Published online Jul 16, 2023. doi: 10.12998/wjcc.v11.i20.4897
Peer-review started: February 28, 2023
First decision: March 28, 2023
Revised: April 11, 2023
Accepted: May 15, 2023
Article in press: May 15, 2023
Published online: July 16, 2023
Processing time: 133 Days and 15.1 Hours
Staphylococcus caprae (S. caprae) is a human commensal bacterium which can be detected in the nose, nails, and skin. It can be responsible for heterogeneous infections such as bacteremia, endocarditis, pneumonia, acute otitis externa, peritonitis, and urinary tract infections. Bone and joint infections due to S. caprae have also been reported, but most of them resulted from the infection of orthopedic devices, especially joint prostheses and internal osteosynthesis devices. Rare cases of primary osteoarticular infections caused by S. caprae have been described, including osteitis, arthritis, or spondylodiscitis.
We report an unusual case of subacute osteomyelitis in a toe phalanx caused by S. caprae in a 14.5-year-old girl.
Subacute S. caprae osteomyelitis is a little-known and probably underestimated community-acquired infectious disease. This microorganism’s pathogenicity should be seen as more than a classic nosocomial orthopedic device infection.
Core Tip:Staphylococcus caprae (S. caprae) is a human commensal bacterium which can be detected in the nose, nails, and skin. It may be responsible for heterogenous infections such as bacteremia, endocarditis, pneumonia, acute otitis externa, peritonitis, and urinary tract infections. Bone and joint infections due to S. caprae have also been reported but most of them resulted from orthopedic device infections, including above all joint prosthesis infections and internal osteosynthesis device infections. Only any rare cases of primary osteoarticular infections caused by S. caprae have been described and consisted in osteitis, arthritis, or spondylodiscitis. We report here the case of an unusual subacute osteomyelitis of a toe phalanx caused by S. caprae in a 14.5-year-old girl.
- Citation: Vazquez O, De Marco G, Gavira N, Habre C, Bartucz M, Steiger CN, Dayer R, Ceroni D. Subacute osteomyelitis due to Staphylococcus caprae in a teenager: A case report and review of the literature. World J Clin Cases 2023; 11(20): 4897-4902
- URL: https://www.wjgnet.com/2307-8960/full/v11/i20/4897.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i20.4897
Coagulase-negative staphylococci (CoNS) are ubiquitous human and animal commensals and form an integral part of healthy human skin microbiota. CoNS are frequently discovered in clinical samples and often considered contaminants because they can become opportunistic pathogens in certain situations. CoNS associated with nosocomial infection are typically characterized by their pronounced antimicrobial resistance, including methicillin-resistant and multidrug-resistant isolates. However, they do not have as much pathogenic potential as coagulase-positive staphylococci such as Staphylococcus caprae (S. caprae).
S. caprae is a commensal coagulase-negative Staphylococcus known to colonize the skin and mammary glands of goats[1], occasionally causing mastitis[2]. In humans, commensal S. caprae can be detected in the nose, nails, and skin[3,4], and it can be the initial cause of heterogeneous infections such as bacteremia[4-13], endocarditis[6], pneumonia[7], acute otitis externa[3,5], peritonitis[14], and urinary tract infections[6,15]. Bone and joint infections due to S. caprae have been reported, but, fortunately, they remain rare[3,8,16-27]. Most osteoarticular S. caprae infections are the result of infected orthopedic devices, especially infected joint prostheses[3,8,17,18,21-27] and internal osteosynthesis devices[17,19,27]. Only very rare cases of primary osteoarticular S. caprae infections have been described, including osteitis[3,8], arthritis[16], or spondylodiscitis[27,28]. It is commonly accepted that the prevalence of human S. caprae infections is underestimated since conventional phenotypic identification systems incorrectly identify many S. caprae strains[3,27,28]. Molecular techniques have improved their identification[9,14,17,20,29,30], becoming essential when standard cultures give negative results[25]. We report a rare and unusual case of subacute osteomyelitis caused by S. caprae in a toe phalanx of a 14.5-year-old girl.
A 14.5-year-old girl was referred to our department by her pediatrician due to persistent pain in her fourth right toe for over 3 mo.
Persistent pain in the fourth right toe for over 3 mo.
There was no past illness for this patient.
There was a history of trivial trauma but no suggestion of broken skin or fever.
At admission, the patient was apyrexial; on examination, the toe showed mild swelling and erythema, and palpation caused discomfort. The girl could freely bear weight on her foot with no limitations.
The patient’s white blood cell count was 7600 cells/mm3, C-reactive protein was less than 0.3 mg/L, and her erythrocyte sedimentation rate was 7 mm/h.
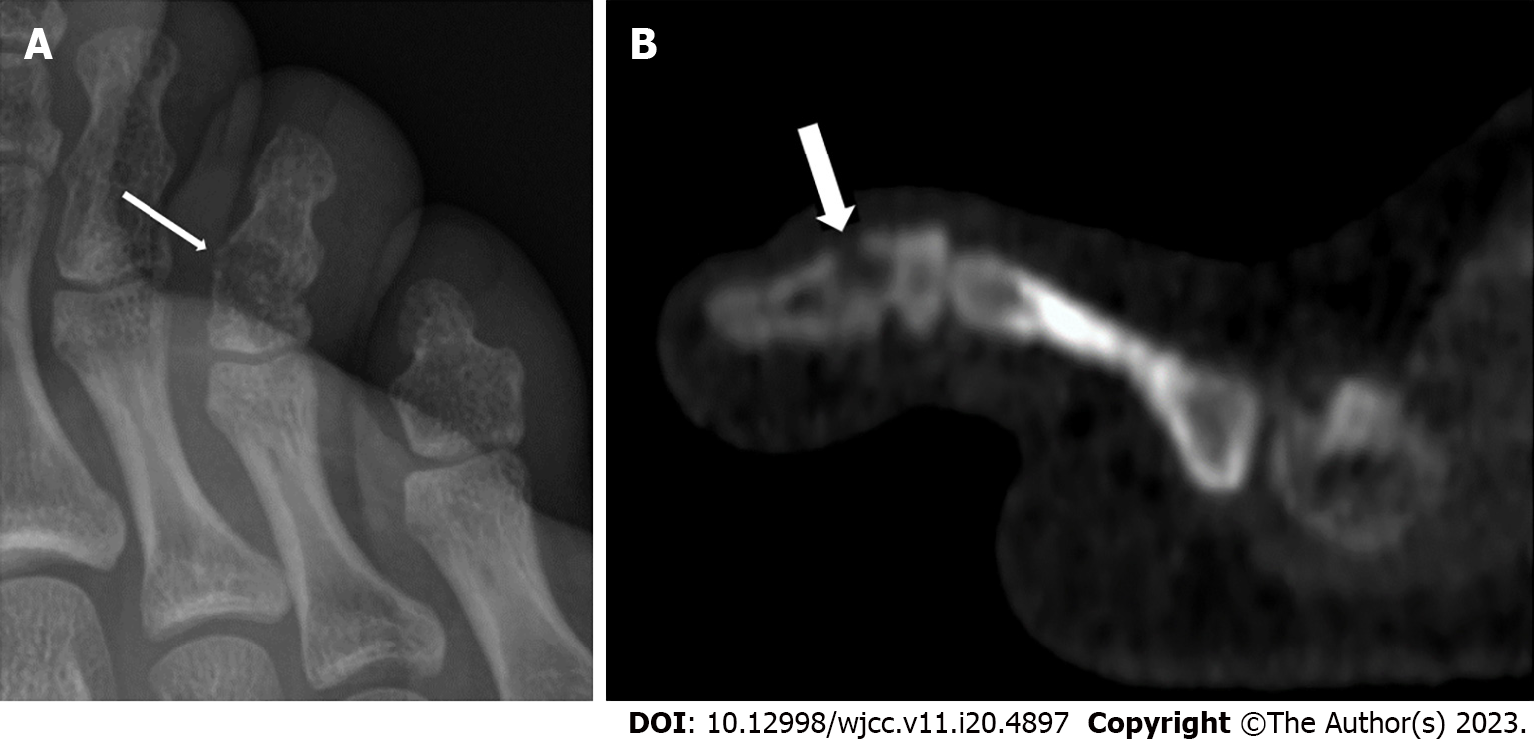
An initial plain radiograph revealed a constitutional fusion of the toe’s distal interphalangeal joint but without any relevant pathology.
Toe phalanx lytic lesion.
The patient was administered oral antibiotics (co-amoxicillin), and this treatment continued for 15 d, although a full resolution of symptoms was achieved after only a few days of treatment, and inflammatory markers remained normal. Three months after surgery, the toe phalanx lytic lesion was completely resolved.
After 2 mo, a new plain radiograph revealed a subtle, ill-defined lytic lesion of the toe phalanx (Figure 1A). A complementary computed tomography (CT) scan of the foot confirmed a lytic lesion with cortical erosion of the metaphysis of the fused toe phalanx (Figure 1B). We performed a direct open biopsy of the toe lytic lesion, involving the debridement and curettage of the pathologic tissue, which resulted in a limited bone defect (3-4 mm). Microbiological cultures of the material removed were made on a solid medium, and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry was used to identify the bacteria, revealing S. caprae.
S. caprae is a commensal coagulase-negative Staphylococcus that may become a human pathogen in community-acquired or nosocomial infections. Most osteoarticular S. caprae infections result from infected orthopedic devices, especially joint prostheses[3,8,17,18,21-27] and internal osteosynthesis devices[17,19,27]. Genome analysis has demonstrated that S. caprae is closely related to S. epidermidis and S. capitis at the species level, especially in its ability to form biofilms, which may explain the virulence of S. caprae infections[31]. The formation of a biofilm is considered an essential step in the pathogenesis of CoNS. Another important step in the induction of an infection is the adhesion of bacterial cells to host tissues and their ability to grow into a biofilm[32]. The genetic determinants of biofilm formation include the icaADBC operon, which codes for the biosynthetic enzymes involved in producing polysaccharide intercellular adhesin[33,34]. S. caprae expresses the ica operon providing the pathogen with the ability to form a biofilm on orthopedic osteosynthesis devices, thus conferring the bacterium with resistance to the immune system and antibiotics[17,35].
Despite this, S. caprae has never been reported to cause subacute osteomyelitis. The present case is thus the first to show that S. caprae can be responsible for subacute osteomyelitis even when no orthopedic device is present. Subacute osteomyelitis is an osseous infection with a duration of more than 3 wk without acute symptoms. Subacute osteomyelitis may result from the inadequate treatment of acute osteomyelitis or may occur in settings displaying strong host resistance to infection, an illness due to less virulent organisms, prior exposure to antibiotics, or a combination of all these factors[36,37]. We hypothesize that S. caprae was one of the few virulent pathogens that could have become an opportunistic pathogen in this case, but the subject managed to maintain it relatively well-controlled. Indeed, S. caprae is recognized as being less virulent than S. aureus and other CoNS[27]. Nevertheless, S. caprae has the bacterial characteristics required for the development of subacute osteomyelitis.
Subacute S. caprae osteomyelitis is a little-known and probably underestimated community-acquired infectious disease. This microorganism’s pathogenicity should be seen as more than a classic nosocomial orthopedic device infection. S. caprae is closely related to S. epidermidis and S. capitis at the species level, especially in its ability to form biofilms, which may explain the virulence of these pathogens. The difficulty in detecting S. caprae is attributable to the fact that conventional phenotypic identification systems still misidentify it. S. caprae should therefore be included in the list of organisms that can cause subacute osteomyelitis, such as S. aureus, Kingella kingae, Salmonella and Streptococcus species, and Mycobacterium tuberculosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Switzerland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lei P, China; Schwan WR, United States S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Yu HG
| 1. | Devriese LA, Poutrel B, Kilpper-BäLz R, Schleifer KH. Staphylococcus gallinarum and Staphylococcus caprae, Two New Species from Animals. Int J Syst Evol Microbiol 1983; 33: 480-486. [RCA] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Deinhofer M, Pernthaner A. Staphylococcus spp. as mastitis-related pathogens in goat milk. Vet Microbiol. 1995;43:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Shuttleworth R, Behme RJ, McNabb A, Colby WD. Human isolates of Staphylococcus caprae: association with bone and joint infections. J Clin Microbiol. 1997;35:2537-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Ross TL, Fuss EP, Harrington SM, Cai M, Perl TM, Merz WG. Methicillin-resistant Staphylococcus caprae in a neonatal intensive care unit. J Clin Microbiol. 2005;43:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Roland PS, Stroman DW. Microbiology of acute otitis externa. Laryngoscope. 2002;112:1166-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Vandenesch F, Eykyn SJ, Bes M, Meugnier H, Fleurette J, Etienne J. Identification and ribotypes of Staphylococcus caprae isolates isolated as human pathogens and from goat milk. J Clin Microbiol. 1995;33:888-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Barelli C, Minto EC, Martinez R, Darini AL. Evaluation of the antimicrobial susceptibilities of coagulase-negative staphylococci by E-test. Rev Latinoam Microbiol. 1999;41:67-72. [PubMed] |
| 8. | Darrieutort-Laffite C, André V, Leautez S, Tanguy G, Cormier G. [Staphylococcus caprae arthritis]. Med Mal Infect. 2013;43:131-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Fujita S, Senda Y, Iwagami T, Hashimoto T. Rapid identification of staphylococcal strains from positive-testing blood culture bottles by internal transcribed spacer PCR followed by microchip gel electrophoresis. J Clin Microbiol. 2005;43:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Spellerberg B, Steidel K, Lütticken R, Haase G. Isolation of Staphylococcus caprae from blood cultures of a neonate with congenital heart disease. Eur J Clin Microbiol Infect Dis. 1998;17:61-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Kato J, Mori T, Sugita K, Murata M, Ono Y, Yamane A, Shimizu T, Okamoto S. Central line-associated bacteremia caused by drug-resistant Staphylococcus caprae after chemotherapy for acute myelogenous leukemia. Int J Hematol. 2010;91:912-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Abdul Rahman Z, Hamzah SH, Hassan SA, Osman S, Md Noor SS. The significance of coagulase-negative staphylococci bacteremia in a low resource setting. J Infect Dev Ctries. 2013;7:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Kini GD, Parris AR, Tang JS. A Rare Presentation of Sepsis from Staphylococcus caprae. Open Microbiol J. 2009;3:67-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Shin JH, Kim SH, Jeong HS, Oh SH, Kim HR, Lee JN, Yoon YC, Kim YW, Kim YH. Identification of coagulase-negative staphylococci isolated from continuous ambulatory peritoneal dialysis fluid using 16S ribosomal RNA, tuf, and SodA gene sequencing. Perit Dial Int. 2011;31:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Kanda K, Suzuki E, Hiramatsu K, Oguri T, Miura H, Ezaki T, Yokota T. Identification of a methicillin-resistant strain of Staphylococcus caprae from a human clinical specimen. Antimicrob Agents Chemother. 1991;35:174-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Elsner HA, Dahmen GP, Laufs R, Mack D. Intra-articular empyema due to Staphylococcus caprae following arthroscopic cruciate ligament repair. J Infect. 1998;37:66-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Allignet J, Galdbart JO, Morvan A, Dyke KGH, Vaudaux P, Aubert S, Desplaces N, Solh NE. Tracking adhesion factors in Staphylococcus caprae strains responsible for human bone infections following implantation of orthopaedic material. Microbiology (Reading). 1999;145 (Pt 8):2033-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Blanc V, Picaud J, Legros E, Bes M, Etienne J, Moatti D, Raynaud MF. [Infection after total hip replacement by Staphylococcus caprae. Case report and review of the literature]. Pathol Biol (Paris). 1999;47:409-413. [PubMed] |
| 19. | Lang S, Livesley MA, Lambert PA, Elliott J, Elliott TS. The genomic diversity of coagulase-negative staphylococci associated with nosocomial infections. J Hosp Infect. 1999;43:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Sivadon V, Rottman M, Chaverot S, Quincampoix JC, Avettand V, de Mazancourt P, Bernard L, Trieu-Cuot P, Féron JM, Lortat-Jacob A, Piriou P, Judet T, Gaillard JL. Use of genotypic identification by sodA sequencing in a prospective study to examine the distribution of coagulase-negative Staphylococcus species among strains recovered during septic orthopedic surgery and evaluate their significance. J Clin Microbiol. 2005;43:2952-2954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Arciola CR, Campoccia D, An YH, Baldassarri L, Pirini V, Donati ME, Pegreffi F, Montanaro L. Prevalence and antibiotic resistance of 15 minor staphylococcal species colonizing orthopedic implants. Int J Artif Organs. 2006;29:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Achermann Y, Vogt M, Spormann C, Kolling C, Remschmidt C, Wüst J, Simmen B, Trampuz A. Characteristics and outcome of 27 elbow periprosthetic joint infections: results from a 14-year cohort study of 358 elbow prostheses. Clin Microbiol Infect. 2011;17:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Roux AL, Sivadon-Tardy V, Bauer T, Lortat-Jacob A, Herrmann JL, Gaillard JL, Rottman M. Diagnosis of prosthetic joint infection by beadmill processing of a periprosthetic specimen. Clin Microbiol Infect. 2011;17:447-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Bajwa AS, Villar RN. Arthroscopy of the hip in patients following joint replacement. J Bone Joint Surg Br. 2011;93:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Xu Y, Rudkjøbing VB, Simonsen O, Pedersen C, Lorenzen J, Schønheyder HC, Nielsen PH, Thomsen TR. Bacterial diversity in suspected prosthetic joint infections: an exploratory study using 16S rRNA gene analysis. FEMS Immunol Med Microbiol. 2012;65:291-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Campoccia D, Baldassarri L, An YH, Kang QK, Pirini V, Gamberini S, Pegreffi F, Montanaro L, Arciola CR. Automated ribotyping to distinguish the different non Sau/ non Sep staphylococcal emerging pathogens in orthopedic implant infections. Int J Artif Organs. 2006;29:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Seng P, Barbe M, Pinelli PO, Gouriet F, Drancourt M, Minebois A, Cellier N, Lechiche C, Asencio G, Lavigne JP, Sotto A, Stein A. Staphylococcus caprae bone and joint infections: a re-emerging infection? Clin Microbiol Infect. 2014;20:O1052-O1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Gowda A, Pensiero AL, Packer CD. Staphylococcus caprae: A Skin Commensal with Pathogenic Potential. Cureus. 2018;10:e3485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 29. | Drancourt M, Raoult D. rpoB gene sequence-based identification of Staphylococcus species. J Clin Microbiol. 2002;40:1333-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 215] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Hirotaki S, Sasaki T, Kuwahara-Arai K, Hiramatsu K. Rapid and accurate identification of human-associated staphylococci by use of multiplex PCR. J Clin Microbiol. 2011;49:3627-3631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Watanabe S, Aiba Y, Tan XE, Li FY, Boonsiri T, Thitiananpakorn K, Cui B, Sato'o Y, Kiga K, Sasahara T, Cui L. Complete genome sequencing of three human clinical isolates of Staphylococcus caprae reveals virulence factors similar to those of S. epidermidis and S. capitis. BMC Genomics. 2018;19:810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Hashem YA, Amin HM, Essam TM, Yassin AS, Aziz RK. Biofilm formation in enterococci: genotype-phenotype correlations and inhibition by vancomycin. Sci Rep. 2017;7:5733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Fluckiger U, Ulrich M, Steinhuber A, Döring G, Mack D, Landmann R, Goerke C, Wolz C. Biofilm formation, icaADBC transcription, and polysaccharide intercellular adhesin synthesis by staphylococci in a device-related infection model. Infect Immun. 2005;73:1811-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Stevens NT, Tharmabala M, Dillane T, Greene CM, O'Gara JP, Humphreys H. Biofilm and the role of the ica operon and aap in Staphylococcus epidermidis isolates causing neurosurgical meningitis. Clin Microbiol Infect. 2008;14:719-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Allignet J, Aubert S, Dyke KG, El Solh N. Staphylococcus caprae strains carry determinants known to be involved in pathogenicity: a gene encoding an autolysin-binding fibronectin and the ica operon involved in biofilm formation. Infect Immun. 2001;69:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Hamdy RC, Lawton L, Carey T, Wiley J, Marton D. Subacute hematogenous osteomyelitis: are biopsy and surgery always indicated? J Pediatr Orthop. 1996;16:220-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Spyropoulou V, Dhouib Chargui A, Merlini L, Samara E, Valaikaite R, Kampouroglou G, Ceroni D. Primary subacute hematogenous osteomyelitis in children: a clearer bacteriological etiology. J Child Orthop. 2016;10:241-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |