Published online Jan 16, 2023. doi: 10.12998/wjcc.v11.i2.322
Peer-review started: September 19, 2022
First decision: November 14, 2022
Revised: November 28, 2022
Accepted: January 5, 2023
Article in press: January 5, 2023
Published online: January 16, 2023
Processing time: 114 Days and 17.3 Hours
The review focuses on the most important areas of cell therapy for spinal cord injuries. Olfactory mucosa cells are promising for transplantation. Obtaining these cells is safe for patients. The use of olfactory mucosa cells is effective in restoring motor function due to the remyelination and regeneration of axons after spinal cord injuries. These cells express neurotrophic factors that play an important role in the functional recovery of nerve tissue after spinal cord injuries. In addition, it is possible to increase the content of neurotrophic factors, at the site of injury, exogenously by the direct injection of neurotrophic factors or their delivery using gene therapy. The advantages of olfactory mucosa cells, in combination with neurotrophic factors, open up wide possibilities for their application in three-dimensional and four-dimensional bioprinting technology treating spinal cord injuries.
Core Tip: The development of an optimal strategy for the treatment of spinal cord injuries is a relevant and topical issue in modern medicine. Olfactory mucosa cells and neurotrophic factors showed their effectiveness in transplantation into the area of the injured spinal cord. In this review, the authors discuss the possibility of their application in four-dimensional bioprinting to create transplants that would have a complex impact on the transplant-mediated repair of the damaged area of the spinal cord.
- Citation: Stepanova OV, Fursa GA, Andretsova SS, Shishkina VS, Voronova AD, Chadin AV, Karsuntseva EK, Reshetov IV, Chekhonin VP. Prospects for the use of olfactory mucosa cells in bioprinting for the treatment of spinal cord injuries. World J Clin Cases 2023; 11(2): 322-331
- URL: https://www.wjgnet.com/2307-8960/full/v11/i2/322.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i2.322
Therapy for spinal cord injury is a relevant issue in modern neurobiology and medicine because the mechanical injury of the spinal cord can lead to irreversible changes in the neural tissue. Spinal cord injuries often lead to disabilities and sometimes can have a lethal outcome[1].
One of the current strategies for the treatment of spinal cord injury is cell therapy. The most optimal source of cells for transplantation may be olfactory mucosa cells. Obtaining olfactory cells is an atraumatic procedure for a patient, which makes the application of this tissue in therapy a promising field of study in personalised medicine. For cell transplantation of the olfactory mucosa cells, neural stem/progenitor cells (NSPCs), olfactory ensheathing cells (OECs), and mesenchymal stem cells (MSCs) are obtained. It was shown that the transplantation of these cells contributed to the reparation of the injured tissue of the spinal cord[2]. Besides, spinal cord therapy involves the application of various neurotrophic factors. Neurotrophins exert an antiapoptotic effect and contribute to the survival of mature neural cells, which is especially important at the site of injury[3]. The application of neurotro
| BDNF | NT-3 | NGF | |
| NSPCs | Improved hindlimb function[75] | Promoted differentiation into neurons[76]; promoted axon regeneration and functional recovery[77] | Reduced oligodendrocyte loss, improved functional recovery, preservation of motor neurons, attenuation of astrocytosis[78] |
| OECs | Improved cell survival[79]; promotes cell migration[80] | Improved cell survival[79]; improved limb mobility and; improvement of growth promoting properties[81] | Improved cell; survival[79] |
| MSCs | Enhanced recovery of motor function, reduced damage of spinal neurons[82] Improved hindlimb function[83] | Improvement of motor function[84], structural and functional recovery of SCI[85,86] and axonal regeneration[86] | Improvement of proliferation, differentiation, immunomodulatory[87]; promoted the growth of blood vessels[88]; increase of expression of genes related to neural differentiated cells[89] |
In the last few years, experimental medicine research included the application of various variants of stable polymers that can deliver cells and neurotrophic factors as a three-dimensional (3D) scaffold[4]. This method has shown its safety in various clinical studies[5,6]. The 3D scaffold provides support for the transplanted cells in more native conditions, which contributes to their survival in the area of injury[7,8]. However, such 3D scaffolds have low adaptivity to the changes in the area of injury and limited changes in shape. The solution to this problem is the development of conceptually novel “smart” materials. The application of such materials will allow specialists to create four-dimensional (4D) scaffolds that will not only combine the advantages of 3D scaffolds, but can also adapt to changes in the area of injury, and respond to external signals[9].
Thus, the creation of structures that have all the advantages of 4D bioprinting and can deliver olfactory mucosa cells and neurotrophic factors will be a breakthrough in the treatment of spinal cord injuries.
The olfactory mucosa of the nose contains several cell types that can be successfully used in cell therapy for spinal cord injuries: NSPCs, ECs, and MSCs.
Despite the fact that cell-therapy based on olfactory mucosa cells is one of the most promising treatments for spinal cord injuries there are some limitations to this approach. Transplantation of OECs from olfactory mucosa significantly improves motor function recovery and reduces the size of cysts[10,11]. However, there is a problem with OEC culture heterogeneity because it is currently difficult to purify these cells to a 100% pure culture, so there might be some side effects[12-14]. Mesenchymal stromal cells can also be obtained from olfactory mucosa. As it was mentioned in the manuscript, studies show that mesenchymal stromal cells transplantation has side effects such as neuropathic pain[15]. The use of neurospheres containing NSPCs from olfactory mucosa is associated with difficulties in calculating the number of cells and the percentage of NSPCs in suspension.
Safety of cell-based treatments is a fundamental concern for regenerative medicine. Efficacy is usually the main focus, however, the safety of each treatment is also tested during the trials[16]. Clinical observations show that OEC transplantation is safe for patients and there were no serious adverse reactions in all cases[17]. Clinical trials of NSPCs and MSCs from the olfactory mucosa have not been performed to date.
NSPCs were discovered in the spinal cord and brain (in the subventricular area and the hippocampus), as well as the olfactory mucosa layer. These cells can differentiate into glial cells and mature neurons and encode neurotrophic factors for successful neuroregeneration[18-20].
The treatment of modelled spinal cord injuries includes suspension cell cultures in the form of neurospheres containing NSPCs. Studies have shown the effectiveness of neurospheres, from the olfactory mucosa, in the treatment of spinal cord injuries. Their transplantation in the acute phase of rat spinal cord injury contributes to the restoration of motor activity of the hind limbs and regeneration of axons of the rubrospinal tract[21,22].
Chronic injury of the spinal cord can be associated with pathological processes that lead to the formation of a posttraumatic cyst. The cyst can enlarge and compress the spinal cord, which complicates the processes of regeneration and mediation of nervous impulses to the limbs[23]. However, the application of these neurospheres in the chronic phase of spinal cord injury remains understudied. In the authors’ laboratory, the first transplantation of neurospheres from the olfactory mucosa was performed in rats with modelled posttraumatic cysts. It was shown that transplantation contributed to the improvement of motor activity of the hind limbs and the reduction of the cyst size[24]. The proven effectiveness of neurospheres, obtained from olfactory mucosa in the experimental studies, can provide grounds for preclinical studies and further application in the treatment of spinal cord injuries in patients.
OECs obtained from the olfactory mucosa are a unique type of glial cell found in the peripheral nervous system. In an adult organism, these cells manage the growth of new axons in the olfactory epithelium and protect a growing axon from growth-inhibiting factors, which allows an axon to grow to the size of an olfactory bulb and form a synapsis[25]. Besides, in vitro and in vivo studies have shown that OECs of an adult organism secreted molecules associated with myelinization: Protein zero (P0) and myelin basic protein[26]. It is suggested that this type of cells can maintain the growth and integrity of axons throughout an organism’s life and contribute to the formation of the myelin sheath around demyelinated axons[27,28]. OECs can also express neurotrophic factors that promote neuroregeneration[29].
It has been shown that transplantation of OECs in the acute phase of spinal cord injury contributed to the regeneration of the nervous tissue and remyelination of axons, which leads to the restoration of limb motor activity. Furthermore, introducing these cells in the acute phase contributes to a reduction of posttraumatic astrogliosis[30]. Transplantation of OECs in the chronic phase promotes the recovery and growth of the damaged axons and improves limb motor activity[31]. In the authors’ laboratory, for the first time, the therapeutic effect of OECs was shown in the treatment of posttraumatic spinal cord cysts. The transplantation of OECs contributed to a decrease in the volume of cysts and restoration of limb motor activity[32,33].
Preclinical studies have demonstrated the safety and efficacy of the use of olfactory mucosa cells in the treatment of acute and subacute stages of spinal cord injury[34-36]. The conducted clinical studies also demonstrated the safe use of autologous OECs in the treatment of patients with spinal cord injuries[37-39]. Thus, OECs from the olfactory mucosa can be considered the optimal cell material for personalised cell therapy in such patients.
Experimental studies have shown that the transplantation of MSCs, derived from olfactory mucosa, contributed to the growth of axons in the injured rat nerves. A positive effect of these cells on the myelinization of axons was also evident in vitro[40]. However, MSCs derived from olfactory mucosa remain understudied in in vivo conditions.
MSCs obtained from bone marrow were also used in the treatment of spinal cord injuries. Even though the transplantation of these cells showed positive results in experimental models, clinical studies did not prove their effectiveness[41]. Besides, some studies describe the side effects of MSC transplantation, including neuropathic pain[15]. The application of MSCs obtained from olfactory mucosa can have the same consequences, which makes the other two types of olfactory mucosa cells (OECs and NSPCs) the most promising for cell therapy.
Another promising method for the treatment of spinal cord injuries is exogenous neurotrophin therapy since the endogenous neurotrophic factor count is insufficient for the regeneration of the injured tissue[42]. Neurotrophic factors, including nerve growth factor (NGF), neurotrophin-3 (NT-3), NT-4, and brain-derived neurotrophic factor (BDNF), play an important role in the processes of neuroregeneration and axon growth after injury[43].
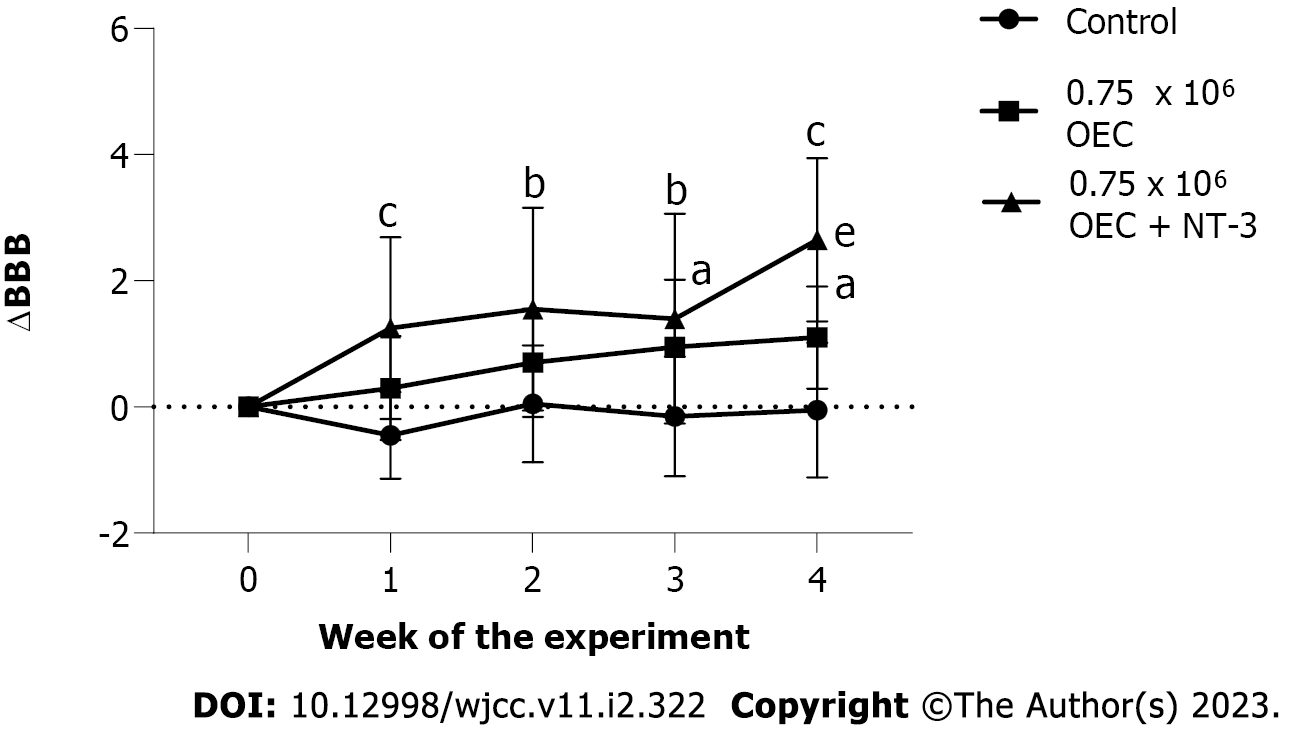
One of the methods for delivering exogenous neurotrophins to the area of injury is injection. Studies conducted at the end of the last century have shown that the delivery of NGF for two weeks after resection of the spinal cord fragment in rats promoted the regeneration of the nervous tissue of the spinal cord[44]. Later, it was shown that the delivery of BDNF in the acute and chronic phases of rat spinal cord injury also contributed to nervous tissue regeneration[45,46]. Further in vitro studies showed that BDNF expressed by the OECs contributed to the transplant-mediated axon growth[47]. For the first time, the authors of this paper have demonstrated that the combined introduction of OECs and exogenous NT-3 to the modelled cysts in the rat spinal cord improved the motor activity of the hind limbs and reduced the cyst size[32]. The use of NT-3 improves the effects obtained from cell therapy and the data are presented in Figure 1[48].
However, after neurotrophins are directly administered to the area of injury, they degrade quickly. Currently, the most promising method of neurotrophin delivery to the area of injury is the use of adenoviral vectors[49]. There are direct and indirect deliveries of adenoviral vectors. Direct delivery involves the injection of the vector to the area of injury or nearby, resulting in the transduction of neurons, astrocytes, oligodendrocytes, macrophages, lymphocytes, and microglia. With indirect delivery, an adenoviral vector is injected intramuscular or into the peripheral nervous system.
Gene therapy ex vivo is a variant of indirect gene delivery. This therapy includes obtaining cells, genetic modification of these cells in vitro, and transplantation of a gene-cell construct into the patient[50]. Experimental studies investigated gene-cell constructs based on Schwann cells[51], MSCs[52,53], NSPCs[54,55], and OECs obtained from olfactory bulb[56]. In the authors’ laboratory, an OEC-based gene-cell construct from the olfactory mucosa was studied. It was shown that transplantation of OECs obtained from olfactory mucosa, transduced by an adenoviral vector encoding a mature form of BDNF, into a post-traumatic cyst could have a therapeutic effect. This is observed not only due to increased secretion of neurotrophin but also due to the regenerative potential of the cells themselves[32]. Other authors showed the effectiveness of OECs obtained from olfactory mucosa transduced with adenoviral vector transcoding NT-3. This construct intensely encoded NT-3, which contributed to the growth of injured axons[57].
Thus, gene-cell constructs are being actively studied, however, it is necessary to continue the investigation of various combinations of cells and adenoviral vectors to create an optimal drug for gene-cell therapy. Special attention should be paid to the safety of this technology. Adenoviral vectors are the most studied in this respect. They are successfully used in the creation of vaccines and treatment of oncological diseases. At present, they are being actively studied for use in regenerative medicine. Although great success has been achieved in this field, it is necessary to improve the immune system response, the life span of the virus, and the packing ability of the vectors. However, the evolution of the adenoviral vector as a tool for the transfer of genetic material has revolutionized how doctors and scientists can approach the treatment of even the most debilitating diseases[58].
Another promising method for delivering cells and neurotrophins to the area of injury is 3D bioprinting. The development of tissue-specific scaffolds that imitate the natural architecture of the studied tissue is the most important task of modern medicine. To create such structures, the field of 3D bioprinting is being actively explored. Scaffolds are printed layer-by-layer, using 3D printers, and each of the layers can be filled with cells and neurotrophic factors[9]. This technology has been actively studied for some years and has already been applied in the treatment of a wide range of pathologies, including spinal cord injuries[59].
The effect of 3D polymers on the restoration of nervous tissue is currently being studied in experimental models. For example, the transplantation of a printed 3D scaffold from biodegradable polyurethane, which contains mature neural stem cells (NSCs) of a mouse, positively affected the regeneration of the central nervous system of Danio-rerio embryos. Transplantation of this construct to adults with brain injury increased their survival rate and had a positive effect on the restoration of motor activity [60]. In addition, there are studies available on the application of printed agarose tubes in combination with mouse bone marrow stem cells after sciatic nerve injury in rats. It was shown that the application of this transplant was more effective in the restoration of motor and sensory activity than an injection of collagen or transplantation of nervous tissue into the area of injury. These studies showed that a solid scaffold increased cell survival rates and positively affected the regeneration of the damaged areas of nervous tissue[61]. The study of the application of a 3D printed polyethylene glycol gelatin methacrylate scaffold with rat neural progenitor cells (NPCs) also showed that transplantation of a scaffold with cells led to a better recovery of hind limbs motor activity in injured rats compared to groups that received cells without a scaffold or a scaffold without cells[62]. To improve the performance of 3D matrices, a combination of germanium phosphide and hyaluronic acid was developed. This construct significantly improved the recovery of motor activity of the hind limbs in rats with modelled spinal cord injury and also increased the migration of rat NPCs in the spinal cord to the area of injection[63].
A promising direction is the study of 3D printing based on OECs obtained from olfactory mucosa, since, as noted earlier, the production and application of these cells do not pose any danger to the patient. 3D microbeads were obtained from OECs and Matrigel. OECs survived the printing process and retained their phenotype. It is assumed that such a 3D construct can have a therapeutic effect when transplanted into the spinal cord of animals and promote the growth of axons along the scaffold[64].
Furthermore, there was a study made on the printed multichannel poly (propylene- fumarate)-collagen scaffold containing neurotrophin NT-3. Implantation of this construct into the spinal cord of an injured rat had a significant neurotrophic effect and contributed to the growth and regeneration of the damaged axons. The porous structure of the scaffold facilitated the migration of endogenous NSCs in rats and promoted neuronal regeneration[65].
However, materials used for 3D printing can rarely respond to the changes in the injured area or adapt to the changing shape, which is a limitation of such therapies. To remove these limitations, 4D bioprinting is being explored.
The development of more complex and “smart” materials will allow researchers to create novel polymers that have distinctive features: they change shape, can self-assemble, are capable of autonomous activation, and can capture a wide range of signals[66]. This type of polymer is used in 4D printing. Currently, these technologies are widely used in bone and skin grafting[67-70]. However, further research into the possibilities of 4D bioprinting will allow the use of four-dimensional polymers in the treatment of spinal cord injuries as well.
At the moment, a matrix has been developed that combines the developments of 3D printing with the advantages of novel technologies. Hybrid gelatin methacrylate-microcapsule hydrogel, in combination with polylactide-clicolide capsules with NT-3, can secrete growth factors at a certain level for 20 d. This, in turn, contributed to the recovery of the spinal cord after injury, differentiation of NSCs located in the spinal cord, and improvement in motor activity of the hind limbs in rats with spinal cord injury[71].
In parallel, a polymer based on epoxydated acrylate of soybean oil in combination with graphene and human MSCs was developed based on laser stereolithography technology[72]. In the present study, cells in the 4D polymer were placed in the medium for neural differentiation. Two weeks later, MSCs on the polymer showed a significantly higher level of neurogenic genes in comparison with the same cells cultivated on a regular 3D polymer. In addition, such material could reversibly change the structure and shape of memory[72]. These results allowed the authors to study MSCs on NSCs in vitro. A later study, by the same authors, focused on the design of polymer microwells with a memory function[73]. This was achieved by combining laser stereolithography, a polydimethylsiloxane mould, and organic glass for the formation of wells 400-800 µm in diameter. This approach allowed the authors to create a micro surrounding of a cell that could change its shape and achieve more favourable conditions for cell cultivation. NSCs in polymer showed a significant increase in the expression of markers of neural and glial differentiation compared to cultures cultivated on plastic and glass surfaces[73]. Another research group used technology based on polymer melt modelling and obtained an effect that was similar to that described above, from the cultivation of NSCs in combination with a polymer from polyurethane, nanoparticles, and gelatin. The resulting polymer also had a memory effect, was capable of maintaining its shape, and was suitable for cryo conservation[74].
As already discussed in this review, olfactory mucosa cells have great potential in the treatment of spinal cord injuries and are optimal for personalised cell therapy. Four-dimensional bioprinting is a novel, actively developing direction in regenerative medicine. The application of olfactory mucosa cells and neurotrophins in the creation of 4D constructs will allow researchers to design unique transplants that will have a complex effect on damaged tissue. It is necessary to study 4D constructs based on both cells with the addition of a neutrophilic factor and transduced cells, which will intensively secrete various neurotrophic factors in the area of injury. Particular attention should be paid to constructs capable of adapting to changes in shape and size, which is especially important in the treatment of post-traumatic cysts.
The most promising vector for the development of spinal cord injury therapy is the development of smart 4D constructs. Such constructs can contain both neurotrophins and cells. The most optimal source of cells for 4D printing is olfactory mucosa due to its atraumatic production and proven therapeutic efficacy of the cells obtained from it. The study of 4D bioprinting based on olfactory mucosa cells and neurotrophic factors is necessary for developing an optimal strategy for the treatment of spinal cord injury.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Society of Regenerative Medicine (Russia), No. ID594.
Specialty type: Neurosciences
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oliva J, United States; Sun Z, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Yip PK, Malaspina A. Spinal cord trauma and the molecular point of no return. Mol Neurodegener. 2012;7:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Lindsay SL, Riddell JS, Barnett SC. Olfactory mucosa for transplant-mediated repair: a complex tissue for a complex injury? Glia. 2010;58:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Skaper SD. Neurotrophic Factors: An Overview. Methods Mol Biol. 2018;1727:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 4. | Ghane N, Beigi MH, Labbaf S, Nasr-Esfahani MH, Kiani A. Design of hydrogel-based scaffolds for the treatment of spinal cord injuries. J Mater Chem B. 2020;8:10712-10738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Deng WS, Ma K, Liang B, Liu XY, Xu HY, Zhang J, Shi HY, Sun HT, Chen XY, Zhang S. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen Res. 2020;15:1686-1700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Xiao Z, Tang F, Zhao Y, Han G, Yin N, Li X, Chen B, Han S, Jiang X, Yun C, Zhao C, Cheng S, Zhang S, Dai J. Significant Improvement of Acute Complete Spinal Cord Injury Patients Diagnosed by a Combined Criteria Implanted with NeuroRegen Scaffolds and Mesenchymal Stem Cells. Cell Transplant. 2018;27:907-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 7. | Gilmour AD, Reshamwala R, Wright AA, Ekberg JAK, St John JA. Optimizing Olfactory Ensheathing Cell Transplantation for Spinal Cord Injury Repair. J Neurotrauma. 2020;37:817-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Liu X, Hao M, Chen Z, Zhang T, Huang J, Dai J, Zhang Z. 3D bioprinted neural tissue constructs for spinal cord injury repair. Biomaterials. 2021;272:120771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 9. | Tamay DG, Dursun Usal T, Alagoz AS, Yucel D, Hasirci N, Hasirci V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front Bioeng Biotechnol. 2019;7:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 10. | Stepanova OV, Voronova АD, Chadin AV, Valikhov MP, Abakumov MA, Reshetov IV, Chekhonin VP. Isolation of Rat Olfactory Ensheathing Cells and Their Use in the Therapy of Posttraumatic Cysts of the Spinal Cord. Bull Exp Biol Med. 2018;165:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Voronova AD, Stepanova OV, Chadin AV, Fursa GA, Karsuntseva EK, Valikhov MP, Semkina АS, Reshetov IV, Chekhonin VP. The Effect of Transplantation of Olfactory Ensheathing Cells on the Size of Posttraumatic Spinal Cord Cysts. Bull Exp Biol Med. 2021;171:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Reshamwala R, Shah M, Belt L, Ekberg JAK, St John JA. Reliable cell purification and determination of cell purity: crucial aspects of olfactory ensheathing cell transplantation for spinal cord repair. Neural Regen Res. 2020;15:2016-2026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Oieni F, Reshamwala R, St John J. Olfactory Ensheathing Cells for Spinal Cord Injury: The Cellular Superpowers for Nerve Repair. Neuroglia. 2022;3:139-143. [DOI] [Full Text] |
| 14. | Hu XC, Lu YB, Yang YN, Kang XW, Wang YG, Ma B, Xing S. Progress in clinical trials of cell transplantation for the treatment of spinal cord injury: how many questions remain unanswered? Neural Regen Res. 2021;16:405-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Ichim TE, Solano F, Lara F, Paris E, Ugalde F, Rodriguez JP, Minev B, Bogin V, Ramos F, Woods EJ, Murphy MP, Patel AN, Harman RJ, Riordan NH. Feasibility of combination allogeneic stem cell therapy for spinal cord injury: a case report. Int Arch Med. 2010;3:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Bartlett RD, Burley S, Ip M, Phillips JB, Choi D. Cell Therapies for Spinal Cord Injury: Trends and Challenges of Current Clinical Trials. Neurosurgery. 2020;87:E456-E472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Cheng Z, Wang R, Cao K, Wang G, Qin J, Li H, Li J, Wang D, He X. Ten years of clinical observation of olfactory ensheathing cell transplantation in patients with spinal cord injury. J Neurorestoratology. 2021;9:106-116. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | de Oliveira A, Sánchez J, Hurtado J. Neural stem cell transplantation and mechanisms for functional recovery. J Stem Cell Res Ther. 2016;2:59-71. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Yousefifard M, Rahimi-Movaghar V, Nasirinezhad F, Baikpour M, Safari S, Saadat S, Moghadas Jafari A, Asady H, Razavi Tousi SM, Hosseini M. Neural stem/progenitor cell transplantation for spinal cord injury treatment; A systematic review and meta-analysis. Neuroscience. 2016;322:377-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 20. | Yuan T, Liu Q, Kang J, Gao H, Gui S. High-Dose Neural Stem/Progenitor Cell Transplantation Increases Engraftment and Neuronal Distribution and Promotes Functional Recovery in Rats after Acutely Severe Spinal Cord Injury. Stem Cells Int. 2019;2019:9807978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Xiao M, Klueber KM, Lu C, Guo Z, Marshall CT, Wang H, Roisen FJ. Human adult olfactory neural progenitors rescue axotomized rodent rubrospinal neurons and promote functional recovery. Exp Neurol. 2005;194:12-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Xiao M, Klueber KM, Guo Z, Lu C, Wang H, Roisen FJ. Human adult olfactory neural progenitors promote axotomized rubrospinal tract axonal reinnervation and locomotor recovery. Neurobiol Dis. 2007;26:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Young W. Spinal cord regeneration. Cell Transplant. 2014;23:573-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Voronova AD, Stepanova OV, Valikhov MP, Chadin AV, Semkina AS, Chekhonin VP. Neural Stem/Progenitor Cells of Human Olfactory Mucosa for the Treatment of Chronic Spinal Cord Injuries. Bull Exp Biol Med. 2020;168:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Rieger A, Deitmer JW, Lohr C. Axon-glia communication evokes calcium signaling in olfactory ensheathing cells of the developing olfactory bulb. Glia. 2007;55:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Ursavas S, Darici H, Karaoz E. Olfactory ensheathing cells: Unique glial cells promising for treatments of spinal cord injury. J Neurosci Res. 2021;99:1579-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Kato T, Honmou O, Uede T, Hashi K, Kocsis JD. Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia. 2000;30:209-218. [PubMed] [DOI] [Full Text] |
| 28. | Radtke C, Kocsis JD. Peripheral nerve injuries and transplantation of olfactory ensheathing cells for axonal regeneration and remyelination: fact or fiction? Int J Mol Sci. 2012;13:12911-12924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Bartolomei JC, Greer CA. Olfactory ensheathing cells: bridging the gap in spinal cord injury. Neurosurgery. 2000;47:1057-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | García-Alías G, López-Vales R, Forés J, Navarro X, Verdú E. Acute transplantation of olfactory ensheathing cells or Schwann cells promotes recovery after spinal cord injury in the rat. J Neurosci Res. 2004;75:632-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | López-Vales R, Forés J, Navarro X, Verdú E. Chronic transplantation of olfactory ensheathing cells promotes partial recovery after complete spinal cord transection in the rat. Glia. 2007;55:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Stepanova OV, Voronova AD, Sosnovtseva AO, Stepanenko AA, Chadin AV, Karsuntseva EK, Fursa GA, Valikhov MP, Semkina AS, Vorobyev PO, Reshetov IV, Chekhonin VP. Study of the Therapeutic Efficiency of Transduced Olfactory Ensheathing Cells in Spinal Cord Cysts. Stem Cells Dev. 2022;31:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Stepanova OV, Voronova AD, Chadin AV, Valikhov MP, Semkina AS, Karsuntseva EK, Chekhonin IV, Shishkina VS, Reshetov IV, Chekhonin VP. Efficiency of Human Olfactory Ensheathing Cell Transplantation into Spinal Cysts to Improve Mobility of the Hind Limbs. Stem Cells Dev. 2019;28:1253-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Muniswami DM, Tharion G. Functional Recovery Following the Transplantation of Olfactory Ensheathing Cells in Rat Spinal Cord Injury Model. Asian Spine J. 2018;12:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Ramer LM, Au E, Richter MW, Liu J, Tetzlaff W, Roskams AJ. Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J Comp Neurol. 2004;473:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 36. | Yui S, Fujita N, Chung CS, Morita M, Nishimura R. Olfactory ensheathing cells (OECs) degrade neurocan in injured spinal cord by secreting matrix metalloproteinase-2 in a rat contusion model. Jpn J Vet Res. 2014;62:151-162. [PubMed] |
| 37. | Féron F, Perry C, Cochrane J, Licina P, Nowitzke A, Urquhart S, Geraghty T, Mackay-Sim A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128:2951-2960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 337] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 38. | Tabakow P, Jarmundowicz W, Czapiga B, Fortuna W, Miedzybrodzki R, Czyz M, Huber J, Szarek D, Okurowski S, Szewczyk P, Gorski A, Raisman G. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 2013;22:1591-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 39. | Zamani H, Soufizomorrod M, Oraee-Yazdani S, Naviafar D, Akhlaghpasand M, Seddighi A, Soleimani M. Safety and feasibility of autologous olfactory ensheathing cell and bone marrow mesenchymal stem cell co-transplantation in chronic human spinal cord injury: a clinical trial. Spinal Cord. 2022;60:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 40. | Lindsay SL, Johnstone SA, Mountford JC, Sheikh S, Allan DB, Clark L, Barnett SC. Human mesenchymal stem cells isolated from olfactory biopsies but not bone enhance CNS myelination in vitro. Glia. 2013;61:368-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Bhanot Y, Rao S, Ghosh D, Balaraju S, Radhika CR, Satish Kumar KV. Autologous mesenchymal stem cells in chronic spinal cord injury. Br J Neurosurg. 2011;25:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Muheremu A, Shu L, Liang J, Aili A, Jiang K. Sustained delivery of neurotrophic factors to treat spinal cord injury. Transl Neurosci. 2021;12:494-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | McCall J, Weidner N, Blesch A. Neurotrophic factors in combinatorial approaches for spinal cord regeneration. Cell Tissue Res. 2012;349:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Oudega M, Hagg T. Nerve growth factor promotes regeneration of sensory axons into adult rat spinal cord. Exp Neurol. 1996;140:218-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 142] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Namiki J, Kojima A, Tator CH. Effect of brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 on functional recovery and regeneration after spinal cord injury in adult rats. J Neurotrauma. 2000;17:1219-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Kwon BK, Liu J, Messerer C, Kobayashi NR, McGraw J, Oschipok L, Tetzlaff W. Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci U S A. 2002;99:3246-3251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Pastrana E, Moreno-Flores MT, Avila J, Wandosell F, Minichiello L, Diaz-Nido J. BDNF production by olfactory ensheathing cells contributes to axonal regeneration of cultured adult CNS neurons. Neurochem Int. 2007;50:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Stepanova OV, Voronova AD, Chadin AV, Fursa GA, Karsuntseva EK, Valikhov MP, Semkina AS, Reshetov IV, Chekhonin VP. Neurotrophin-3 Enhances the Effectiveness of Cell Therapy in Chronic Spinal Cord Injuries. Bull Exp Biol Med. 2022;173:114-118. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 49. | Betz VM, Sitoci-Ficici KH, Uckermann O, Leipnitz E, Iltzsche A, Thirion C, Salomon M, Zwipp H, Schackert G, Betz OB, Kirsch M. Gene-activated fat grafts for the repair of spinal cord injury: a pilot study. Acta Neurochir (Wien). 2016;158:367-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Sosnovtseva AO, Stepanova OV, Stepanenko AA, Voronova AD, Chadin AV, Valikhov MP, Chekhonin VP. Recombinant Adenoviruses for Delivery of Therapeutics Following Spinal Cord Injury. Front Pharmacol. 2021;12:777628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 51. | Zhang X, Zeng Y, Zhang W, Wang J, Wu J, Li J. Co-transplantation of neural stem cells and NT-3-overexpressing Schwann cells in transected spinal cord. J Neurotrauma. 2007;24:1863-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Koda M, Kamada T, Hashimoto M, Murakami M, Shirasawa H, Sakao S, Ino H, Yoshinaga K, Koshizuka S, Moriya H, Yamazaki M. Adenovirus vector-mediated ex vivo gene transfer of brain-derived neurotrophic factor to bone marrow stromal cells promotes axonal regeneration after transplantation in completely transected adult rat spinal cord. Eur Spine J. 2007;16:2206-2214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Pu Y, Meng K, Gu C, Wang L, Zhang X. Thrombospondin-1 modified bone marrow mesenchymal stem cells (BMSCs) promote neurite outgrowth and functional recovery in rats with spinal cord injury. Oncotarget. 2017;8:96276-96289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Falk A, Holmström N, Carlén M, Cassidy R, Lundberg C, Frisén J. Gene delivery to adult neural stem cells. Exp Cell Res. 2002;279:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Hwang K, Jung K, Kim IS, Kim M, Han J, Lim J, Shin JE, Jang JH, Park KI. Glial Cell Line-derived Neurotrophic Factor-overexpressing Human Neural Stem/Progenitor Cells Enhance Therapeutic Efficiency in Rat with Traumatic Spinal Cord Injury. Exp Neurobiol. 2019;28:679-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Ruitenberg MJ, Plant GW, Hamers FP, Wortel J, Blits B, Dijkhuizen PA, Gispen WH, Boer GJ, Verhaagen J. Ex vivo adenoviral vector-mediated neurotrophin gene transfer to olfactory ensheathing glia: effects on rubrospinal tract regeneration, lesion size, and functional recovery after implantation in the injured rat spinal cord. J Neurosci. 2003;23:7045-7058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Ruitenberg MJ, Levison DB, Lee SV, Verhaagen J, Harvey AR, Plant GW. NT-3 expression from engineered olfactory ensheathing glia promotes spinal sparing and regeneration. Brain. 2005;128:839-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, Zhao C, Zheng Z, Shu Y, Wu X, Lei J, Li Y, Zhang W, Yang C, Wu K, Wu Y, Ho S, Athiviraham A, Lee MJ, Wolf JM, Reid RR, He TC. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis. 2017;4:43-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 458] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 59. | Bedir T, Ulag S, Ustundag CB, Gunduz O. 3D bioprinting applications in neural tissue engineering for spinal cord injury repair. Mater Sci Eng C Mater Biol Appl. 2020;110:110741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 60. | Hsieh FY, Lin HH, Hsu SH. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials. 2015;71:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 287] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 61. | Owens CM, Marga F, Forgacs G, Heesch CM. Biofabrication and testing of a fully cellular nerve graft. Biofabrication. 2013;5:045007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 62. | Koffler J, Zhu W, Qu X, Platoshyn O, Dulin JN, Brock J, Graham L, Lu P, Sakamoto J, Marsala M, Chen S, Tuszynski MH. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med. 2019;25:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 420] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 63. | Xu C, Chang Y, Wu P, Liu K, Dong X, Nie A, Mu C, Liu Z, Dai H, Luo Z. Two‐Dimensional‐Germanium Phosphide‐Reinforced Conductive and Biodegradable Hydrogel Scaffolds Enhance Spinal Cord Injury Repair. Adv Funct Mater. 2021;31:2104440. [DOI] [Full Text] |
| 64. | Othon CM, Wu X, Anders JJ, Ringeisen BR. Single-cell printing to form three-dimensional lines of olfactory ensheathing cells. Biomed Mater. 2008;3:034101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Chen X, Zhao Y, Li X, Xiao Z, Yao Y, Chu Y, Farkas B, Romano I, Brandi F, Dai J. Functional Multichannel Poly(Propylene Fumarate)-Collagen Scaffold with Collagen-Binding Neurotrophic Factor 3 Promotes Neural Regeneration After Transected Spinal Cord Injury. Adv Healthc Mater. 2018;7:e1800315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 66. | Invernizzi M, Turri S, Levi M, Suriano R. 4D printed thermally activated self-healing and shape memory polycaprolactone-based polymers. Eur Polym J. 2018;101:169-176. [RCA] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 67. | Arif ZU, Khalid MY, Ahmed W, Arshad H. A review on four-dimensional (4D) bioprinting in pursuit of advanced tissue engineering applications. Bioprinting. 2022;27:e00203. [DOI] [Full Text] |
| 68. | Lei Z, Wang Q, Wu P. A multifunctional skin-like sensor based on a 3D printed thermo-responsive hydrogel. Mater Horiz. 2017;4:694-700. [DOI] [Full Text] |
| 69. | Midha S, Dalela M, Sybil D, Patra P, Mohanty S. Advances in three-dimensional bioprinting of bone: Progress and challenges. J Tissue Eng Regen Med. 2019;13:925-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 70. | Qasim M, Chae DS, Lee NY. Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. Int J Nanomedicine. 2019;14:4333-4351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 71. | Chiang MY, Cheng HW, Lo YC, Wang WC, Chang SJ, Cheng CH, Lin YC, Lu HE, Sue MW, Tsou NT, Li SJ, Kuo CH, Chen YY, Huang WC, Chen SY. 4D spatiotemporal modulation of biomolecules distribution in anisotropic corrugated microwrinkles via electrically manipulated microcapsules within hierarchical hydrogel for spinal cord regeneration. Biomaterials. 2021;271:120762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 72. | Miao S, Cui H, Nowicki M, Xia L, Zhou X, Lee SJ, Zhu W, Sarkar K, Zhang Z, Zhang LG. Stereolithographic 4D Bioprinting of Multiresponsive Architectures for Neural Engineering. Adv Biosyst. 2018;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 73. | Miao S, Cui H, Esworthy T, Mahadik B, Lee SJ, Zhou X, Hann SY, Fisher JP, Zhang LG. 4D Self-Morphing Culture Substrate for Modulating Cell Differentiation. Adv Sci (Weinh). 2020;7:1902403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 74. | Wu SD, Hsu SH. 4D bioprintable self-healing hydrogel with shape memory and cryopreserving properties. Biofabrication. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 75. | Chang DJ, Cho HY, Hwang S, Lee N, Choi C, Lee H, Hong KS, Oh SH, Kim HS, Shin DA, Yoon YW, Song J. Therapeutic Effect of BDNF-Overexpressing Human Neural Stem Cells (F3.BDNF) in a Contusion Model of Spinal Cord Injury in Rats. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 76. | Lin XY, Lai BQ, Zeng X, Che MT, Ling EA, Wu W, Zeng YS. Cell Transplantation and Neuroengineering Approach for Spinal Cord Injury Treatment: A Summary of Current Laboratory Findings and Review of Literature. Cell Transplant. 2016;25:1425-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 77. | Tang S, Liao X, Shi B, Qu Y, Huang Z, Lin Q, Guo X, Pei F. The effects of controlled release of neurotrophin-3 from PCLA scaffolds on the survival and neuronal differentiation of transplanted neural stem cells in a rat spinal cord injury model. PLoS One. 2014;9:e107517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | Wang L, Gu S, Gan J, Tian Y, Zhang F, Zhao H, Lei D. Neural Stem Cells Overexpressing Nerve Growth Factor Improve Functional Recovery in Rats Following Spinal Cord Injury via Modulating Microenvironment and Enhancing Endogenous Neurogenesis. Front Cell Neurosci. 2021;15:773375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 79. | Wright AA, Todorovic M, Tello-Velasquez J, Rayfield AJ, St John JA, Ekberg JA. Enhancing the Therapeutic Potential of Olfactory Ensheathing Cells in Spinal Cord Repair Using Neurotrophins. Cell Transplant. 2018;27:867-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 80. | Wang Y, Teng HL, Gao Y, Zhang F, Ding YQ, Huang ZH. Brain-derived Neurotrophic Factor Promotes the Migration of Olfactory Ensheathing Cells Through TRPC Channels. Glia. 2016;64:2154-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 81. | Ma YH, Zhang Y, Cao L, Su JC, Wang ZW, Xu AB, Zhang SC. Effect of neurotrophin-3 genetically modified olfactory ensheathing cells transplantation on spinal cord injury. Cell Transplant. 2010;19:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Uchida S, Hayakawa K, Ogata T, Tanaka S, Kataoka K, Itaka K. Treatment of spinal cord injury by an advanced cell transplantation technology using brain-derived neurotrophic factor-transfected mesenchymal stem cell spheroids. Biomaterials. 2016;109:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 83. | Lee SH, Kim Y, Rhew D, Kim A, Jo KR, Yoon Y, Choi KU, Jung T, Kim WH, Kweon OK. Impact of local injection of brain-derived neurotrophic factor-expressing mesenchymal stromal cells (MSCs) combined with intravenous MSC delivery in a canine model of chronic spinal cord injury. Cytotherapy. 2016;. [PubMed] [DOI] [Full Text] |
| 84. | Stewart AN, Kendziorski G, Deak ZM, Bartosek NC, Rezmer BE, Jenrow K, Rossignol J, Dunbar GL. Transplantation of mesenchymal stem cells that overexpress NT-3 produce motor improvements without axonal regeneration following complete spinal cord transections in rats. Brain Res. 2018;1699:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Zhang W, Yan Q, Zeng YS, Zhang XB, Xiong Y, Wang JM, Chen SJ, Li Y, Bruce IC, Wu W. Implantation of adult bone marrow-derived mesenchymal stem cells transfected with the neurotrophin-3 gene and pretreated with retinoic acid in completely transected spinal cord. Brain Res. 2010;1359:256-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 86. | Dong Y, Yang L, Zhao H, Zhang C, Wu D. Transplantation of neurotrophin-3-transfected bone marrow mesenchymal stem cells for the repair of spinal cord injury. Neural Regen Res. 2014;9:1520-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Zha K, Yang Y, Tian G, Sun Z, Yang Z, Li X, Sui X, Liu S, Zhao J, Guo Q. Nerve growth factor (NGF) and NGF receptors in mesenchymal stem/stromal cells: Impact on potential therapies. Stem Cells Transl Med. 2021;10:1008-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 88. | Luo W, Gong Y, Qiu F, Yuan Y, Jia W, Liu Z, Gao L. NGF nanoparticles enhance the potency of transplanted human umbilical cord mesenchymal stem cells for myocardial repair. Am J Physiol Heart Circ Physiol. 2021;320:H1959-H1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 89. | Morys J, Borkowska P, Zielinska A, Kowalski J. Study of the influence of NGF-β gene overexpression in human mesenchymal stem cells on the expression level of SOX1 and neural pathway genes. Mol Biol Rep. 2022;49:4435-4441. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |