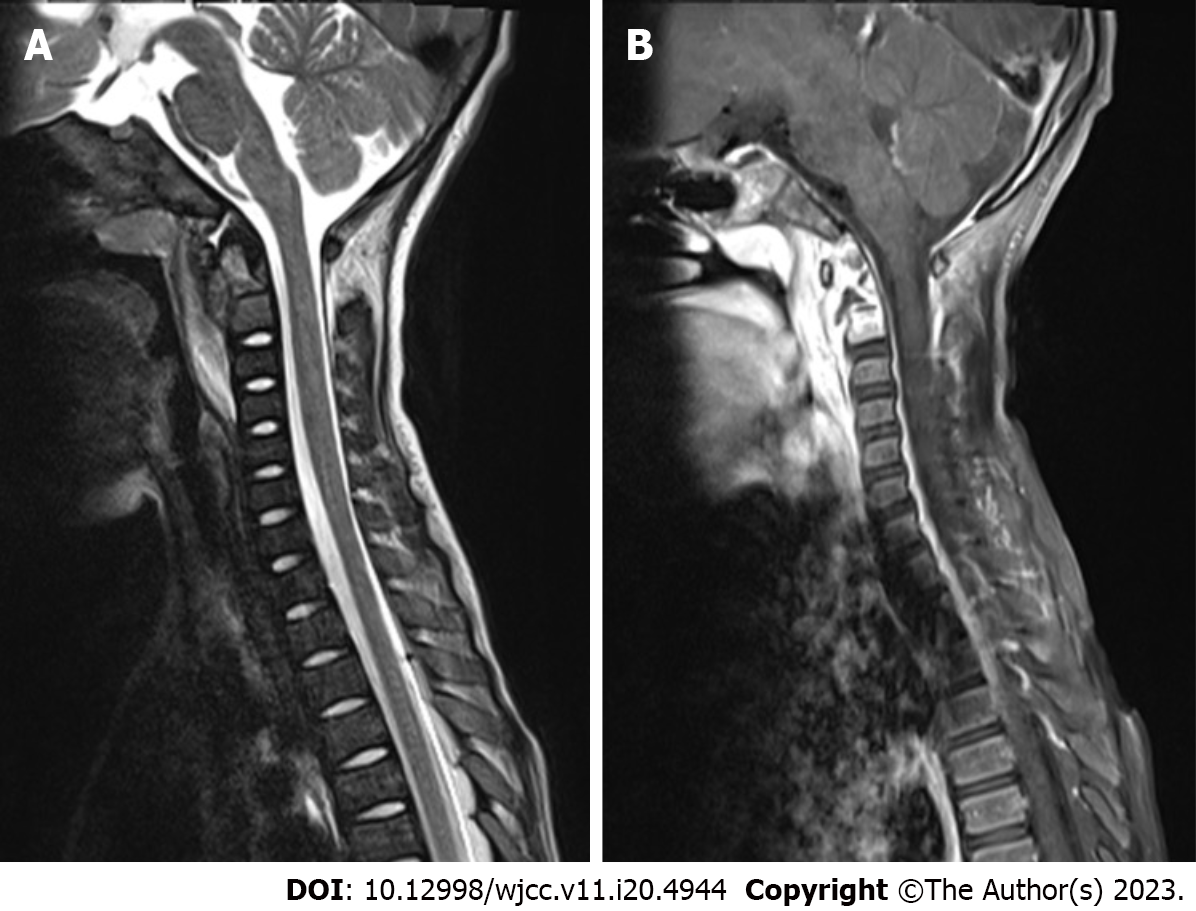
Axial bone texture abnormalities, a decline in local bone density, and enlargement of the atlantoaxial joint space were observed on X-rays (Figure 1). The odontoid process and the upper margin of the axial vertebral body were damaged, and the atlantoaxial joint was dislocated, according to a computed tomography (CT) scan (Figure 2). Cervical spine magnetic resonance imaging (MRI) with contrast enhancement revealed odontoid destruction and abnormal signals in the retropharyngeal and prevertebral spaces (Figure 3).
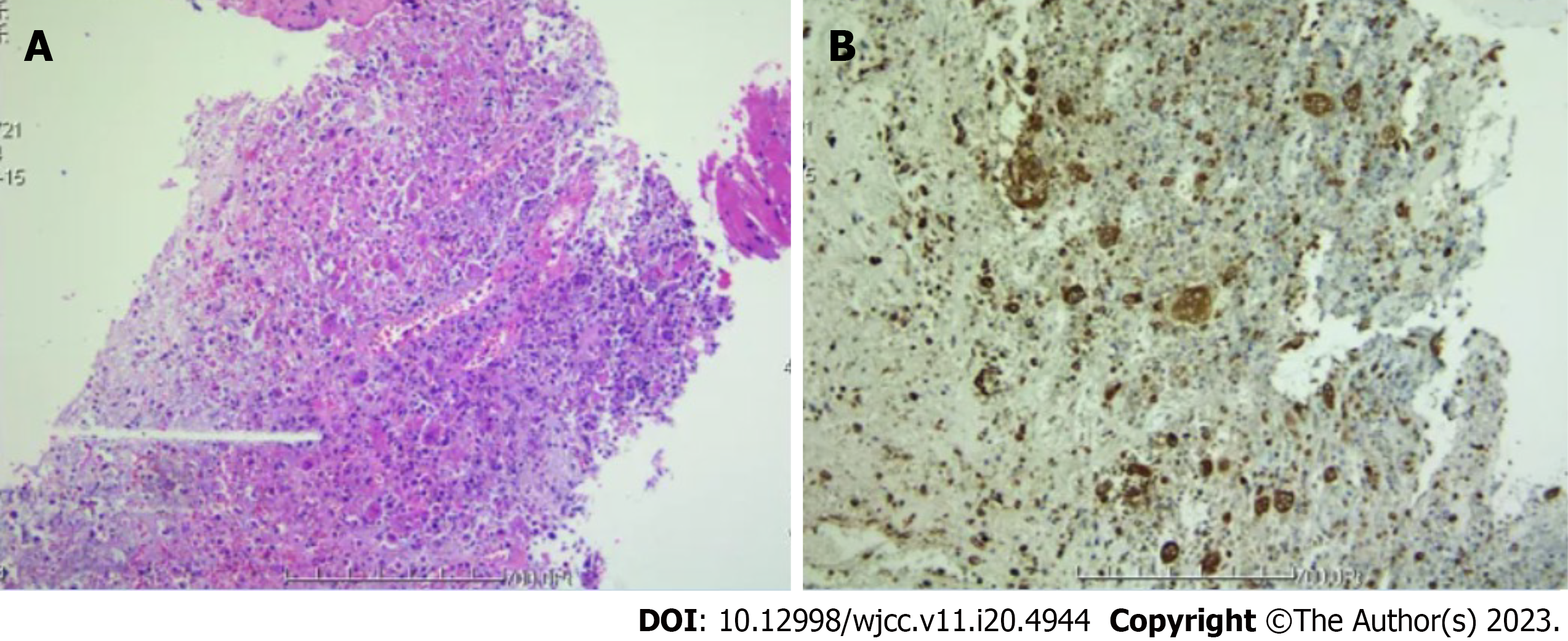
Pharyngitis was discovered during electronic laryngoscopy. An EG was suspected after an oral endoscopic biopsy of a lesion in the posterior pharyngeal wall indicated granulomatous inflammation with necrosis (Figure 4). No bacteria, viruses, fungi, parasites, mycobacterium tuberculosis complex, mycoplasma, or chlamydia were found in the specimens sent for high-throughput genome sequencing.
Combining the above findings with the patient's auxiliary examination, the following diagnosis was made: Axial vertebral body EG and atlantoaxial dislocation.
The patient was admitted to complete the relevant examinations and underwent biopsy to clarify the cause. Figure 5 shows the hyperextended position in which cranial traction reduction was carried out. Regular bedside X-rays were taken to assess the decrease in atlantoaxial joint dislocation following traction, and the traction line and weight were promptly adjusted. According to the categorization of atlantoaxial joint dislocation given by Wang et al[4], the child had Type II (reducible dislocation) and atlantoaxial instability and required surgical therapy. We employed pedicle screws to regain spinal stability while simultaneously administering local corticosteroid injections to treat the EG intraoperatively.
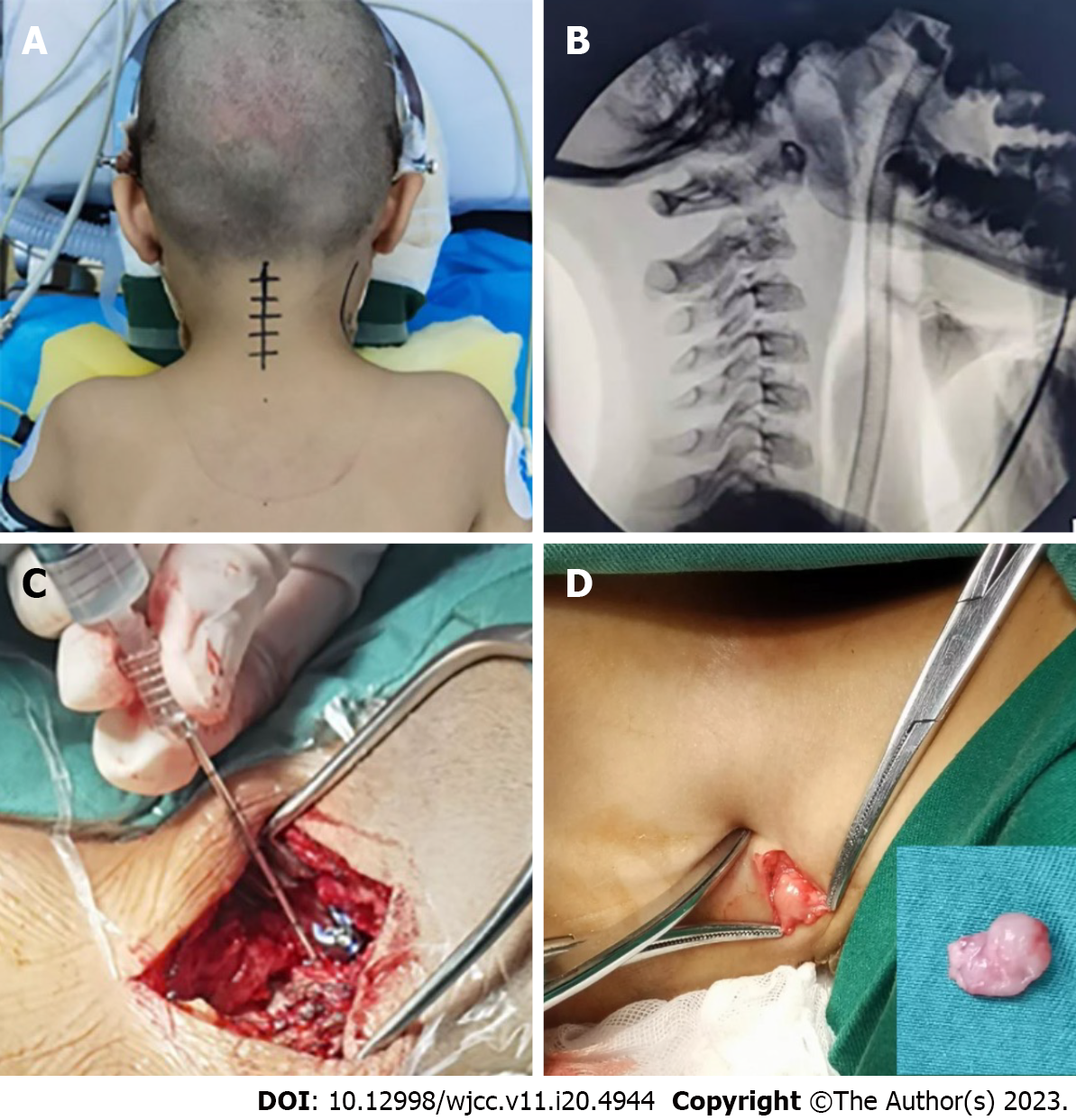
The child was positioned in a prone position with a "U"-shaped cushion on the chest, the head was placed on a specially built plaster bed, the cervical spine was gently bent by 15°-20°, and the skull traction was kept at approximately 2 kg after general anesthesia with endotracheal intubation (Figure 6A). After positioning the child, a C-arm X-ray machine was used to determine the improvement of the atlantoaxial dislocation and perform the operation while maintaining the reset state (Figure 6B). To expose the bilateral lamina and subperiosteal dissection to expose the posterior arch of the atlas to the midline of the lateral mass of the axis (2 mm), we made an 8 cm longitudinal incision from the middle of the back of the neck. The neurovascular plexus behind the lateral mass joints of the atlantoaxial vertebrae was reserved, and the upper and lower portions of the posterior arch of the atlas were individually investigated. Care was taken to avoid injuring the vertebral artery, as well as the lateral atlantoaxial joint, the lower part of the atlas posterior arch, and the upper part of the atlas isthmus. We fully exposed the atlas pedicle to see whether there was any rotation or structural abnormalities by using the nerve stripper to examine from the medial wall of the lateral mass of the atlas inward. We used the posterior arch tubercle of the atlas as the needle entrance mark, opened the area approximately 20 mm lateral to the midline, removed a tiny piece of cortical bone with a burr, and maintained a 10°-15° head tilt in both the coronal and sagittal planes in the direction of screw insertion. Then, a positioning pin was inserted, perspective observation was used to confirm the placement point and orientation, and the drill hole was gently widened. The needle entry point of the axis was 2 mm above the midpoint of the inferior articular process of the axis, and the horizontal inward was tilted approximately 20° and gently drilled. The sagittal plane was angled upward approximately 30°. Reaming was finished after accurate fluoroscopy. Forty milligrams of methylprednisolone sodium succinate injection, for a total of 80 mg on both sides, was administered into the C2 vertebral body lesion through the pedicle screw channels (Figure 6C). The connecting rod was installed, the pedicle screw with a 3.5 mm diameter was fastened, and the screw was locked to use the force of the screw rod to restore the cervical spine's natural curvature. The boy's vital signs were constantly monitored, and nerve electrophysiological monitoring was employed during the procedure to reduce the danger of cervical spinal cord and nerve damage. The ilium was utilized for bone grafting at the lesion of the axis after fluoroscopy indicated that the pedicle screw was secured in place and the cervical spine was properly reset. The surgical wound was then stitched up after the drainage tube was inserted. Larger cervical lymph nodes were removed concurrently for postoperative biopsy (Figure 6D).
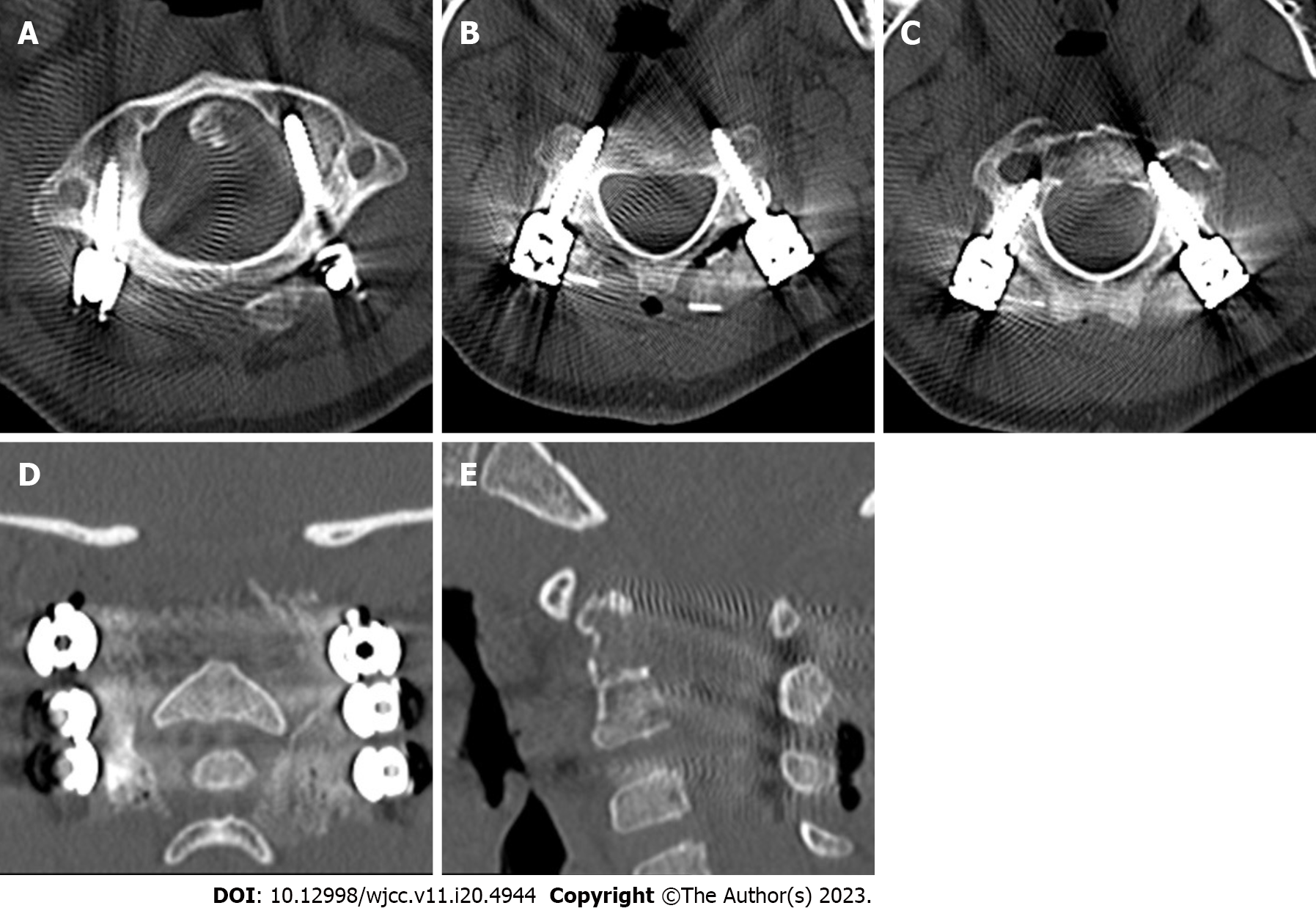
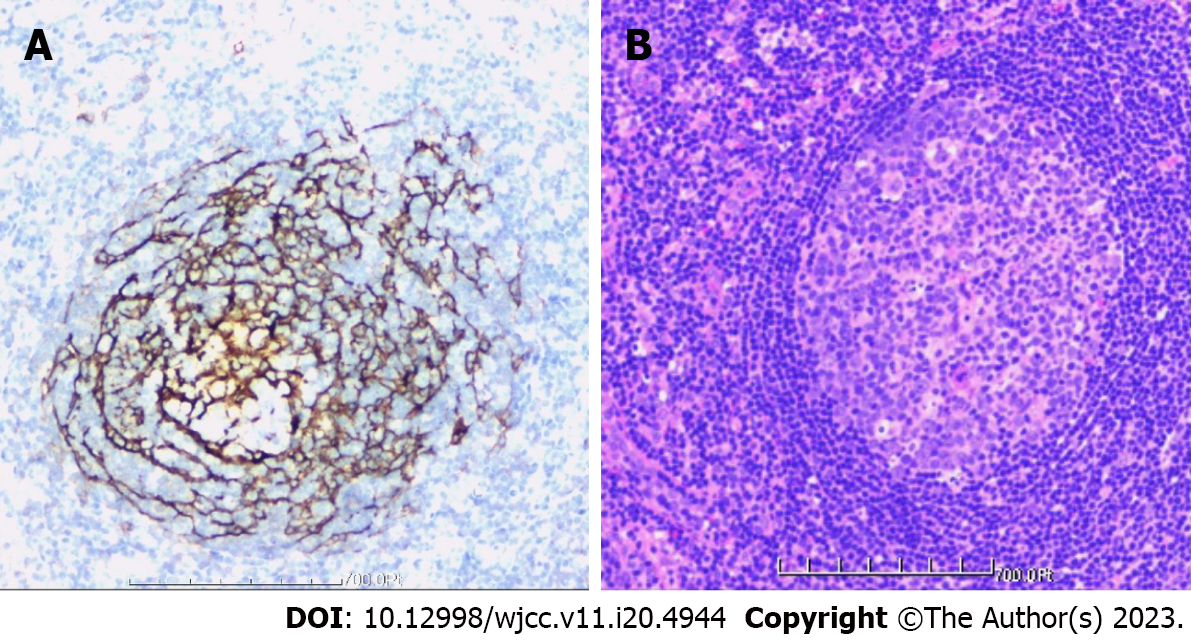
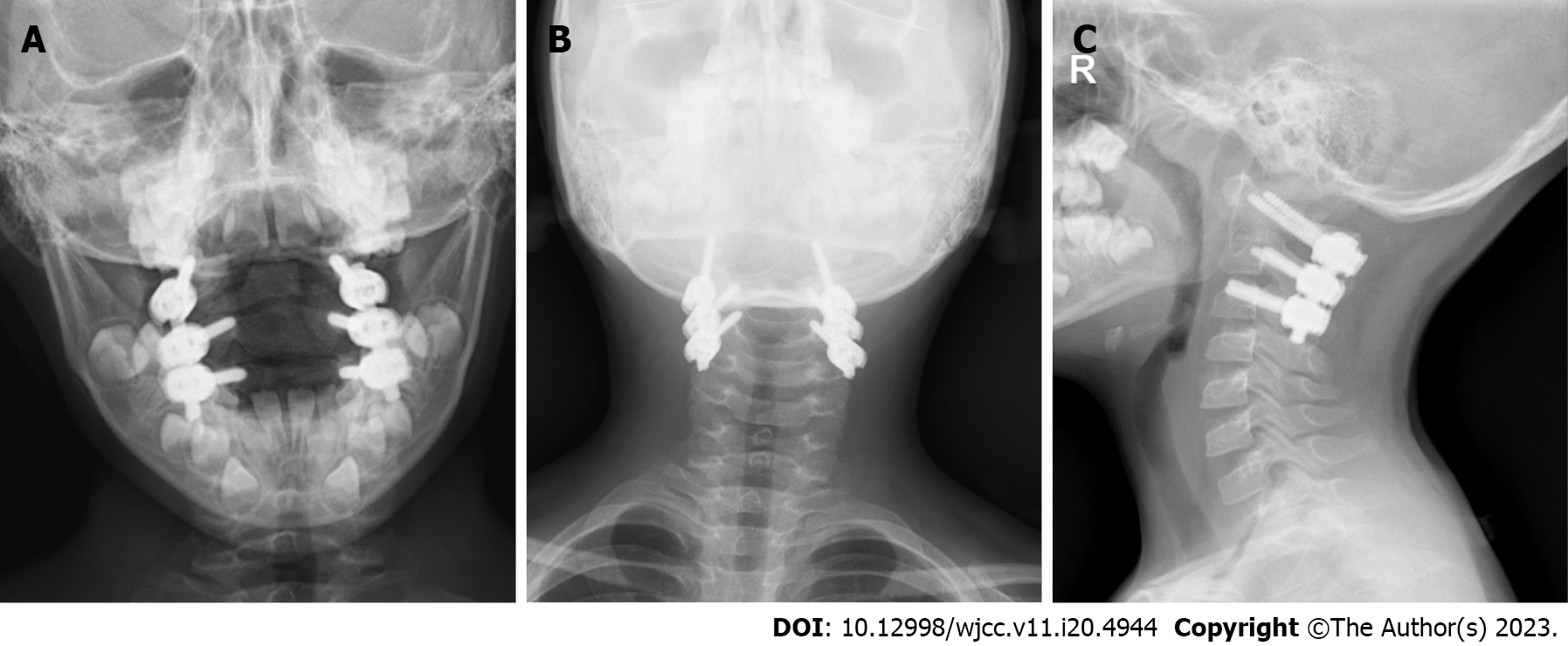
The patient was moved to the intensive care unit for observation and care after the procedure. His vital signs and neurological function were continuously monitored, and he was given symptomatic treatments, including analgesics, routine antibiotics to prevent infections, wound dressings, and enhanced respiratory tract management. After more than 48 h, the drainage tube was withdrawn. The patient began to walk progressively on the third day following surgery while wearing a head-neck-chest brace that was worn for 10 to 12 wk. Posterior atlantoaxial pedicle screws effectively corrected the atlantoaxial joint dislocation, according to the postoperative X-ray analysis (Figure 7). The atlantoaxial joint dislocation decreased, the screw position was good, and the bone graft had good embedding, according to the postoperative CT scan (Figure 8). The atlantoaxial joint dislocation was decreased, the screw position was good, there was no spinal canal stenosis, the shape of the spinal cord was regular, the signal was uniform, and no abnormal enhancement was identified in the spinal canal, according to the postoperative MRI (Figure 9). The tissue was primarily hyperplastic and included degenerating fibrous tissue and degenerating bone tissue, with flaky distribution, partial necrosis of bone tissue, interstitial vascular hyperplasia and dilatation with inflammatory cell infiltration, patchy areas of necrosis (pink), and a significant amount of multinucleated giant-cell infiltration, according to the postoperative pathological reports (Figure 10). Plasma cell CD38 (focus +), CD138, lymphocyte LCA (+), Ki-67 approximately 10% graded CK (-), EMA (-), Smur100 (+), CD68 (histiocyte +), CD1a (histiocyte +), SMA (vascular +), and CD34 (vascular +) were all detected in the tissue by immunohistochemistry. The diagnosis of EG was confirmed by immunohistochemical staining with histology (Figure 10). After the surgery, the patient recovered well and was released two weeks later. An X-ray revealed that the atlantoaxial dislocation had been corrected and that the joint was in a good position one year after the treatment (Figure 11). One year after the procedure, the atlantoaxial dislocation was still corrected, the internal fixation was in a good position, and the iliac bone transplant had successfully fused (Figure 12). The MRI one year after the operation showed that the axis lesion had reduced significantly (Figure 13).
A form of Langerhans cell histiocytosis (LCH) known as EG is characterized by eosinophil infiltration and histiocytosis[7]. Although the lesions can involve bones throughout the body, the spine is one of the disease predilection sites, whereas the cervical vertebrae are relatively rarely involved[8]. Osteolysis is the primary presentation in 80% of cases, most of which involve asymmetrical and osteolytic changes. Bone lesions are the most common manifestation of the disease, while extraosseous involvement is less frequent[1,8,9]. Classification of Histiocytic Disorders in Children: Its histological characteristics include the proliferation of many Langerhans cells and the infiltration of eosinophils and lymphocytes in the diseased tissue[1,4,10]. Bone EG has a low occurrence, at approximately 1/1500000. The majority of cases affect children between the ages of 5 and 14 years, with a male-to-female ratio of approximately 2 to 5:1 and a spinal prevalence of 6.5% to 25%[4-6]. The gradual nature of spinal EG onset is reflected in its early clinical signs, which frequently include localized soreness, restricted spinal function, and a lack of specificity[2,6]. The incidence of neurological symptoms is approximately 33%[6,11]. Laboratory findings are nonspecific and show anemia, increased ESR, an increased white blood cell count or eosinophil count, and increased alkaline phosphatase. Spinal EG imaging signs vary as the disease progresses, and it can be challenging to distinguish EGs from infections, TB, and tumors[6]. Because of the disease's low prevalence, lack of specificity of clinical signs and vague radiological findings, it is often ignored or misdiagnosed.
Needle biopsy and histological examination are required as the sole method of obtaining a conclusive diagnosis[1,6]. Histopathologically, it is distinguished by mostly yellow-brown soft granulation tissue that has a variable number of lymphocytes and eosinophils infiltrating it. At the margin of the osteolytic necrotic region, multinucleated giant cells can also be detected. The majority of them are CD1a positive and S100 positive, according to an immunohistochemical investigation[4,12]. The diagnosis of LCH can be made either by means of histological analysis of the lesions (presumed diagnosis) or by immunohistochemical analysis. Under optical microscopy, a monoclonal neoplastic infiltrate of Langerhans cells is observed with a lobulated nucleus of thin chromatin and moderately abundant eosinophilic cytoplasm with some nuclear atypia and variable mitotic activity. In contrast, an immunohistochemical study revealed the presence of Birbeck granules and positivity for S-100 and CD1a (representing diagnostic certainty)[13-15]. The main clinical manifestation of this 6-year-old male child was neck pain and obvious limitation of neck movement, and the misdiagnosis time extended to half a year. Routine blood and procalcitonin levels were normal, C-reactive protein: 21.78 mg/L and ESR: 78 mm/h. Immunohistochemistry shows histiocytes positive for CD1a and S100. These findings are consistent with previous literature.
The notion of customized treatment should be followed because there are currently few studies relating to various treatments for spinal EG because of its uncommon occurrence. The prognosis can be considerably improved with early diagnosis and therapy. Depending on the patient's health and the risks of surgical intervention, either conservative treatment or surgical treatment may be considered[16]. At present, spinal EG is considered self-limited, but its natural outcome is quite different. The prognosis is poorer when more vertebral bodies are implicated and the patient is younger. Early-stage patients with no visible neurological symptoms or spinal instability may benefit from conservative treatments such bed rest, bracing, traction, and medication[2,17]. Bed rest and brace immobilization have obvious effects on patients with mild vertebral lesions, can restore the height of the collapsed vertebral body and relieve pain and mild neurological symptoms, and thus can be used as the initial treatment of spinal EG[1,18]. However, after immobilization therapy, CT and MRI findings should be reexamined regularly to determine whether EG develops further. Regardless of the presence of clinical symptoms, additional therapy is required if imaging results reveal growth of the lesions. When signs of nerve compression arise during braking treatment, the patient needs to be operated on immediately. Because of the risk of secondary malignant tumors, damage to the spinal cord, destruction of the cartilage endplates of the upper and lower vertebral bodies, and effects on vertebral body growth, radiotherapy and chemotherapy are currently not recommended for children unless the disease is difficult to control[1]. In cases of significant compression of the EG vertebrae of the spine, percutaneous vertebroplasty and percutaneous kyphoplasty are frequently performed[19]. For individuals with spinal EG who additionally have nerve compression, spinal instability, or other complications, such as the dislocation of the atlantoaxial joint in the present case, this technique of treatment is useless. Surgery is usually utilized as treatment when specific circumstances, such as spinal instability, a lack of clarity in the diagnosis, significant neurological problems, evident deformities, agonizing pain, and constrained range of motion, occur. Patients with or without persistent spinal subluxation should also receive surgical care. The surgical strategy is determined by the focus's location and the extent of surrounding involvement. Curettage and biopsy, subtotal corpectomy, corpectomy with fusion, laminectomy, or posterior fusion are among the surgical options[1,2,6,17,20].
A local steroid infusion has a positive impact on spinal EG[1,2,21,22]. In clinical settings, CT guidance is typically used to inject steroids into the target. Local steroid injection can minimize edema, stop capillary and fibroblast growth, and prevent the infiltration of inflammation and exudation. This prevents the development of granulation tissue[21]. Corticosteroids can reduce the production and release of histamine, serotonin, and other active substances, which can lessen the hyperemia and edema caused by an inflammatory reaction and inhibit inflammatory infiltration and exudation. Histiocytosis is a secondary reaction to infection or inflammation[20]. In the later stage, it may prevent capillaries and fibroblasts from proliferating, which may prevent the development of granulation tissue even more. Forty milligrams of steroids can be administered if the lesion only affects half or less of the vertebral body; 80 mg of steroids can be injected if the lesion affects more than half of the vertebral body[21].
This patient, who was 6 years and 8 mo old, had atlantoaxial joint dislocation as well as an axial EG. The medulla oblongata and upper cervical spinal cord may be compressed as a result of dislocation of the axial odontoid process, which can lead to neurological symptoms such hemiplegia, dyspnea, and limb numbness[23]. Aggressive surgical therapy was planned to stop the disease from progressing further. Currently, the primary therapy for regaining atlantoaxial stability is surgery[4]. In actuality, anterior surgery is unable to provide patients with the best surgical outcomes due to its high risk, complex anatomy, deep operating field, and difficult nursing care. For adult upper cervical disorders, posterior atlantoaxial pedicle screw fixation has been gradually employed in recent years. In the past, there were no internal fixation devices for the pediatric cervical spine, and it was usually thought that children were still developing and that internal fixation might stunt their growth. Additionally, atlantoaxial pedicle internal fixation in children is a somewhat challenging procedure. Without cervical internal fixation, only autologous bone grafts were utilized in the past; nevertheless, transpedicular screw fixation is the most effective internal fixation method for the atlantoaxial joint[24]. Screw placement in the pedicle provides a more consistent clinical outcome than other fixation techniques because it has a stronger holding power, exhibits a firmer biomechanical stability, prevents atlanto-occipital fusion, and maintains the mobility of the atlanto-occipital joints. In a related study by Chen et al[25], children between the ages of 0 and 6 years had axial vertebrae that were anatomically 89.38% similar to those of adults and could securely accept screws with a 3.5 mm diameter. The curvature, growth, and development of children's upper cervical vertebrae have not been significantly affected by atlantoaxial fixation and fusion, according to the most recent long-term follow-up. Considering that children's bones are relatively malleable to expansion, age is not a severe restriction[26]. Therapy using local steroid injections is safe and efficient. In addition to effectively reducing edema in the lesion and relieving pain, local injection has considerably fewer side effects than systemic high-dose steroid therapy. In this instance, CT-guided percutaneous local steroid injection was not appropriate because the child was young, uncooperative, and presented with atlantoaxial joint dislocation upon admission. Therefore, performing local steroid injection through percutaneous puncture under the direction of CT alone was not appropriate.
In general, our research has made a significant contribution to the use of this treatment method for related diseases. Our study revealed significant therapeutic effects of this treatment, indicating broad potential applications. However, we must acknowledge the limitations of this study, primarily reflected in the small sample size. Future research should build upon our findings and continue to collect more cases to further establish the therapeutic efficacy of this treatment and explore optimization methods.
After surgery, the spinal cord compression in the patient was relieved, the stability of the spine was restored, local edema was reduced, and the pain significantly eased. The patient has returned to a normal life at present. Axial EG with dislocation of the atlantoaxial joint in children can be effectively and safely treated with posterior pedicle screw fixation and local steroid injections. Clinically, this course of therapy may be beneficial for children with axial EGs complicated by atlantoaxial joint dislocation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mahmoud MZ, Saudi Arabia; Reis F, Brazil S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Huang W, Yang X, Cao D, Xiao J, Yang M, Feng D, Huang Q, Wu Z, Zheng W, Jia L, Wu S. Eosinophilic granuloma of spine in adults: a report of 30 cases and outcome. Acta Neurochir (Wien). 2010;152:1129-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Zheng W, Wu J, Wu Z, Xiao J. Atlantoaxial instability secondary to eosinophilic granuloma of the axis in adults: long-term follow-up in six cases. Spine J. 2014;14:2701-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Hoover KB, Rosenthal DI, Mankin H. Langerhans cell histiocytosis. Skeletal Radiol. 2007;36:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Wang S, Wang C, Yan M, Zhou H, Dang G. Novel surgical classification and treatment strategy for atlantoaxial dislocations. Spine (Phila Pa 1976). 2013;38:E1348-E1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Ueda Y, Murakami H, Demura S, Kawahara N, Tomita K, Tsuchiya H. Eosinophilic granuloma of the lumbar spine in an adult. Orthopedics. 2012;35:e1818-e1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Huang WD, Yang XH, Wu ZP, Huang Q, Xiao JR, Yang MS, Zhou ZH, Yan WJ, Song DW, Liu TL, Jia NY. Langerhans cell histiocytosis of spine: a comparative study of clinical, imaging features, and diagnosis in children, adolescents, and adults. Spine J. 2013;13:1108-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Fenoy AJ, Greenlee JD, Menezes AH, Donovan KA, Sato Y, Hitchon PW, Chaloupka JC. Primary bone tumors of the spine in children. J Neurosurg. 2006;105:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Jiang L, Liu ZJ, Liu XG, Zhong WQ, Ma QJ, Wei F, Dang GT, Yuan HS. Langerhans cell histiocytosis of the cervical spine: a single Chinese institution experience with thirty cases. Spine (Phila Pa 1976). 2010;35:E8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Schmidt S, Eich G, Geoffray A, Hanquinet S, Waibel P, Wolf R, Letovanec I, Alamo-Maestre L, Gudinchet F. Extraosseous langerhans cell histiocytosis in children. Radiographics. 2008;28:707-26; quiz 910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Histiocytosis syndromes in children. Writing Group of the Histiocyte Society. Lancet. 1987;1:208-209. [PubMed] |
| 11. | Prasad GL, Divya S. Eosinophilic Granuloma of the Cervical Spine in Adults: A Review. World Neurosurg. 2019;125:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Feng F, Tang H, Chen H, Jia P, Bao L, Li JJ. Percutaneous vertebroplasty for Langerhans cell histiocytosis of the lumbar spine in an adult: Case report and review of the literature. Exp Ther Med. 2013;5:128-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Duda-Szymańska J, Wierzchniewska-Ławska A. Langerhans cell histiocytosis in a 3-year-old girl: a case report and literature review. Pol J Pathol. 2009;60:134-137. [PubMed] |
| 14. | Grois N, Tsunematsu Y, Barkovich AJ, Favara BE. Central nervous system disease in Langerhans cell histiocytosis. Br J Cancer Suppl. 1994;23:S24-S28. [PubMed] |
| 15. | Iyeyasu JN, Vaz ACM, Reis F, Altemani J, Queiroz LS, Carvalho KM. Langerhans cell histiocytosis diagnosed in an elderly patient. Radiol Bras. 2012;45:241-243. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Denaro L, Longo UG, Papalia R, Di Martino A, Maffulli N, Denaro V. Eosinophilic granuloma of the pediatric cervical spine. Spine (Phila Pa 1976). 2008;33:E936-E941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Zhong N, Xu W, Meng T, Yang X, Yan W, Xiao J. The surgical strategy for eosinophilic granuloma of the pediatric cervical spine complicated with neurologic deficit and/or spinal instability. World J Surg Oncol. 2016;14:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: Diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol. 2018;78:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Gu YF, Tian QH, Li YD, Wu CG, Song HM, He CJ. Percutaneous vertebroplasty in the treatment of malignant vertebral compression fractures with epidural involvement. J Interv Med. 2018;1:240-246. [PubMed] |
| 20. | Bertram C, Madert J, Eggers C. Eosinophilic granuloma of the cervical spine. Spine (Phila Pa 1976). 2002;27:1408-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Rimondi E, Mavrogenis AF, Rossi G, Ussia G, Angelini A, Ruggieri P. CT-guided corticosteroid injection for solitary eosinophilic granuloma of the spine. Skeletal Radiol. 2011;40:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Yan J, Zhou S, Li Y. Benign orbital tumors with bone destruction in children. PLoS One. 2012;7:e32111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Yang SY, Boniello AJ, Poorman CE, Chang AL, Wang S, Passias PG. A review of the diagnosis and treatment of atlantoaxial dislocations. Global Spine J. 2014;4:197-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 24. | Kumar KK, Parikh B, Jabarkheel R, Dirlikov B, Singh H. Fluoroscopic versus CT-guided cortical bone trajectory pedicle screw fixation: Comparing trajectory related complications. J Clin Neurosci. 2021;89:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Chen ZD, Wu J, Lu CW, Zeng WR, Huang ZZ, Lin B. C1-C2 Pedicle Screw Fixation for Pediatric Atlantoaxial Dislocation: Initial Results and Long-term Follow-up. J Pediatr Orthop. 2020;40:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Zhang YH, Shao J, Chou D, Wu JF, Song J, Zhang J. C1-C2 Pedicle Screw Fixation for Atlantoaxial Dislocation in Pediatric Patients Younger than 5 Years: A Case Series of 15 Patients. World Neurosurg. 2017;108:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |