Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4397
Peer-review started: March 14, 2023
First decision: April 19, 2023
Revised: April 28, 2023
Accepted: May 19, 2023
Article in press: May 19, 2023
Published online: June 26, 2023
Processing time: 104 Days and 7.3 Hours
Septic arthritis of the manubriosternal joint is a diagnostic challenge due to its rarity and anatomical characteristic. Conventional ultrasound, plain radiographs, and computed tomography are not able to confirm or even suspect arthritis early. Superb microvascular imaging is a new advanced Doppler technique in eva
A 34-year-old immunocompetent woman presented with a fever and a dull ache in the chest radiating to the right arm. Traumatic injury and the most common respiratory and cardiac disorders were ruled out. Blood cultures came back positive for Staphylococcus aureus, and sepsis was confirmed. A small lump was noted on the chest during the first week of hospitalization. Superb microvascular imaging was performed and septic arthritis of the manubriosternal joint was detected. MRI confirmed the diagnosis and showed septic arthritis of the manubriosternal joint with several localized abscesses behind the sternum. The patient was treated for three weeks with intravenous antibiotics and the outcome was favorable: Inflammatory markers became normal, and the lump disappeared. Three months later, the patient was examined for a new episode of mild pain in the sternum and was diagnosed with persistent perichondritis by ultrasound in comparison with MRI.
Superb microvascular imaging is a useful tool for the early diagnosis of septic arthritis of the manubriosternal joint and following-up.
Core Tip: Septic arthritis is a diagnostic challenge and requires a methodical approach. The atypical course and rarity of septic arthritis of the manubriosternal joint are due to its anatomy (symphysis): The cartilaginous joint does not have a typical synovial lined capsule. Radiological imaging plays the most important role in the diagnosis. Chest X-ray and computed tomography is the least specific in the early stages. Superb microvascular imaging allows for clearer detection of synovial hypertrophy and slow flow vascularity at the early and late stages of the disease than power Doppler. Magnetic resonance imaging helps to exclude abscesses in cases with high inflammatory markers or sepsis.
- Citation: Seskute G, Kausaite D, Chalkovskaja A, Bulotaite E, Butrimiene I. Diagnostic use of superb microvascular imaging in evaluating septic arthritis of the manubriosternal joint: A case report. World J Clin Cases 2023; 11(18): 4397-4405
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4397.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4397
Septic arthritis is a severe, life-threatening condition that is still a diagnostic challenge due to its unpredictable, acute onset and course. The incidence of septic arthritis is low, with only 4-10 new cases per 10000 population in Europe[1]. Septic arthritis of the manubriosternal (MS) joint is a rare pathology, with only thirteen cases identified in literature since 1985[2]. All of these cases could be classified into one of three groups: primary with risk factors[3-6], secondary due to other diseases[7-11], and completely idiopathic in immunocompetent patients[12-14]. Accurate and early diagnosis can be challenging and requires a methodic approach with various diagnostic measures. Early diagnosis and treatment can circumvent the need for surgical intervention. Sternal radiographs and/or computed tomography (CT) scans are the first usually performed tests, but the least sensitive in the early stages[6,7,8-11,13,14]. Also, it is often mistaken for another disease process such as cardiac or pulmonary causes of chest pain and a lot of other tests are performed in parallel[4,10,13]. Due to wide differential diagnosis, magnetic resonance imaging (MRI) performing is commonly delayed[4,5,10,13]. Also, the use of MRI is often prohibited because of its high cost and limited availability. There is only one case that demonstrates the value of ultrasound for evaluating septic arthritis of the MS joint with massive effusion by B mode, but at the early stage of the disease[12].
Ultrasound imaging is increasingly effective in the diagnosis of musculoskeletal conditions when done by an experienced operator. It has many advantages including real-time and cross-sectional imaging with excellent spatial resolution. Compared to MRI, ultrasound assesses joint effusion, the severity of the Doppler-positive synovitis, and the presence of bone and cartilage damage or irregularity in the joint. Conventional Doppler techniques have limitations in detecting low-velocity blood flow[15]. Superb microvascular imaging (SMI) is a new Doppler technique that suppresses the noise caused by motion artifacts with an innovative filter system without removing the weak signal arising from small vessel blood flow, thus achieving a greater sensitivity than power Doppler (PD)[16,17]. SMI presents two modes: Color (cSMI, which demonstrates B-mode and color information simultaneously) and monochrome (mSMI, which focuses only on the vasculature). Growing evidence indicates that SMI imaging could provide a non-invasive and lower-cost tool for the assessment of inflammatory arthritides. There is limited data available on the use of SMI in evaluating septic arthritis.
Following is a case presentation of a young, previously healthy woman with severe chest pain without any clinical signs of arthritis. The role of SMI in the early diagnosis and follow-up of the patient is discussed.
In July 2021, a 34-year-old woman came to the emergency department with a five-day history of fever and a dull ache in the chest radiating to the right arm.
She visited her family doctor one week before because the ache in the chest was given a visual analogue scale score of 9-10 and gradually worsened. There was no redness or irregularity in the skin of the chest area. Blood tests were normal. She was given nonsteroidal anti-inflammatory drugs and opioids for pain relief, but the condition did not improve, therefore she came to our hospital for emergency treatment.
There is no relevant history of past illness, intravenous drug abuse, inflammatory joint alterations, trauma, and spreading from a source of infection (dental, skin areas).
The patient was a non-smoker. She claimed to have no allergies to food or medicines, no operations were performed. The patient denied any family history of arthritis.
Body mass index - 22.4 kg/m2. The vital signs were as follows: Body temperature - 36.9℃; blood pressure - 98/60 mmHg; heart rate - 100 beats per minute; respiratory rate - 19 breaths per min. During palpation, there were no swollen or painful lumps and lymph nodes in the typical axilla, neck, or groin areas; the chest was painless.
Primary laboratory tests showed significantly increased inflammatory markers: C-reactive protein (CRP) 256.0 mg/L (Ref ≤ 5 mg/L), leukocytosis white blood cells (WBCs) - 14.20 × 109/L (Ref from 4.5 to 11.0 × 109/L); elevated procalcitonin 0.69 µg/L (Ref < 0.1 ng/mL) and liver enzymes: Aspartate aminotransferase - 239 U/ L (Ref ≤ 40), alanine aminotransferase - 334 U/L (Ref ≤ 40); normal troponin - 0 ng/L (Ref ≤ 16), and elevated D-dimers 1725 µg/L (Ref < 250 µg/L). No abnormality was found in urine analyses.
The electrocardiogram showed only sinus tachycardia - 103 bpm. Pulmonary embolism, aortic aneurysm, pneumonia, pneumothorax, and even rib fracture were suspected. Thoracic CT angiography, non-contrast chest CT, and X-ray of the thoracic spine, ribs, and sternum were performed, as well as diagnostic ultrasound with PD (by an abdominal sonographer) of the soft tissues at the sternum, but no pathology was detected.
The patient was hospitalized and an empiric course of intravenous antibiotic treatment (amoxicillin/clavulanic acid 1.2 g, q.i.d) was prescribed. During the second day of hospitalization, the blood culture showed growth of methicillin-susceptible Staphylococcus aureus, and sensitivity for empiric treatment was confirmed.
The patient was diagnosed with sepsis (fever, tachycardia, and leukocytosis), though the cause was unknown.
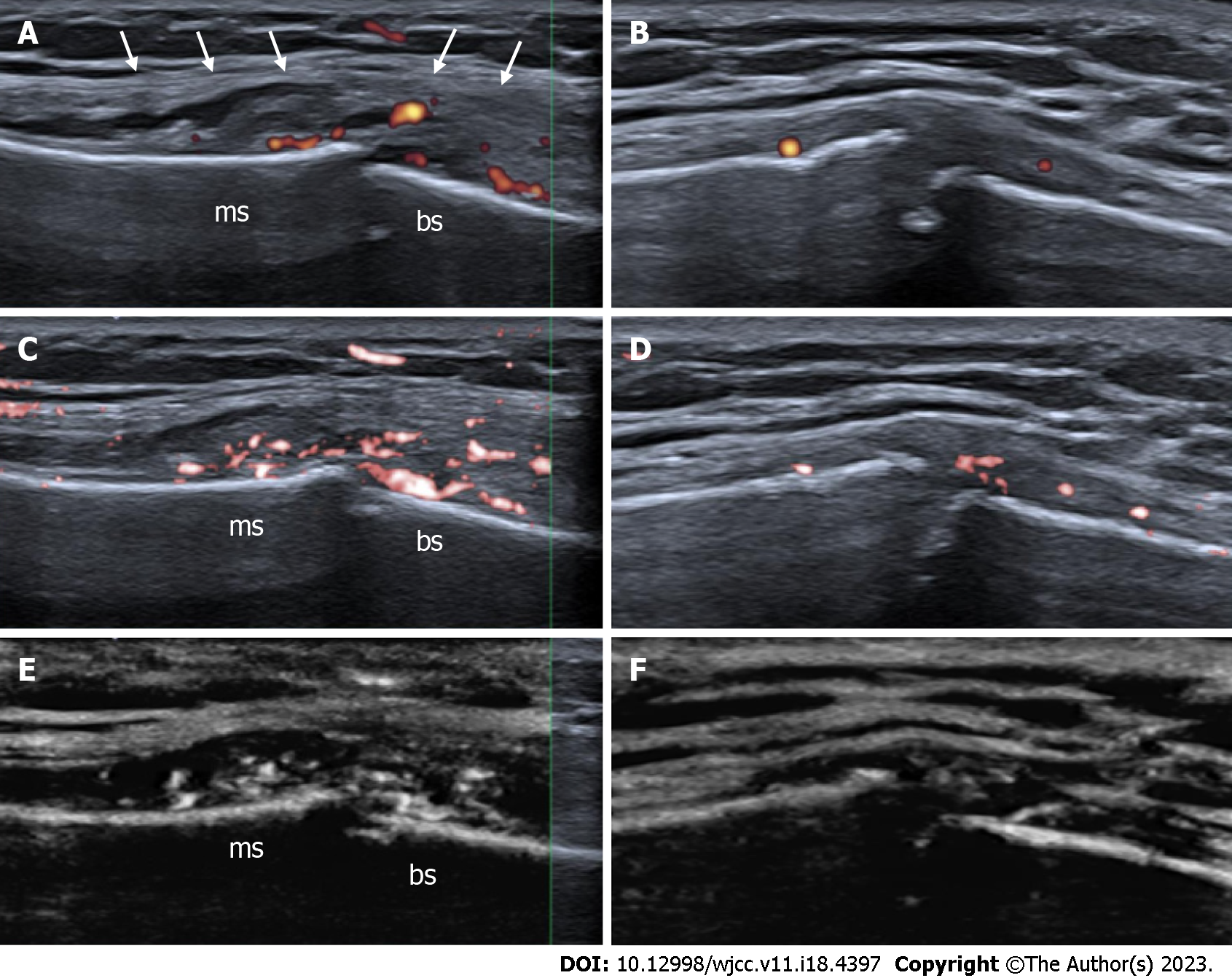
During the third day of hospitalization, the patient experienced numbness on the left side of the face and began to feel dizzy. She felt a small and painless lump in the MS joint region with overlying erythema (Figure 1A and B). The pain in the chest progressed. She was consulted by a neurologist, who recommended CT angiography of the head to detect any ischemic changes due to venous sinus thrombosis or carotid artery dissection. However, the CT angiography was normal and the dizziness disappeared the next day. Serological testing for human immunodeficiency virus and hepatitis (B, and C virus) was negative. Infective endocarditis was ruled out by a transthoracic echocardiogram at the end of the first week of hospitalization. No other findings in the transthoracic echocardiogram were detected. The first soft tissue diagnostic - ultrasound in the sternum did not show any changes, but it was repeated five days later by a rheumatologist, and active MS joint arthritis was detected (Figure 2A, C and E). The pseudo-capsular layer formed by surrounded ligaments and soft tissues became separated from the irregular bone surface by intra-articular effusion and synovial hypertrophy with remarkable PD signals suggestive of active MS arthritis (Figure 2A). Colour SMI modes show higher vascularity (Figure 2C), and monochrome SMI focuses only on the vasculature, excluding artifacts (Figure 2E). A joint puncture was not performed due to minor fluid accumulation (approximately 3 mm × 0.6 mm in size).
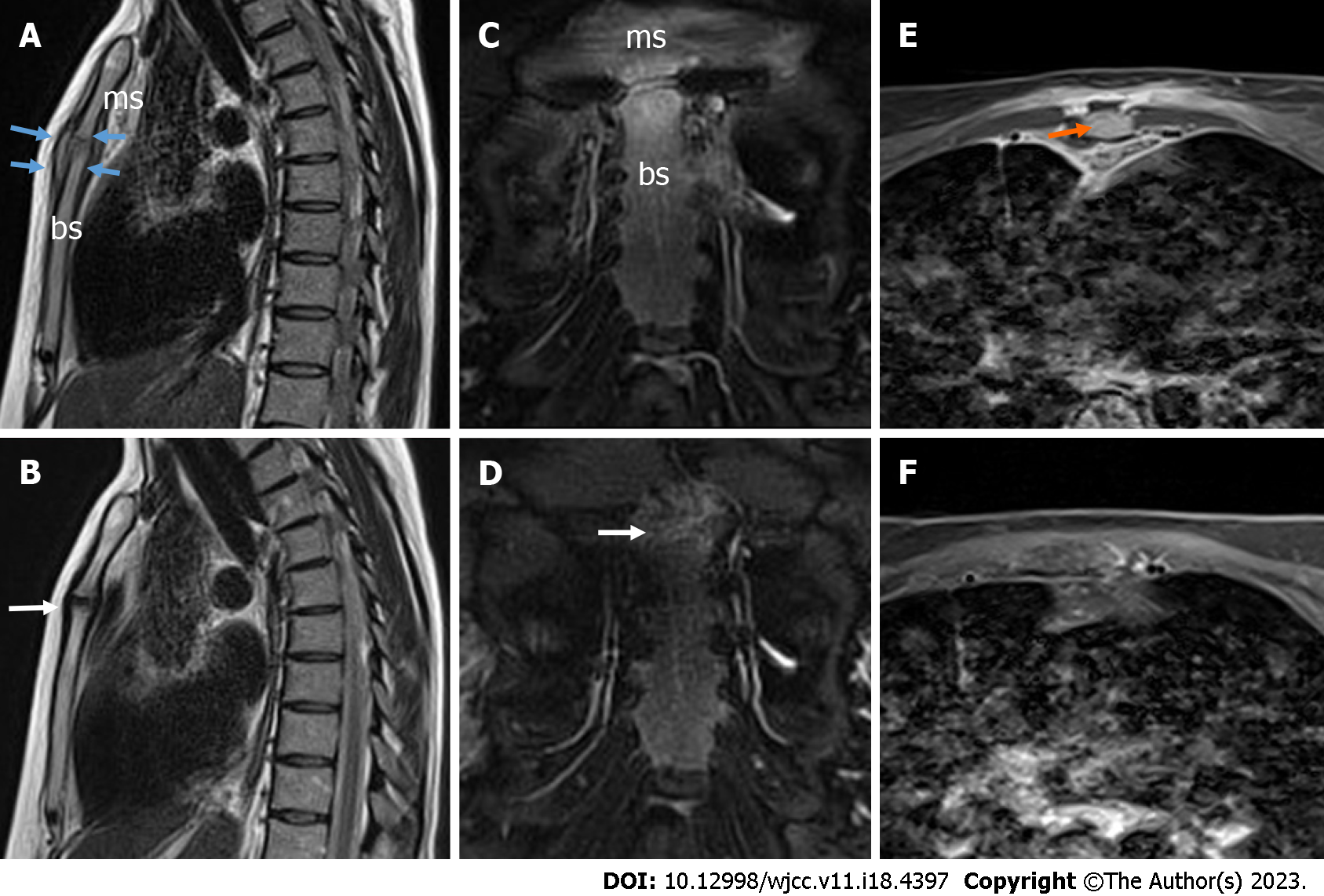
Axial post-gadolinium contrast-enhanced sternal MRI was performed to rule out mediastinitis and showed signs of sternal arthritis with localized abscesses up to 12 mm × 4 mm × 44 mm in front of the left first rib and up to 8 mm × 5 mm × 18 mm in the MS joint (Figure 3A, C and E). After 10 days of antibiotic treatment, the patient’s condition improved, with significantly less chest pain and decreased inflammatory markers (CRP 8.77 mg/L). The repeated blood cultures were negative.
We diagnosed the patient with MS septic arthritis complicated by an abscess and sepsis.
She was advised to continue amoxicillin/clavulanic acid perorally for two weeks after hospitalization.
Three months later, the patient began feeling mild pain in the MS region. During the physical examination, no objective signs of relapse were observed. X-ray of the sternum showed perichondritis and ultrasound imaging detected mild and residual joint synovial hypertrophy with vascularity and more eroded MS joint margins (Figure 2B, D and F). MRI showed positive dynamics (Figure 3B, D and F) with no signs of abscesses and confirmed ultrasound results because mild perichondritis with mild bone marrow was detected. Inflammatory markers were normal (CRP - 3.69 mg/L, WBCs - 7.81 × 109/L). A decision was taken to prescribe a three-week course of antibiotics (doxycycline 100 mg b.i.d) to avoid the relapsing course of arthritis.
Septic arthritis usually presents with acute onset monoarticular joint pain and swelling, but in the present case, arthritis began without any objectively classical signs. The atypical course and rarity of septic arthritis in the MS joint are due to its anatomy: Being a symphysis, the rates of septic arthritis are massively lower than in synovial joints[18]. The joint does not have a capsule and is not attached by any ligaments[19]. Sometimes, this joint is mistakenly described as a capsulated joint, which is anatomically incorrect. The pectoralis major and pectoralis fascia originate from the anterior surface of the sternum, and therefore, include the sternal angle[20]. It forms a pseudo capsule because it is separated from the irregular bone surface by intra-articular synovial hypertrophy and/or inflammatory fluid retention, as well as effusion. This explanation reveals the causes of mild and atypical clinical manifestations of arthritis.
Because of the rarity of this condition, little is known about its pathogenesis, and there are many possible causes. MS joint arthritis is common in healthy males with an average age of 44 and risk factors such as sports like rugby or working as a disc jockey[5,12]. Septic arthritis of the MS joint in an immunocompetent patient without any suspicion of trauma is a very rare phenomenon and there is only one published case[13]. Other causes that are written up are intravenous drug use and systemic lupus erythematosus[2,3,8]. Our patient was immunocompetent without any risk factors. The wide differential diagnosis of acute pain in the chest often delays clear diagnosis[21].
History and clinical examination findings alone have been found to be poorly sensitive for the diagnosis of acute septic arthritis[22]. Radiological imaging plays the most important role in the diagnosis of MS arthritis. Chest X-ray is the least specific in the early stages. CT scans are more likely to be positive at an advanced stage. Our case demonstrates the value of CT in the early stage of arthritis: only mild marginal erosions could be suspected and depend on the radiologist's accuracy. The findings were too mild to consider as a reason for strong pain in the chest. Ultrasound can detect early changes and be used to guide joint aspiration procedures but is often unsuccessful due to the paucity of fluid in the small MS joint[23]. Ultrasound is a cheaper, quicker, and more easily accessible tool for early diagnosis than MRI or CT.
Although ultrasound imaging of septic arthritis cannot be used as an absolute diagnostic modality, it enables early identification of both intra-articular and extra-articular abnormalities before significant cartilage lysis occurs. Ultrasound imaging or greyscale ultrasound of an intra-articular anechoic effusion of multiple internal echoes with distention of the joint capsule is highly suggestive of septic arthritis of the synovial joints[24,25]. The thickness of the synovia and the joint capsule is not specific to septic arthritis, but intra-articular synovial hypertrophy with remarkable PD signals suggests acute active synovitis[26,27]. Hypoechogenicity of surrounding muscles with loss of normal muscular fibrillar pattern could be suggestive of early muscle affection[25]. The early appearance of bone erosion of the joint suggests that arthritis is suppurative[27]. To sum up, ultrasound findings such as joint effusion, intra-articular synovial hypertrophy with remarkable PD signals, and irregularity of the bony margins may support the clinical suspicion of infection[24-27].
Apart from the MS joint, there are more symphyses such as pubic, intervertebral, sacrococcygeal, mentalis, and xiphisternal. The pubic symphysis is readily visualized with ultrasound, yet the sonographic findings of septic arthritis in the joint have not been documented[28]. Septic arthritis of the pubic symphysis is mostly suspected or diagnosed by CT or MRI scans of the pelvis. Pelvic radiographs are relatively insensitive for the diagnosis of septic arthritis and osteomyelitis of the symphysis pubis, especially early in the course of the disease[29]. The lack of experience in the evaluation of symphyses by ultrasound could be explained by the rarity, atypical course of the disease, and mimicry of other diseases.
SMI allows for clearer detection of synovial hypertrophy and active vascularity at an early stage of the disease than conventional PD. All possible ultrasound changes according to the literature[1,12,24-27], our own experience, and this case data are summarised in Table 1 (we recommend additional studies to determine the sensitivity and specificity of SMI in diagnosing septic arthritis). At the early stage of confirming a diagnosis, vascularity needs to be checked by an experienced musculoskeletal sonographer. MRI plays the most important role in excluding abscesses connected to the joint, especially when high inflammatory markers are found. As our case showed, MRI helps to exclude complications of arthritis and must be performed in cases with sepsis or high inflammatory markers.
| Disease course | |||
| Early - acute | Subacute | Chronic residual | |
| Effusion | +++ | +++ | -/+ |
| Synovial thickening | + | +++ | +/++ |
| Active vascularity: | |||
| PD | -/+ | ++ | -/+ |
| SMI | ++ | +++ | +/++ |
| Bone erosions | -/+ | ++/+++ | +++ |
Regarding management, in the early stages of the disease, empiric administration (mostly penicillins according to the literature[13] or a combination[12]) of intravenous antibiotics is the mainstay until the response of blood or synovial fluid cultures. Intravenous antibiotics with joint drainage are indicated for more than 60% of cases[30]. Synovial thickening, cellulitis, and bone edema may persist even after the eradication of the infection[30]. After three months we performed an ultrasound with MRI due to the risk of a persistent abscess. Both investigations confirmed late erosions in the joint margins and mild arthritis. However, if the MRI at the early stage did not detect an abscess, then ultrasound imaging in combination with a chest X-ray could be sufficient for the follow-up, therefore more studies are needed.
The case demonstrates the role of a wide spectrum of radiological tests in evaluating rare pathology - septic arthritis of the manubriosternal joint and the use of advanced ultrasound for follow-up persistent changes. Furthermore, ultrasound findings of septic arthritis of the manubriosternal joint are analyzed in detail in all stages of the disease. There is a lack of information in the literature about the management of septic arthritis of symphysis by ultrasound, the best choice of investigation in the differential diagnosis from early to late disease course and follow-up of these patients. Ultrasound provides the clinician with tools to promptly manage such a case, especially when more costly methods are not available. There is a task to accrue more data on ultrasound use for practical application in septic arthritis generally and purulent process in symphyses.
SMI in comparison with other radiological tests demonstrates the diagnostic usefulness for the early diagnosis and follow-up monitoring of patients with septic arthritis of the MS joint, especially in the subclinical case at the beginning of the disease, and for screening in the late course. It provides objective findings that assist a clinician in making a time-sensitive diagnosis.
Authors would like to thank the patient and Vilnius University Hospital Santaros Clinics for giving informed consent and providing the images for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: EMEUNET; Lithuanian Rheumathologists Association.
Specialty type: Rheumatology
Country/Territory of origin: Lithuania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mahmoud MZ, Saudi Arabia; Rezus E, Romania S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Gaigneux E, Cormier G, Varin S, Mérot O, Maugars Y, Le Goff B. Ultrasound abnormalities in septic arthritis are associated with functional outcomes. Joint Bone Spine. 2017;84:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Gruber BL, Kaufman LD, Gorevic PD. Septic arthritis involving the manubriosternal joint. J Rheumatol. 1985;12:803-804. [PubMed] |
| 3. | López-Longo FJ, Monteagudo I, Vaquero FJ, Martinez Moreno JL, Carreño L. Primary septic arthritis of the manubriosternal joint in a heroin user. Clin Orthop Relat Res. 1986;230-231. [PubMed] |
| 4. | Sinha S, Sinha A, Nagarajah K, Oei EL, Critchley P, McNally MA. Chronic sternal osteomyelitis complicating primary manubriosternal septic arthritis. Clin Rheumatol. 2006;25:934-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Peng EW, McKillop G, Prasad S, Walker WS. Septic arthritis of the manubriosternal joint. Ann Thorac Surg. 2007;83:1190-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Nwaejike N, Unsworth-White MJ. Manubriosternal subluxation/dislocation can lead to manubriosternal septic arthritis in patients with kyphoscoliosis. Ann R Coll Surg Engl. 2010;92:W35-W37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Van Linthoudt D, De Torrente A, Humair L, Ott H. Septic manubriosternal arthritis in a patient with Reiter's disease. Clin Rheumatol. 1987;6:293-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Kruper LL, Low DW, Bucky LP. Immediate pectoralis flap closure following septic arthritis of the manubriosternal joint. Plast Reconstr Surg. 2001;107:997-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Patel P, Gray RR. Tuberculous osteomyelitis/arthritis of the first costo-clavicular joint and sternum. World J Radiol. 2014;6:928-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Zaheen A, Siemieniuk RA, Gudgeon P. An atypical cause of atypical chest pain. Can J Infect Dis Med Microbiol. 2014;25:253-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Gorospe L, Ayala-Carbonero AM, Rodríguez-Díaz R, García Latorre R, Muñoz-Molina GM, Cabañero-Sánchez A. Tuberculosis of the manubriosternal joint and concurrent asymptomatic active pulmonary tuberculosis in a patient presenting with a chest wall mass. Clin Imaging. 2015;39:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Carnevale A, Righi R, Maniscalco P, Labaj O, Occhionorelli S, Benea G, Giganti M. Primary septic arthritis of the manubriosternal joint in an immunocompetent young patient: A case report. Radiol Case Rep. 2017;12:682-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | O'Connor A, Furtado S. Septic arthritis of the manubriosternal joint presenting as a chest-wall swelling in an immunocompetent patient. J Surg Case Rep. 2019;2019:rjz261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Honda H, Yaita K. Suppurative Arthritis of the Manubriosternal Joint. Intern Med. 2019;58:2903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Park AY, Seo BK. Up-to-date Doppler techniques for breast tumor vascularity: superb microvascular imaging and contrast-enhanced ultrasound. Ultrasonography. 2018;37:98-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Fu Z, Zhang J, Lu Y, Wang S, Mo X, He Y, Wang C, Chen H. Clinical Applications of Superb Microvascular Imaging in the Superficial Tissues and Organs: A Systematic Review. Acad Radiol. 2021;28:694-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 18. | Ross JJ, Shamsuddin H. Sternoclavicular septic arthritis: review of 180 cases. Medicine (Baltimore). 2004;83:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Monkhouse WS. Last’s Anatomy, Regional and Applied. 10th ed. Sinnatamby C, editor. Edinburgh: Churchill Livingstone. 1999: 539. |
| 20. | Gray Henry T, Pickering P, Robert H. Anatomy, descriptive and surgical. 39th ed. New York: Bounty Books, 1977. |
| 21. | Long B, Koyfman A, Gottlieb M. Evaluation and Management of Septic Arthritis and its Mimics in the Emergency Department. West J Emerg Med. 2019;20:331-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Carpenter CR, Schuur JD, Everett WW, Pines JM. Evidence-based diagnostics: adult septic arthritis. Acad Emerg Med. 2011;18:781-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 23. | Womack J. Septic arthritis of the sternoclavicular joint. J Am Board Fam Med. 2012;25:908-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Kim S, Baradia H, Sambasivan A. The Use of Ultrasonography in Expediting Septic Joint Identification and Treatment: A Case Report. Am J Phys Med Rehabil. 2020;99:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Manar AB, Hanan AH, Wesam AM. Role of high resolution ultrasonography in diagnosing septic hip arthritis in premature neonates admitted to the neonatal intensive care unit. Egypt J Radiology Nucl Med. 2017;48:971-975. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Mnif J, Khannous M, Keskes H, Louati N, Damak J, Kechaou MS. [Ultrasonography in the diagnostic approach of septic arthritis]. Rev Chir Orthop Reparatrice Appar Mot. 1997;83:148-155. [PubMed] |
| 27. | Kawashiri SY, Edo Y, Kawakami A. Early Detection of Inflammation and Joint Destruction Revealed by Ultrasound in a Patient with Sternoclavicular Septic Arthritis. Intern Med. 2019;58:865-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Becker I, Stringer MD, Jeffery R, Woodley SJ. Sonographic anatomy of the pubic symphysis in healthy nulliparous women. Clin Anat. 2014;27:1058-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 29. | Ross JJ, Hu LT. Septic arthritis of the pubic symphysis: review of 100 cases. Medicine (Baltimore). 2003;82:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Bierry G, Huang AJ, Chang CY, Torriani M, Bredella MA. MRI findings of treated bacterial septic arthritis. Skeletal Radiol. 2012;41:1509-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (1)] |