Published online Jul 6, 2023. doi: 10.12998/wjcc.v11.i19.4601
Peer-review started: March 22, 2023
First decision: May 12, 2023
Revised: May 16, 2023
Accepted: June 2, 2023
Article in press: June 2, 2023
Published online: July 6, 2023
Processing time: 100 Days and 6.3 Hours
Severe acute pancreatitis (AP) is one of the most common diseases of the gastrointestinal tract and carries a significant financial burden with high disability and mortality. There are no effective drugs in the clinical management of severe AP, and there is an absence of evidence-based medicine concerning the treatment of severe AP.
To explore whether ulinastatin (UTI) can improve the outcome of severe AP.
The present research included patients who were hospitalized in intensive critical care units (ICUs) after being diagnosed with severe AP. Patients received UTI (400000 IU) or placebos utilizing computer-based random sequencing (in a 1:1 ratio). The primary outcome measures were 7-d mortality, clinical efficacy, inflammatory response, coagulation function, infection, liver function, renal function, and drug-related adverse effects were evaluated.
A total of 181 individuals were classified into two groups, namely, the placebo group (n = 90) and the UTI group (n = 91). There were no statistically significant differences in baseline clinical data between the two groups. The 7-d mortality and clinical efficacy in the UTI group were remarkably improved compared with those in the placebo group. UTI can protect against hyperinflammation and improve coagulation dysfunction, infection, liver function, and renal function. UTI patients had markedly decreased hospital stays and hospitalization expenditures compared with the placebo group.
The findings from the present research indicated that UTI can improve the clinical outcomes of patients with severe AP and has fewer adverse reactions.
Core Tip: One of the most prevalent gastrointestinal tract gastrointestinal disorders, severe acute pancreatitis (AP), is the leading cause of hospitalization due to gastrointestinal diseases. The present study suggests that ulinastatin (UTI) can reduce the risk of overall 7-d mortality in severe AP patients and is associated with a higher total effective rate vs the placebo control group. We also found that UTI can protect against hyperinflammation. It also did not significantly increase the incidence rate of severe adverse effects.
- Citation: Wang SQ, Jiao W, Zhang J, Zhang JF, Tao YN, Jiang Q, Yu F. Ulinastatin in the treatment of severe acute pancreatitis: A single-center randomized controlled trial. World J Clin Cases 2023; 11(19): 4601-4611
- URL: https://www.wjgnet.com/2307-8960/full/v11/i19/4601.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i19.4601
One of the most prevalent gastrointestinal tract gastrointestinal disorders, severe acute pancreatitis (AP), is the leading cause of hospitalization due to gastrointestinal diseases, and carries a significant financial burden[1-3]. The incidence of AP worldwide is approximately 34 per 100000 people per year and continues to rise, and the health care system costs huge billion annually[4]. Although it is generally considered a mild disease with a good prognosis in most AP patients, 15% to 20% of AP patients develop a severe condition with high morbidity and mortality with systemic and local complications[5,6]. Pancreatitis is aggravated by inappropriate treatment, which is responsible for over 25% of mortality associated with severe AP[7,8]. Although surgical treatment is currently the mainstay in clinical practice, a recent meta-analysis confirmed the presence of multiple complications, postoperative body pain, and prolonged postoperative recovery duration after surgical intervention[9]. More researchers are calling for conservative treatment with drugs, especially for severe AP patients who prefer not to undergo surgery, which might be a good try[1,5,7,10,11].
The pathological mechanisms of AP include immune system mediation and complex cascade reactions of inflammatory activation[12]. Severe stress may trigger systemic inflammatory reactions, and hyperinflammation might disrupt coagulation functioning, leaving the patient at a greater risk of diseases. Long-term patient prognosis is affected by disease progression and multiple organ dysfunction syndromes (MODS) caused by an imbalance and high intensity of the systemic inflammatory response syndrome and the compensatory anti-inflammatory response syndrome 13. The ability to prevent and alleviate MODS and successfully manage hyperinflammation after severe AP are crucial early-stage therapy goals[13].
Ulinastatin (UTI) is an inhibitor of serine proteases that has a molecular weight of 67000 and is purified from human urine. This compound's most important pharmacological properties include anti-inflammation, immunomodulation, and protection of organs[14-16]. In the management of acute inflammatory diseases like sepsis and ischemia-reperfusion damage, pharmaceuticals may be used as antiapoptotic and anti-inflammatory agents[17]. By conducting a meta-analysis that included 15 randomized controlled trials (RCTs), He et al[18] found that patients who underwent cardiac surgery could gain benefit from UTI by suppressing postoperative inflammation and offering lung protection[18]. In China, UTIs are extensively utilized in the management of inflammatory diseases, organ preservation during surgery, and the management of shock[19]. A retrospective study enrolled 130 SAP patients and showed that UTI can improve the clinical outcomes of SAP patients, but efficacy varies with the dosage[1]. Another retrospective study enrolled 78 SAP patients and showed that UTI combined with glutamine is effective in treating severe pancreatitis, which efficiently facilitates the recovery of immune, metabolic, and liver functions[5]. Yang and Zhao[7] also reported a retrospective study that enrolled 100 severe AP patients and showed that somatostatin plus UTI is a viable alternative in the treatment of severe AP patients. A meta-analysis of RCTs suggested that UTI demonstrated a favorable benefit in treating acute respiratory distress syndrome patients, but this conclusion has not been verified without a larger sample size of RCTs[20].
However, the effectiveness of UTI following severe AP is still uncertain since these studies had limited sample numbers and there are no evidence-based clinical trials with large sample sizes. Consequently, the focus of this research was to determine whether or not treating severe AP with UTIs would improve patient outcomes and attenuate hyperinflammation.
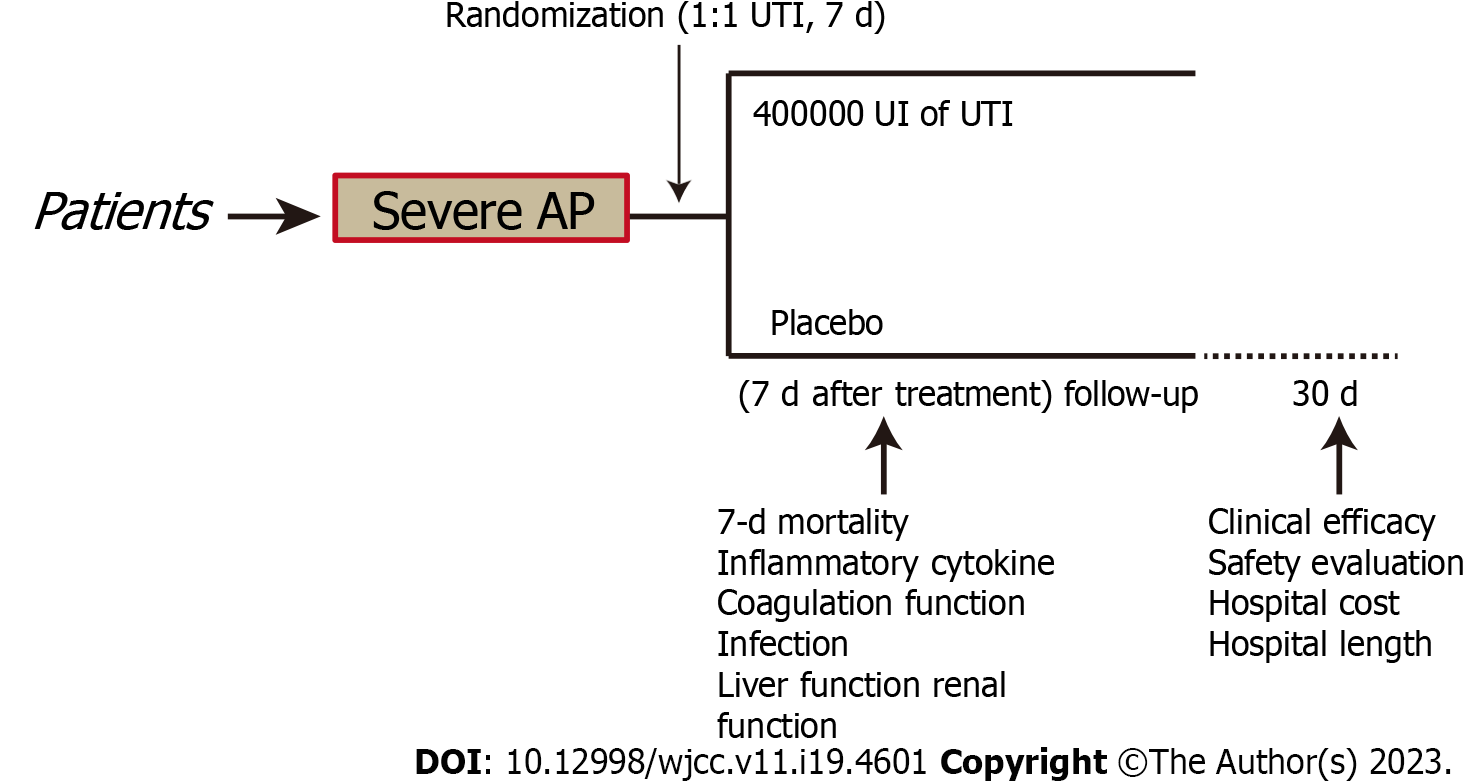
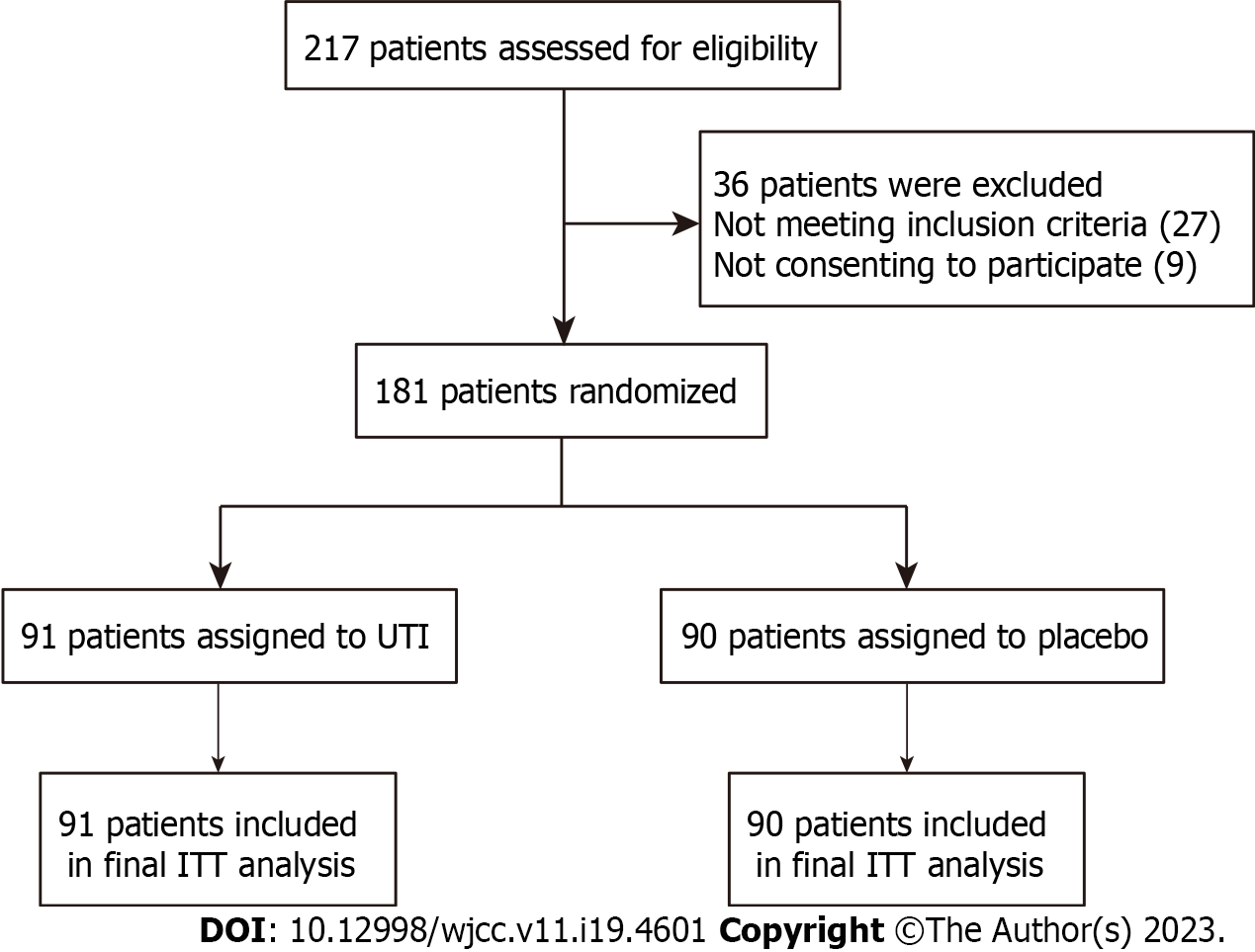
A placebo-controlled, parallel-arm, randomized experiment was carried out in Jiangsu from October 2018 to December 2021. A total of 217 patients were screened over this period, and 181 of these were initially enrolled in the study to form the ITT population. To determine if the intervention is superior, the present research was conducted. It was registered with the registration number CWXH-IPR-2018015 (date: 9/Sep/2018) with protocol approval from the Clinical Research Ethics Committees of the 904th Hospital of PLA endorsed the methodology used in the present research (2018-YXLL-097) and was following the Declaration of Helsinki. The protocol for the research was subjected to approval granted by the Ethics Committees of all the collaborating centers. Those patients whose competence could be demonstrated by their comprehensive awareness of time, place, and person, along with their comprehension of the investigator's explanation of the trial, were asked to obtain written informed consent for the study. In addition, patients were allocated at random (1:1) and were administered an intravenous infusion of either 400000 IU UTI (Guangdong Tianpu Biochemical Pharmaceutical Co., Ltd., H20040506, 2 mL:100000 IU) or placebos dissolved in 250 mL of 0.9% saline given intravenously over 1 h every 12 h for 7 d, the total daily dose is 800000 units (400000 units administered twice)[16]. Infusion could be interrupted for 1 d if there was a greater than the threefold-fold increase in liver enzymes over baseline levels following the procedure (Figure 1). All other treatments were the same. The last check-up was performed 30 d following the procedure.
Patients were included in the present research in the ICU. SAP was diagnosed following the criteria of the Revised Atlanta Classification[21]. The following were the criteria for inclusion: (1) Patients aged 25 to 70 years; (2) patients who met the diagnostic criteria of severe AP[2]; (3) patients with complete data; and (4) patients who were randomly assigned to receive either UTI or a placebo. The exclusion criteria were as follows: (1) Patients who were unlikely to be salvaged upon admission; (2) allergic to UTI; (3) received anticoagulant medication within 48 h before hospitalization; (4) pregnant women and patients with malignant tumors; (5) treatment with immunosuppressive drugs; (6) multiple organ dysfunction before illness; and (7) other explanations were discovered by researchers.
With the aid of SPSS software (version: 14.0) (SPSS Institute, Hefei, Anhui Medical University), permuted-block randomization was carried out based on a computer system that used an allotment list to produce random numbers (in a one-to-one ratio). This was carried out by a statistician who was not a member of the research team to maintain the integrity and blinding of the research. The outcomes of the random sampling process were enclosed in prenumbered envelopes and kept at the location of the research until the study's conclusion was reached. The study medicines were delivered by a research nurse following the random assignment sequence. Both the research participants and the patients were unaware of which medicine was being applied in the trial. In the event of an emergency, such as acute hepatic failure, two experts might recommend that the treatment allotment be unmasked and that the study medicine be adjusted or discontinued if needed, according to the protocol. All of the occurrences were recorded in detail. Then, we acquired information on the patient's demographics, medical histories, and pertinent investigation findings.
All clinical and imaging data and treatment were subjected to assessment by a masked independent diagnostic and assessment committee. This committee included two researchers who were trained before the start of the present research and did not engage in the clinical care of patients. The primary endpoint of this study was 7-d mortality. Additionally, we also evaluated the clinical efficacy, and the specific evaluation methods referred to in the previous literature[7] included cured, effective, and ineffective. Cured as follows: Clinical symptoms (including fever, abdominal distension, abdominal pain, etc.) disappeared basically, blood amylase level returned to positive Ranson scores < 3 and Acute Physiology and Chronic Health Evaluation (APACHE)-II scores < 8, vital signs are stable; Effective as follows: The clinical symptoms improved obviously, and the blood amylase level decreased, Ranson score and APACHE-II score decreased significantly. Ineffective as clinical symptoms, laboratory indicators and related scores not improvement. Total effectiveness included cure and effectiveness. The secondary endpoints included: (1) Inflammatory cytokine levels (pretherapy and posttreatment), such as serum tumor necrosis factor-α and interleukin-6; (2) coagulation function, such as the plasma prothrombin time, activated partial thromboplastin time and fibrinogen levels, was measured using an automatic coagulation analyzer; (3) serum infection index levels, such as white blood cell count, C-reactive protein, and procalcitonin (PCT); (4) liver function index levels, such as alanine aminotransferase and aspartate aminotransferase; and (5) renal function index levels, such as creatinine and blood urea nitrogen.
We kept track of the length of time spent in the intensive care unit, and the most prevalent adverse effects of UTI included granulocytopenia and abnormal liver enzymes. Other rare complications include diarrhea, vomiting, and allergies. All complications were confirmed and recorded by physical examination after two doctors and nurses; granulocytopenia was diagnosed by routine blood level detection. Abnormal liver enzymes were diagnosed by liver function tests. Finally, we check the related index every two days over the first 14 d.
Extended hospital stays and greater healthcare costs have previously been recorded for individuals who need ICU[22,23]. This study thus compared the two groups in terms of how long they stayed in the hospital and how much their treatment ultimately cost.
The primary endpoint of a previous study demonstrated that the 7-d mortality rate among those who received the UTI treatment was 5.36%, but it was 26.92% among those who received the placebo. Our decision to recruit 92 patients (n = 46 per group) was based on the results of a calculation that determined the sample size using an alpha value of 0.05 and a statistical power of 80%. We decided to enroll 181 participants in the study (n = 90 per group). Eventually, 91 patients were recruited in the UTI category, whereas 90 were recruited in the placebo category. Every participant's baseline information was recorded in the study database, and the outcomes were recorded by a research nurse.
Baseline data along with outcome measurements were imported into the database with the aid of a research nurse. The data were collected using paper forms and submitted to a digital database that was secured by passwords. The mean ± SD are the reporting formats for any continuous variables. Analyses of statistical data were executed with the SPSS 19.0 statistical tool (SPSS, Inc., Chicago, United States). M (Q1, Q3) was used to denote measurement data that follow a nonnormal distribution. Quantitative data were evaluated utilizing independent-sample t-tests. The χ2 test or Fisher's exact t-test was conducted to evaluate the qualitative data. The criterion for significance was established at P < 0.05.
From October to December 2021, we assessed 217 elderly patients. In total, 181 participants were randomized at a 1:1 ratio and given either UTI treatment (n = 91) and receiving a placebo (n = 90). No cases of opening blindness were noted during the research period. Also, no remarkable variations in the preliminary data were found between the two cohorts (Table 1). Within the scope of this study, no patients were ever lost to follow-up. Overall, all the patients were included in the final intention-to-treat analysis (Figure 2). We had a concluding appointment with the last patient chosen at random on February 1, 2022.
| UTI group (n = 91) | Placebo group (n = 90) | P value | |
| Age (year, mean ± SD) | 52.7 ± 3.8 | 53.5 ± 4.1 | 0.175 |
| Gender, n (%) | 0.705 | ||
| Male | 67 (73.63) | 64 (71.11) | |
| Female | 24 (26.37) | 26 (28.88) | |
| BMI (kg/cm2, mean ± SD) | 22.6 ± 2.1 | 23.1 ± 2.5 | 0.147 |
| Cause of disease, n (%) | 0.582 | ||
| Biliary | 61 (67.03) | 57 (63.33) | |
| Hyperlipidemic | 18 (19.78) | 19 (21.11) | |
| Alcoholic | 12 (13.09) | 14 (15.56) | |
| Smoking history, n (%) | 0.483 | ||
| Yes | 53 (58.24) | 57 (63.33) | |
| No | 38 (41.76) | 33 (36.67) | |
| Drinking history, n (%) | 0.371 | ||
| Yes | 58 (58.24) | 63 (70.00) | |
| No | 33 (41.76) | 27 (30.00) | |
| Living environment, n (%) | 0.604 | ||
| Town | 59 (64.84) | 55 (61.11) | |
| Countryside | 32 (35.16) | 35 (38.89) | |
| Past medical history, n (%) | |||
| Hypertension | 31 (34.07) | 29 (32.22) | 0.792 |
| Hyperlipidemia | 35 (38.46) | 37 (41.11) | 0.716 |
| Diabetes | 28 (30.77) | 29 (32.22) | 0.833 |
| Cholelithiasis | 17 (18.68) | 16 (17.78) | 0.875 |
| Previous pancreatitis, n (%) | 9 (9.89) | 10 (11.11) | 0.789 |
| APACHE-II score on admission (mean ± SEM) | 12.6 ± 0.8 | 12.2 ± 0.8 | 0.724 |
| Ranson score on admission (mean ± SEM) | 3.3 ± 0.3 | 3.2 ± 0.4 | 0.842 |
| Necrotizing pancreatitis | 23 (25.27) | 20 (22.22) | 0.727 |
| Time from the onset to diagnosis (hours) | 15.2 ± 6.5 | 14.6 ± 7.1 | 0.950 |
| Endoscopic bile duct drainage | 37 (40.66) | 42 (46.67) | 0.455 |
The data showed that the total 7-d mortality rate was 14.36% (26/181). Within the UTI category, the 7-d mortality rate was 7.69% (7/91) in contrast with 21.11% (19/90) within the placebo category. A significantly higher 7-d mortality was seen in the placebo category in contrast with the UTI control (P = 0.010, Table 2). When comparing the UTI category to the placebo category, the total effective rate was greater (P = 0.008, Table 2).
| UTI group (n = 91) | Placebo group (n = 90) | P value | |
| 7-d mortality | 7 (7.69) | 19 (21.11) | 0.010 |
| Efficacy | 0.001 | ||
| Cured | 55 (60.44) | 34 (37.78) | |
| Effective | 28 (30.77) | 35 (38.89) | |
| Ineffective | 8 (8.79) | 21 (23.33) | |
| Total effectiveness | 83 (91.21) | 69 (76.67) | 0.008 |
Before UTI or placebo treatment, there was no variation between the UTI and placebo categories in kidney function index levels, hepatic function index levels, serum infection index levels, coagulation functions, or inflammatory cytokine levels (P > 0.05), but these indices were substantially increased in the UTI category relative to the placebo category (P < 0.05, Table 3).
| Before treatment | After treatment | |||||
| UTI group (n = 91) | Placebo group (n = 90) | P value | UTI group (n = 91) | Placebo group (n = 90) | P value | |
| Inflammatory cytokine, mean ± SD | ||||||
| TNF-α (pg/mL) | 77.28 ± 19.43 | 76.91 ± 18.27 | 0.895 | 29.67 ± 11.19 | 43.17 ± 12.08 | < 0.001 |
| IL-6 (pg/mL) | 89.37 ± 16.28 | 87.91 ± 16.74 | 0.553 | 50.18 ± 12.24 | 66.35 ± 14.26 | < 0.001 |
| Coagulation function, mean ± SD | ||||||
| PT (s) | 21.05 ± 3.44 | 20.62 ± 3.19 | 0.385 | 12.16 ± 2.84 | 16.71 ± 3.15 | < 0.001 |
| APTT (s) | 39.22 ± 4.26 | 40.15 ± 4.39 | 0.150 | 32.26 ± 3.73 | 37.25 ± 4.02 | < 0.001 |
| D-D (mg/L) | 4.26 ± 1.12 | 4.33 ± 1.27 | 0.694 | 1.75 ± 0.59 | 2.94 ± 0.79 | < 0.001 |
| FIB (g/L) | 1.07 ± 0.23 | 1.12 ± 0.26 | 0.172 | 3.46 ± 0.60 | 3.28 ± 0.55 | 0.037 |
| Infection index levels, mean ± SD | ||||||
| CRP (mg/L) | 31.29 ± 4.16 | 30.98 ± 4.07 | 0.613 | 5.19 ± 0.97 | 8.34 ± 1.33 | < 0.001 |
| PCT (ng/L) | 2.05 ± 0.21 | 2.10± 0.19 | 0.095 | 0.75 ± 0.23 | 0.82 ± 0.38 | 0.135 |
| WBC (× 109/L) | 18.05 ± 9.11 | 17.56 ± 8.97 | 0.716 | 12.74 ± 6.41 | 15.19 ± 7.29 | 0.017 |
| Liver function index levels, mean ± SD | ||||||
| ALT (U/L) | 32.20 ± 3.74 | 33.15 ± 3.68 | 0.087 | 48.19 ± 4.27 | 53.11 ± 4.53 | < 0.001 |
| AST (U/L) | 34.17 ± 2.83 | 34.42 ± 2.99 | 0.564 | 49.67 ± 3.88 | 50.27 ± 4.19 | 0.230 |
| Renal function index levels, mean ± SD | ||||||
| Scr | 81.26 ± 9.91 | 79.64 ± 9.12 | 0.254 | 84.22 ± 10.16 | 105.42 ± 11.20 | < 0.001 |
| BUN | 5.37 ± 1.94 | 5.19 ± 1.73 | 0.511 | 8.06 ± 2.99 | 10.19 ± 3.28 | < 0.001 |
Granulocytopenia and abnormal liver enzymes are the two most common negative outcomes that are caused by UTI. Results showed that granulocytopenia affected 8 patients (8.79%) in the UTI category and 4 patients (4.44%) in the placebo category. Hepatic enzyme abnormalities were more common in the UTI category (34.07% vs 27.77%) than in the control group. No substantial variation in the incidence of granulocytopenia (P = 0.240) or abnormal liver enzymes (P = 0.360) existed between the UTI category and the placebo category (Table 4). Additionally, as illustrated in Table 4, the potential UTI-induced adverse events such as diarrhea (20.00% vs 29.67%, P = 0.132) and vomiting (12.22% vs 20.88%, P = 0.085) were not shown to vary significantly between the two groups. The UTI category had a greater rate of allergic reactions than the placebo category (24.18% vs 10.00%, P = 0.029, Table 4). Throughout the intervention, no blind cases were revealed.
| UTI (n = 91) | Placebo (n = 90) | P value | |
| Granulocytopenia | 8 (8.79) | 4 (4.44) | 0.240 |
| Abnormal liver enzymes | 31 (34.07) | 25 (27.77) | 0.360 |
| Diarrhea | 27 (29.67) | 18 (20.00) | 0.132 |
| Vomiting | 19 (20.88) | 11 (12.22) | 0.117 |
| Allergies | 22 (24.18) | 9 (10.00) | 0.029 |
| Hospitalization stays, day, mean ± SD | 17.8 ± 4.2 | 19.6 ± 5.3 | 0.012 |
| Hospitalization costs, CNY, mean ± SD | 3.94 ± 0.82 | 3.82 ± 0.99 | 0.376 |
There was a significant variation in the length of hospital stay between the UTI category (17.8 d) and the placebo category (19.6 d) (P = 0.012). Costs for inpatient treatment were much lower in the UTI category (38200 RMB) in contrast with the placebo category (39400 RMB), although the variation was insignificant (P = 0.376, Table 4).
The present research demonstrate that UTI is linked to a higher total effective rate compared to the placebo treatment, which could lead to a considerable reduction in the incidence of total 7-d mortality in patients with severe AP. We also discovered that UTI has the potential to enhance kidney function, hepatic function, and coagulation function while also protecting against hyperinflammation and infection. Although the synergistic impact after the simultaneous administration of other medications in certain patients could raise the percentage of patients who develop allergies, UTI did not substantially elevate the incidence rate of serious complications other than allergies, which is worth noting. In addition, it reduced the time patients spent in the hospital.
Our results indicated a total 7-d mortality rate of 14.36%, which is in line with previous data[1,24-26]. Severe AP can cause substantial complications with high mortality in up to 25% of those affected with hospitalization periods in an ICU within two weeks[27]. Wang et al[24] reported that the mortality in the basic treatment was 20%, while the mortality was 4.8% in the somatostatin + UTI group. Many causes of severe AP have been recorded in the literature, but the pathogenetic theories and the specific cause of severe AP are still controversial. Whitcomb[28] reported that the development and recurrence of AP were linked to composite factors, including genetics, environmental factors, and metabolic processes. Choledocholithiasis and excess alcohol have become the most common causes of severe AP, especially in developed countries and countries with high alcohol intake[29]. With the in-depth study of AP and the development of modern molecular biology, recent studies have shown that gene variants and specific receptor defects may increase the risk of AP in patients, thereby increasing the risk of developing severe AP[27,30]. Intensive care management, infection prevention and identification, nutritional support, and therapeutic drugs are the cornerstones of treating severe AP; surgery is no longer an early intervention and may not be needed[25]. Further progress will also require the development and clinical testing of medications that target disease mechanisms. In our data, no medication has been shown to consistently modulate the outcomes of severe AP. The main negative result may be due to the small number of enrolled cases, such as antioxidants and vitamin C trials[31] and pentoxifylline trials[32]. Hence, large-sample clinical RCTs are urgently needed.
UTI is a 67 kDa glycoprotein isolated from healthy human urine, which is an inhibitor of trypsin and other proteases in the urine applied for the treatment of acute inflammatory diseases, hemorrhagic shock, toxic shock, and sepsis[16,33]. Enhanced splenic proliferative responses and cytokine production after acute AP are two ways in which UTIs aid in the recovery of immune function[34]. Animal research has shown that UTIs can regulate inflammation, oxidative stress, apoptosis, immune regulation, and organ protection[14,35]. UTI treatment was employed to decrease the overall 7-d fatality rate in the current investigation. There are still many controversies in clinical studies in severe AP after UTI treatment. A prospective, randomized, placebo-controlled trial showed that patients at high risk of pancreatitis and hyperamylasemia after endoscopic retrograde cholangiopancreatography (ERCP) did not benefit from low-dose prophylactic treatment with UTI after surgery[36]. Ueki et al[37] also reported that preventive administration of UTI and gabexate mesylate had no significant clinical value on the incidence of post-ERCP pancreatitis. Conclusions from these research reports should be treated with caution, however, because of their limited sample sizes or retrospective nature. Conversely, Wei et al[38] reported that after diabetic ketoacidosis complicated with AP, the recovery period of clinical symptoms and the levels of inflammatory mediators may be reduced by using an insulin pump in conjunction with UTI. Thus, the current investigation approved a large single-center RCT to verify the therapeutic benefits of UTI in severe AP. Additionally, attention should be given to both the prevention and management of allergic responses.
Some drawbacks are present in this research. Since this was an RCT conducted at a single site, the findings may not be generalized. The current study only included a single dose of UTI administration.
The results of this research illustrate that UTI treatment could enhance kidney function, hepatic function, and coagulation function and decrease the risk of infection and death associated with hyperinflammation after severe AP. The length of time patients spent in the hospital and their associated costs also dropped significantly. Additional investigation is warranted among individuals receiving varying doses of AP to fully understand the prospective applicability of UTI in these patients.
Severe acute pancreatitis (AP) is one of the most common diseases of the gastrointestinal tract, is the leading cause of hospitalization due to gastrointestinal diseases. Surgical treatment is currently the mainstay in clinical practice, is associated with multiple complications, postoperative body pain, and delayed postoperative recovery. Ulinastatin (UTI) is a serine protease inhibitor, which seemed to show a beneficial effect for acute respiratory distress syndrome patient treatment but lacked a larger sample size of randomized controlled trials.
To evaluate the clinical value and safety of UTI in severe AP.
This research was conducted to determine whether UTI might be used to improve the outcomes of patients with severe AP.
Patients with severe AP who were transferred to intensive critical care units were enrolled in the current study. Patients were assigned at random (ratio of 1:1) using a computer to receive either a UTI (400000 IU) or a placebo. The seven-day mortality rate, clinical efficacy, and drug-associated side events were evaluated.
No statistically significant differences in baseline clinical data between the two groups. When compared with the results obtained from the placebo category, both the clinical efficacy and the seven-day mortality rate for the UTI category showed significant improvements. UTI therapy was shown to protect against hyperinflammation, attenuate coagulation dysfunction and infection, and even improve liver and kidney functioning. Hospitalization durations for UTI-treated patients were much lower than those in the placebo category.
Treatment with UTI may enhance therapeutic efficacy for individuals with severe AP and is associated with fewer side effects.
A large number study with varied dosages, and long-term outcome follow-up are needed.
We hereby express our gratitude to Jiangsu Brilliant Biological Technology Co., Ltd. for providing technical help.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Chilimuri S, United States; Gaspar R, Portugal; Iwasaki E, Japan; Kitamura K, Japan S-Editor: Fan JR L-Editor: A P-Editor: Zhao S
| 1. | He HW, Zhang H. The efficacy of different doses of ulinastatin in the treatment of severe acute pancreatitis. Ann Palliat Med. 2020;9:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, Ball CG, Parry N, Sartelli M, Wolbrink D, van Goor H, Baiocchi G, Ansaloni L, Biffl W, Coccolini F, Di Saverio S, Kluger Y, Moore E, Catena F. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 430] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 3. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254-272.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1082] [Article Influence: 180.3] [Reference Citation Analysis (1)] |
| 4. | Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 475] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 5. | Zhao L, Ma Y, Li Q, Wang Y. Ulinastatin combined with glutamine improves liver function and inflammatory response in patients with severe acute pancreatitis. Am J Transl Res. 2022;14:918-926. [PubMed] |
| 6. | van Dijk SM, Hallensleben NDL, van Santvoort HC, Fockens P, van Goor H, Bruno MJ, Besselink MG; Dutch Pancreatitis Study Group. Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66:2024-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 298] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 7. | Yang L, Zhao Z. Somatostatin plus Ulinastatin in the Treatment of Severe Acute Pancreatitis and Its Effect on Serum Cytokine Levels. Evid Based Complement Alternat Med. 2022;2022:7223632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Jabłońska B, Mrowiec S. Nutritional Support in Patients with Severe Acute Pancreatitis-Current Standards. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Dutta AK, Goel A, Kirubakaran R, Chacko A, Tharyan P. Nasogastric versus nasojejunal tube feeding for severe acute pancreatitis. Cochrane Database Syst Rev. 2020;3:CD010582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (2)] |
| 10. | Hadi A, Werge M, Kristiansen KT, Pedersen UG, Karstensen JG, Novovic S, Gluud LL. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: Case report on three family members. Pancreatology. 2020;20:665-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 11. | Zhu Q, Zou F, Lin J, Liu X, Luo Y. Effect of continuous renal replacement therapy adjuvant to broad-spectrum enzyme inhibitors on the efficacy and inflammatory cytokines in patients with severe acute pancreatitis. Am J Transl Res. 2021;13:8067-8075. [PubMed] |
| 12. | Shamoon M, Deng Y, Chen YQ, Bhatia M, Sun J. Therapeutic implications of innate immune system in acute pancreatitis. Expert Opin Ther Targets. 2016;20:73-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Greve F, Aulbach I, Mair O, Biberthaler P, Hanschen M. The Clinical Impact of Platelets on Post-Injury Serum Creatinine Concentration in Multiple Trauma Patients: A Retrospective Cohort Study. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Nakanishi K, Takeda S, Sakamoto A, Kitamura A. Effects of ulinastatin treatment on the cardiopulmonary bypass-induced hemodynamic instability and pulmonary dysfunction. Crit Care Med. 2006;34:1351-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Feng X, Ma W, Chen J, Jiao W, Wang Y. Ulinastatin alleviates early brain injury after traumatic brain injury by inhibiting oxidative stress and apoptosis. Acta Cir Bras. 2022;37:e370108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Karnad DR, Bhadade R, Verma PK, Moulick ND, Daga MK, Chafekar ND, Iyer S. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: a multicenter randomized controlled study. Intensive Care Med. 2014;40:830-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Ji J, Hong X, Su L, Liu Z. Proteomic identification of hippocalcin and its protective role in heatstroke-induced hypothalamic injury in mice. J Cell Physiol. 2019;234:3775-3789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | He QL, Zhong F, Ye F, Wei M, Liu WF, Li MN, Li QB, Huang WQ, Sun LB, Shu HH. Does intraoperative ulinastatin improve postoperative clinical outcomes in patients undergoing cardiac surgery: a meta-analysis of randomized controlled trials. Biomed Res Int. 2014;2014:630835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Wan X, Xie X, Gendoo Y, Chen X, Ji X, Cao C. Ulinastatin administration is associated with a lower incidence of acute kidney injury after cardiac surgery: a propensity score matched study. Crit Care. 2016;20:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Zhang X, Zhu Z, Jiao W, Liu W, Liu F, Zhu X. Ulinastatin treatment for acute respiratory distress syndrome in China: a meta-analysis of randomized controlled trials. BMC Pulm Med. 2019;19:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4342] [Article Influence: 361.8] [Reference Citation Analysis (45)] |
| 22. | Xu H, Jiao W, Zhang Y, Deng X, Dai R, Chen L. Effects of ulinastatin therapy in emergency severe multiple trauma: A single-center randomized controlled trial. Medicine (Baltimore). 2023;102:e32905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Horváth IL, Bunduc S, Fehérvári P, Váncsa S, Nagy R, Garmaa G, Kleiner D, Hegyi P, Erőss B, Csupor D. The combination of ulinastatin and somatostatin reduces complication rates in acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2022;12:17979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Wang G, Wen J, Wilbur RR, Wen P, Zhou SF, Xiao X. The effect of somatostatin, ulinastatin and Salvia miltiorrhiza on severe acute pancreatitis treatment. Am J Med Sci. 2013;346:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ. 2019;367:l6227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 26. | Swaroop VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA. 2004;291:2865-2868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 177] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Portelli M, Jones CD. Severe acute pancreatitis: pathogenesis, diagnosis and surgical management. Hepatobiliary Pancreat Dis Int. 2017;16:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology. 2013;144:1292-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 29. | Wang GJ, Gao CF, Wei D, Wang C, Ding SQ. Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol. 2009;15:1427-1430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 178] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (5)] |
| 30. | Papachristou GI, Sass DA, Avula H, Lamb J, Lokshin A, Barmada MM, Slivka A, Whitcomb DC. Is the monocyte chemotactic protein-1 -2518 G allele a risk factor for severe acute pancreatitis? Clin Gastroenterol Hepatol. 2005;3:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Siriwardena AK, Mason JM, Balachandra S, Bagul A, Galloway S, Formela L, Hardman JG, Jamdar S. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut. 2007;56:1439-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Vege SS, Atwal T, Bi Y, Chari ST, Clemens MA, Enders FT. Pentoxifylline Treatment in Severe Acute Pancreatitis: A Pilot, Double-Blind, Placebo-Controlled, Randomized Trial. Gastroenterology. 2015;149:318-20.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Liu S, Xu J, Gao Y, Shen P, Xia S, Li Z, Zhang M. Multi-organ protection of ulinastatin in traumatic cardiac arrest model. World J Emerg Surg. 2018;13:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Ma T, Kang C, Shao H, Qi Q, Hu W. Protective effects of ulinastatin on proliferation and cytokine release of splenocytes from rats with severe acute pancreatitis. Eur Surg Res. 2006;38:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Liu T, Liao XZ, Zhou MT. Ulinastatin alleviates traumatic brain injury by reducing endothelin-1. Transl Neurosci. 2021;12:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Yoo JW, Ryu JK, Lee SH, Woo SM, Park JK, Yoon WJ, Lee JK, Lee KH, Hwang JH, Kim YT, Yoon YB. Preventive effects of ulinastatin on post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients: a prospective, randomized, placebo-controlled trial. Pancreas. 2008;37:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Ueki T, Otani K, Kawamoto K, Shimizu A, Fujimura N, Sakaguchi S, Matsui T. Comparison between ulinastatin and gabexate mesylate for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a prospective, randomized trial. J Gastroenterol. 2007;42:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Wei D, Yin C, Lu S, Xiong J, Zhu L, Yan S, Meng R. The effect of insulin pump combined with ulinastatin on the levels of PCT, TG, PTX-3, and CX3CL1 in patients with diabetic ketoacidosis and pancreatitis. Medicine (Baltimore). 2021;100:e25141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (1)] |