Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4210
Peer-review started: January 10, 2023
First decision: March 26, 2023
Revised: March 31, 2023
Accepted: April 14, 2023
Article in press: April 14, 2023
Published online: June 26, 2023
Processing time: 167 Days and 17.6 Hours
Cannabis, commonly known as marijuana, is a drug extracted from the Cannabis plant known for its psychotropic and medicinal properties. It has been used for healing purposes during ancient times, although its psychoactive components led to its restricted use in medicine. Nonetheless, cannabis is found to have modulatory effects on the endocannabinoid system exhibiting its medicinal role in the gastrointestinal (GI) system. Emerging animal and human studies demon
Core Tip: Cannabis is becoming increasingly popular in the management of a variety of gastrointestinal disorders due to its active role in the endocannabinoid system. It provides anti-inflammatory, anti-emetic and analgesic effects indicating its potential use in treatment and symptom control. There is rising evidence on the therapeutic efficacy and short-term safety profile of cannabis but its long term safety profile remains to be explored. Before gastroenterologists consider prescribing medical marijuana, gaining understanding of the benefits and associated risks and having an open and individualized discussion with patients are essential.
- Citation: Samuel S, Michael M, Tadros M. Should gastroenterologists prescribe cannabis? The highs, the lows and the unknowns. World J Clin Cases 2023; 11(18): 4210-4230
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4210.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4210
Cannabis, also known as marijuana, is a substance derived from the cannabis plant consisting of three species known as Cannabis sativa, Cannabis indica and Cannabis ruderalis. The plant was well-known during the ancient times where it was traditionally used for therapeutic use in many countries. It was used for its anti-inflammatory, anti-septic and anti-epileptic properties written in the Ayurvedic texts from India. The Chinese culture would incorporate it in their tea for the treatment of gout, malaria, rheumatologic disorders and neuropathic pain. Marijuana would be given as a ceremonial sacrifice for Jewish priests[1]. In addition, Ancient Egypt and China utilized cannabis for the treatment of gas
Although three species exist, marijuana is typically extracted from Cannabis sativa. It contains 60 aromatic hydrocarbon compounds known as cannabinoids. Two of the sixty compounds are often used and advertised: Delta-9-tetra-hydrocannabinoil (THC) and cannabidiol (CBD). Each strain of cannabis consists of varying compositions with some higher in CBD while others contain more THC. THC is frequently used for its psychotropic effects while CBD can aid with controlling pain, inflammation and improving motility[1,2].
Cannabis has a complex history in the western culture due to both health and political concerns. It is currently classified as an illegal substance under federal law in the United States. The first cannabinoid receptor was identified in St. Louis, Missouri, United States in 1988 called cannabinoid receptor type 1 (CB1) followed by the discovery of cannabinoid receptor type 2 (CB2) in Cambridge, United Kingdom in 1993[3,4]. Anandamide was the first cannabinoid that was discovered in Israel in 1992, thus forming the endocannabinoid system (ECS)[5]. Since then, over 500 additional cannabinoids which function as neurotransmitters have been identified from the cannabis plant including THC and CBD. In the United States, THC was first authorized for medical use by the Food and Drug Administration (FDA) in 1986 for cancer patients who experienced chemotherapy induced nausea and vomiting or required appetite stimulants. The federal legalization of medical and recreational cannabis remains a divisive political topic but its use has become more widespread as more states continue to legalize the substance.
THC and CBD interact with two receptors, CB1 and CB2. CB1 and CB2 are G-protein-coupled receptors (GPR) found throughout the body but have varying effects that are still being elucidated in ongoing trials[6]. The main neurotransmitter that interacts with CB1 is anandamide and the key neurotransmitter that interacts with CB2 is 2-arachidonoylglycerol[5,7].
CB1 receptors are commonly associated with the psychotropic effects of cannabis and are found in high concentrations in central neurons, including the cortex, hippocampus, basal nuclei and amygdala, and peripheral neurons, including the enteric nervous system and vagal and spinal neurons[3,6]. Large concentrations of CB1 receptors were found in the colonic epithelium, smooth muscle and submucosal myenteric plexus using immunohistochemistry[8]. Aside from psychotropic effects, the activation of CB1 receptors can affect multiple processes including appetite stimulation, pain perception, metabolism and alterations in GI motility, contractility and secretions[9].
CB2 receptors are tightly associated with the immune system and inflammation. They are present in high concentrations in multiple cell lines including macrophages, neutrophils and some B and T lymphocytes[4]. CB2 receptors, when activated, are associated with reduced inflammation and pain and decreased intestinal motility.
THC and CBD carry a similar structure to naturally occurring endogenous neurotransmitters, giving them various effects and potencies. Portions of THC mimic the active binding sites of the endogenous cannabinoid anandamide and CBD similarly mimics 2-arachidonoylglycerol[5,7]. THC is a partial CB1 and CB2 agonist that is available in varying concentrations from 0.5-90+% depending on the product and modality. Metabolites of THC are commonly tested for in urine studies but can also be tested in hair and blood.
CBD effects on CB1 and CB2 receptors promote anti-inflammatory effects. CBD binds weakly to CB1 and CB2 receptors leading to strong antagonist effects, potentially explaining decreased inflammation through inverse agonism of immune cell activation[10]. CBD products are available in varying concentrations from 0.5-90+% depending on mechanism of use and is more readily available as it is not defined as a controlled substance.
The ECS is moderated by lipid-based neurotransmitters in a complicated network of endogenous neurotransmitters and various receptors, including CB1 and CB2, and has effect on the enteric system[8]. Interestingly, the neurotransmitters act in a retrograde direction, meaning they are produced at the post-synaptic membrane and bind to receptors on the pre-synaptic membrane[11]. Cannabinoids are one of the most common retrograde neurotransmitters in the human body[12].
Activation of CB1 in the ECS results in an inhibitory effect on neurons via reduced acetylcholine release[13]. The reduction in acetylcholine can decrease gut motility or secretion and visceral pain sensation, which play a large role in symptom management including nausea, pain or diarrhea. Activation of CB2 is noted to be elevated in inflamed tissues, positing that CB2 plays an immunomodulatory role in gut inflammation[8]. There are also endocannabinoid-like receptors that have been discovered that fall under GPR including transient receptor potential vanilloid 1, peroxisome proliferator-activated receptor α, GPR55, GPR119, among others[9]. In addition to endocannabinoid-like receptors, endocannabinoid-like compounds such as N-palmitoylethanolamine and N-oleoylethanolamine stimulate similar receptors, resulting in anti-inflammatory and analgesic effects[14,15]. Studies demonstrate a reduction in inflammatory cytokines in cannabis users through the activation of its receptors[16,17].
There are many commercially available modalities on the market for use of cannabis and its concentrates. Types of cannabis include cannabis or cannabinoid derivatives (inhaled and oral THC), cannabis derivatives including synthetic THC (dronabinol) or endocannabinoid ligands (palmitoylethanolamide) and phytocannabinoids (CBD oil). The different modes of application are visualized in Figure 1. Inhalation which is available through smoking cannabis flower, cannabis concentrates and vaping THC and/or CBD distillate, is a very common option, especially for patients with severe nausea. Effects after inhalation peak approximately 30 min after use and decrease over the following 2-3 h. Ingestion is another mode of use, available in commercial edibles such as candy or chocolate as well as cannabis infused products such as butter or beverages as well as tinctures. Onset after ingestion normally begins 30-90 min from intake, peaks at 2-3 h after intake and is slowly metabolized over 4-12 h.
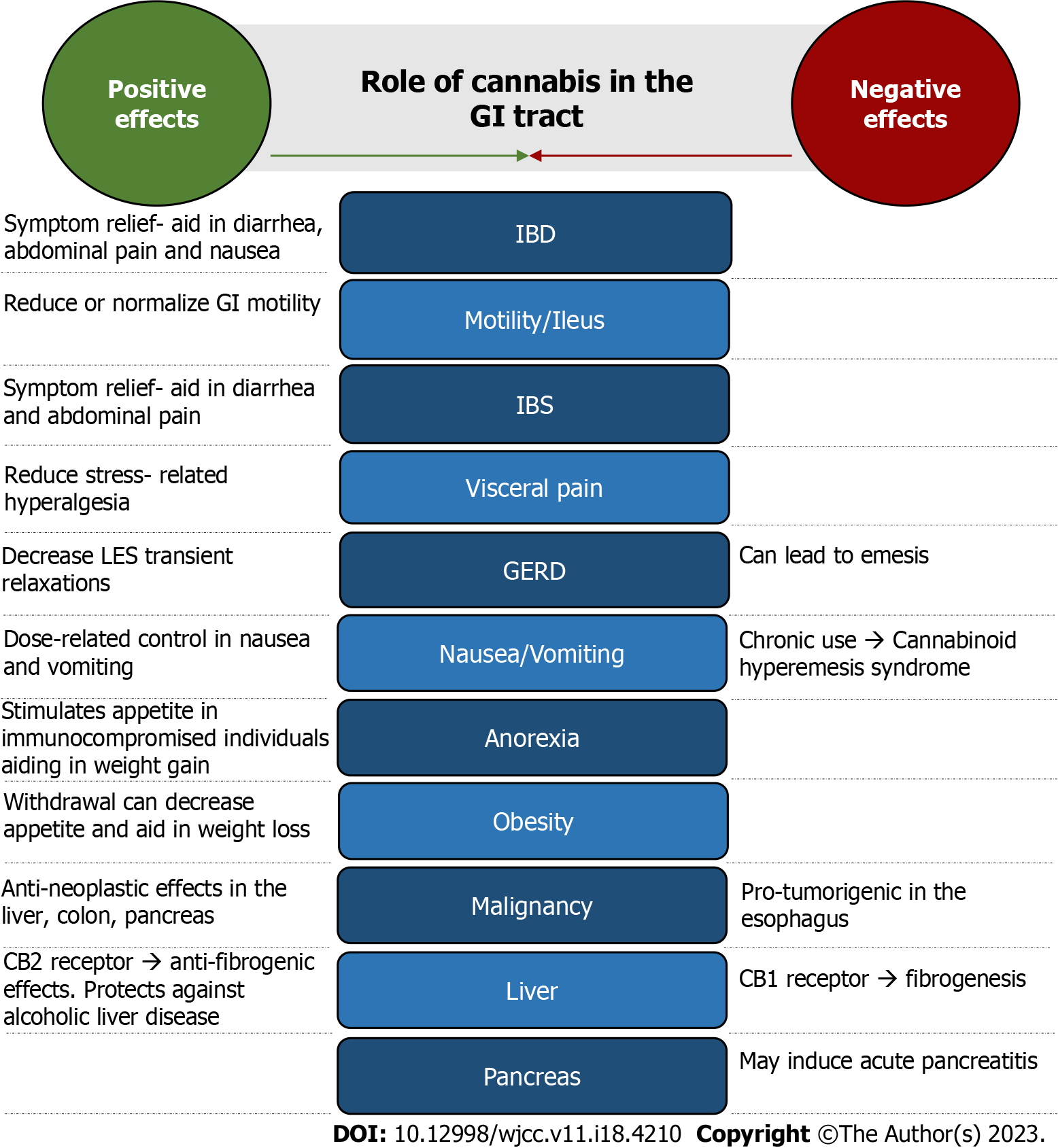
Cannabis is becoming more accepted and widespread for medicinal use. It shows therapeutic benefit in psychiatric and neurologic conditions as well as some GI disorders. Its effects on the GI tract play an important role in a variety of digestive disorders including inflammatory bowel disease (IBD), GI motility, irritable bowel syndrome (IBS), cachexia and anorexia, neoplastic conditions and many more (Figure 2). However, adverse side effects of marijuana are evident including its role in cognition and addictive properties. The long-term safety profile of cannabis is underrecognized and needs further investigation. We conducted an extensive literature review by searching PubMed and Google Scholar databases for current evidence on cannabis effects on GI disorders and symptoms. Citation mining was also utilized. We included 45 studies consisting of surveys, clinical trials, cohort and observational studies and meta-analyses to assess the outcomes and type and modality of cannabis used (Table 1). Additional evidence such as animal models, systematic reviews and case reports were also compared.
| Ref. | GI disorders/symptoms | Study type | Number of subjects | Cannabis type and modality | Outcome |
| Irving et al[32], 2018 | Ulcerative colitis | RCT | 60 | CBD-rich extract (3.2%-4.7% THC) 500 mg/day | Improvement in quality of life, symptoms |
| Naftali et al[148], 2018 | Ulcerative colitis | RCT | 28 | Cannabis cigarettes (11.5 mg THC; 23 mg THC/day daily) | Endoscopic and clinical improvement in moderately active ulcerative colitis |
| Naftali et al[21], 2011 | Crohn’s disease | Retrospective observational | 30 | Inhaled or oral cannabis | Reduction in disease activity index, need for other drugs and surgeries |
| Naftali et al[28], 2013 | Crohn’s disease | Prospective RCT | 21 | Cannabis sativa cigarette (23% THC, 0.5% cannabidiol) | Significant clinical response, no decrease in CRP |
| Naftali et al[29], 2017 | Crohn’s disease | RCT | 19 | Oral CBD 10 mg BID | CBD safe but no beneficial effect |
| Naftali et al[31], 2021 | Crohn’s disease | RCT | 56 | CBD rich oil 160/40 mg/mL (CBD/THC); placebo | Clinical and quality of life improvement without change in inflammatory markers or endoscopic scores |
| Naftali et al[25], 2019 | IBD | Prospective | 127 | 30 g per month or THC 21 mg and CBD 170 mg daily | Clinical improvement, reduced use of medication and slight weight gain |
| Lahat et al[20], 2012 | IBD | Prospective | 13 | Inhaled cannabis 50 g/month | Improved quality of life measurements and disease activity index and weight gain |
| Lal et al[23], 2011 | IBD | Cross-sectional survey | 291 | Cannabis (reported) | Used for symptom relief particularly those with history of abdominal surgery, low quality of life index and/or chronic abdominal pain |
| Storr et al[24], 2014 | IBD | Cross-sectional survey | 313 | Cannabis (reported) | Pain and diarrheal relief but associated with higher risk of surgery |
| Hoffenberg et al[149], 2019 | IBD | Prospective/descriptive | 15 | Oral or sublingual cannabis oil plus other forms (varying contents CBD:THC) | Adolescents and young adults improvement in sleep. Nausea and appetite. No weight gain. |
| Kerlin et al[27], 2018 | IBD | Survey | 1666 | Cannabis (reported) | Improvement in pain, appetite and anxiety but had higher baseline anxiety, IBD symptoms and pain that nonusers |
| Desai et al[30], 2019 | IBD | Retrospective | 7483 | Cannabis (reported) | Decreased length of hospital stay. In Crohn’s disease, lower need for parenteral nutrition but increased risk of intrabdominal abscess or active fistulizing disease |
| Dalavaye et al[22], 2023 | IBD | Prospective case series | 76 | Oral, sublingual or vaping. THC and CBD variable dosages | Improved quality of life including sleep and anxiety |
| Bateman[37], 1983 | Motility | RCT | 7 | THC 0.5 or 1 mg injections | No significance in gastric emptying of liquid |
| McCallum et al[35], 1999 | Motility | RCT | 13 | THC 10 mg/m2 of body surface area (unclear route) | Significant delay in gastric emptying with solid food but no correlation between plasma THC level and delay in gastric emptying |
| Klooker et al[43], 2011 | IBS | RCT | 22 | Oral THC: DRO 5 and 10 mg | No alteration in baseline visceral perception in rectal distension |
| Wong et al[45], 2011 | IBS | RCT | 75 | Oral THC: DRO 2.5 and 5 mg | Increase in colonic compliance, decrease colonic motility index in IBS-D and IBS-A, no effect on sensation or tone |
| Wong et al[44], 2012 | IBS | RCT | 36 | Oral THC: DRO 2.5 and 5 mg BID | No effect on gut transit. DRO delays colonic transit in those with CNR1 genotype variant |
| Patel et al[46], 2020 | IBS | Retrospective study | 31272 | Cannabis (reported) | Higher odds of IBS-hospitalizations and rising trend of cannabis use and related psychiatric comorbidities |
| Desai et al[42], 2020 | IBS | Retrospective study | 9363 | Cannabis (reported) | Decreased healthcare utilization and costs |
| Choi et al[150], 2022 | IBS | Retrospective study | 7163 | Cannabis (reported) | No difference in readmission rates for IBS-specific causes for cannabinoid users and non-users |
| Beaumont et al[51], 2009 | GERD | RCT | 18 | Oral THC: DRO 10 mg/20 mg | Inhibits post-prandial increase in transient LES relaxation and reduces LES basal pressure |
| Smith et al[56], 2015 | Nausea and vomiting | Meta analysis of 23 studies | N/A | Cannabis (reported) | Cannabinoids are effective in treating nausea and vomiting but not concluded as superior to traditional anti-emetics |
| Grimison et al[60], 2020 | Nausea and vomiting | RCT | 81 | Oral THC 2.5 mg/CBD 2.5 mg | Less nausea and vomiting but additional side effects in chemotherapy induced nausea and vomiting |
| Jatoi et al[72], 2002 | Cancer-related anorexia/cachexia | RCT | 469 | Oral THC: DRO 2.5 mg BID | Megestrol acetate was superior to anorexia palliation compared to dronabinol alone |
| Strasser et al[71], 2006 | Cancer-related anorexia/cachexia | RCT | 164 | Oral cannabis extract (2.5 mg THC and 1 mg cannabidiol); or oral 2.5 mg THC | No difference in appetite or quality of life in cancer patients |
| Brisbois et al[70], 2011 | Cancer-related anorexia/cachexia | RCT | 21 | Oral DRO 2.5 mg BID | Improved chemosensory perception, improved appetite and increased protein caloric intake |
| Foltin et al[151], 1998 | Weight gain | RCT | 6 | Smoked marijuana cigarettes (2.3% THC) | 40% increase in daily caloric intake due to increased snacking |
| Timpone et al[69], 1997 | Anorexia in HIV | RCT | 39 | Oral DRO 2.5 mg BID | Megastrol acetate had greater weight gain than dronabinol and combination did not show additional weight gain in HIV patients |
| Haney et al[66], 2005 | Anorexia in HIV | RCT | 30 | Smoked marijuana cigarettes (1.8, 2.9, 3.9% THC); oral DRO 10, 20 and 30 mg | Calorie intake increased in cannabis use in HIV patients |
| Haney et al[67], 2007 | Anorexia in HIV | RCT | 10 | Smoked marijuana cigarettes (2.0, 3.9% THC); oral DRO 5 mg and 10 mg | Calorie intake and weight increased in a dose dependent response in cannabis use in HIV patients |
| Bedi et al[68], 2010 | Anorexia in HIV | RCT | 7 | Oral DRO 10 mg QID | Increased caloric intake but repeated high dose led to selective tolerance without increase in body weight in HIV patients |
| Ngueta et al[79], 2015 | Obesity | Cross sectional/Observational | 786 | Cannabis (reported) | Cannabis use association with lower BMI and lower percent fat mass |
| Jin et al[82], 2017 | Obesity | Observational/Longitudinal | 712 | Cannabis (reported) | No association in adolescent cannabis use and weight change from adolescence to midlife |
| Ross et al[81], 2020 | Obesity | Longitudinal/Observational | 401 | Cannabis (reported) | Higher baseline BMI led to increased cannabis use in adolescents. Increased cannabis use led to small decrease in BMI over 2 years |
| ElTelbany et al[90], 2022 | Hepato-cellular carcinoma | Observational study | 101231036 | Cannabis (reported) | Cannabis users 55% less likely to have hepatocellular carcinoma than nonusers |
| Adejumo et al[99], 2017 | NAFLD | Case control study | 5950391 | Cannabis (reported) | Dose dependent reduction in prevalence of NAFLD with cannabis use |
| Vazquez et al[100], 2019 | NAFLD | Observational longitudinal study | 390 | Cannabis (reported) | Cannabis consumption led to lower fatty liver index demonstrating lower risk of developing NAFLD over 3 yr in psychosis patients |
| Adejumo et al[101], 2018 | Alcohol-associated liver disease | Cross-sectional study | 318514 | Cannabis (reported) | Cannabis use associated with decreased incidence of alcoholic liver disease |
| Nordmann et al[106], 2018 | Hepatic steatosis | Prospective cohort study | 838 | Cannabis (reported) | Cannabis use associated with reduced prevalence of steatosis in HIV-HCV co-infected patients |
| Barré et al[105], 2021 | Hepatic steatosis | Observational longitudinal study | 997 | Cannabis (reported) | Cannabis use associated with reduced risk of elevated fatty liver index in HIV-HCV co-infected patients |
| Ishida et al[97], 2008 | Hepatitis C and fibrosis | Cohort study | 204 | Cannabis (reported) | Daily cannabis use associated with moderate to severe fibrosis in HCV infected individuals |
| Liu et al[104], 2014 | Hepatitis C and fibrosis | Retrospective cross-sectional study | 550 | Cannabis (reported) | No association between cannabis use and fibrosis in HCV infected patients |
| Simons-Linares et al[113], 2018 | Acute pancreatitis | Retrospective cohort study | 460 | Cannabis (reported) | Cannabis has potential association with acute pancreatitis but does not affect mortality or disease severity |
IBD consists of two major chronic autoimmune inflammatory conditions: Crohn’s Disease (CD) and Ulcerative Colitis. They have a predisposition towards males between ages 15 to 30 or greater than 60 years old. Common medications used for treatment, induce remission and prevent reactivation are 5-aminosalicylate drugs, corticosteroids and immunosuppressive agents. Surgical intervention such as colectomy is typically the last line of therapy.
Cannabis has potential analgesic effects for IBD patients. CB1 and CB2 receptor activation may minimize gut sensitivity and exert anti-inflammatory effects in the GI tract[1]. In rodent models studying IBD, CB receptor agonists reduced gut inflammation and improved IBD related symptoms[18]. Although several human studies have evaluated the therapeutic effects on cannabis in IBD patients, evidence is mixed. A meta-analysis of 20 studies revealed that cannabis and cannabinoids improved quality of life in IBD patients with initially lower baseline quality of life and reported symptoms[19]. Supporting literature also demonstrates benefit in disease activity indices and decrease in alternative analgesics[20-22]. Subjective improvement in disease-associated abdominal pain, diarrhea and appetite in addition to anxiety and fatigue were reported in IBD patients[23-27]. In 2011, Naftali et al[21] led the first observational study in CD which demonstrated decreased disease activity scores and less need for corticosteroids and surgery[21]. In a small prospective placebo-controlled study, the authors found 90% of CD individuals using THC-rich cannabis had a greater than 100 point reduction in CD Activity Index. Quality of life, appetite, pain scores and satisfaction also improved[28]. However, a following study by Naftali et al[29] demonstrated no beneficial effects of CBD in CD[29]. It was also proposed CD patients who used cannabis for greater than 6 mo had an increased surgical risk but it was unclear if cannabis was used prior to or after surgery[24]. A nationwide study on inpatient outcomes found that although cannabis users with CD required less parenteral nutrition needs than nonusers (3% vs 4.7%) and had shorter hospital stays due to potential symptomatic improvement, there were higher complications of active fistulas or intraabdominal abscesses compared to nonusers (8.6% vs 5.9%)[30].
Conversely, a meta-analysis revealed cannabis had no effect on complications from CD including stricture and fistula formation, abscesses, anemia, bowel obstruction and need for colectomy. Additionally, cannabis was concluded to be ineffective in achieving remission in IBD[19]. Small randomized controlled trials (RCT) demonstrated CBD-rich oil, although had significant clinical and quality of life improvement, exhibited no significant change in inflammatory parameters (C-reactive protein and calprotectin) or endoscopic score in IBD[31,32]. In majority of the studies, cannabis was generally well-tolerated with some mild increased risk of side effects due to its central actions including dizziness, dry mouth, and anxiety. The most reported reason to stop taking cannabis was dizziness[19]. In 2020, Cochrane reviews by Kafil et al[33] evaluated RCT studies for IBD patients and concluded cannabis safety and efficacy is uncertain so concrete conclusions cannot be made[33]. Overall, cannabis does offer symptomatic relief but there is scarce evidence that can support its use to control inflammation and achieve remission in IBD.
Cannabis may reduce GI motility by its agonistic activity on the CB1 receptors of myenteric neurons. It is involved in presynaptic inhibition of the excitatory neurotransmitters, acetylcholine release and substance P, which decreases smooth muscle peristalsis and contractility. CB2 receptors, particularly during inflammatory states, can also decrease GI motility[34]. It slows down gastric emptying, upper GI transit and colonic propulsion[9]. A small RCT demonstrated THC delayed solid food in gastric emptying by decreasing smooth muscle activity via peripheral effect[35]. In an animal model, CBD reduced gut hypermotility under an inflammatory state by potentially activating the enteric CB1 receptor by fatty acid amide hydrolase inhibition, the enzyme involved in endocannabinoid degradation[36]. However, one study found no change in gastric emptying after administration of cannabis[37]. The foreseen physiologic effect of marijuana in gut motility contrasts with its apparent effect on consti
Paralytic ileus is defined as the impairment of bowel muscle contractions that leads to intestinal obstruction. The pathogenesis of ileus may be affected by the release of endogenous cannabinoids. In vivo studies demonstrated cannabis may reduce or normalize GI motility. An animal model showed improvement in ileus after administration of CBD[40]. Targeted therapy towards endogenous cannabinoids may minimize the occurrence of ileus[9].
IBS is defined by Rome IV criteria as a functional disorder in which changes in bowel habits or defecation is associated with recurrent abdominal pain experienced at least once a week for the past 3 mo[41]. Although the pathophysiology is not completely understood, it is speculated that the gut-brain signaling system is impaired which leads to visceral hypersensitivity and motility disturbance. Alteration in gut microbiota can also lead to inflammation. Patients may experience abdominal discomfort, bloating and inconsistent bowel habits[9]. Cannabis may aid in symptomatic relief in IBS patients due to the effects on gut motility and tone[42]. Cannabinoid receptors are proposed to mediate abdominal visceral sensation. One small RCT study demonstrated lack of cannabis effect on reducing visceral hypersensitivity which contrasts with animal studies that demonstrate visceral pain reduction[43]. Dronabinol, a synthetic form of THC and nonselective cannabinoid receptor agonist, is proposed to ease colonic tone, inhibit gastric emptying and reduce colonic motility, although other studies differ[34,44]. Wong et al[45] concluded dronabinol inhibits colonic motility during the fasting phase and augments colonic compliance in IBS patients with diarrhea (IBS-D) or alternating predominant IBS. It either inhibits activity of excitatory motor mechanisms or activates the inhibitory neural mechanisms[45]. However the following study demonstrated dronabinol’s lack of effect on colonic transit on IBS-D individuals but is postulated to inhibit transit in patients who have a specific genetic variation of CB1[44]. Hospitalized cannabis users with IBS had lower symptomatic burden with shorter length of stay, lower rate of endoscopic procedure utilization and overall decreased hospital cost[42]. Opposing data demonstrated cannabis users had increased odds for IBS-hospitalization and there was a rising trend of cannabis use disorder and related psychiatric comorbidities including anxiety and depression[46].
The ECS is proposed to be involved in the modulation of stress through peripheral mechanisms and centrally by the hypothalamic-pituitary-adrenal axis. Chronic stress is thought to lead to visceral hyperalgesia and chronic pain. Endocannabinoids effect on CB1 as well as inhibitors of endocannabinoid degradation can reduce stress-related hyperalgesia[47]. Many patients with GI disorders such as IBD and gastroparesis are burdened by abdominal pain in which cannabis is shown to provide symptomatic relief[18,48]. Conversely a small phase 2 clinical trial demonstrated THC was not superior to placebo in patients with chronic abdominal pain[49]. Large clinical studies are needed to evaluate the efficacy of cannabis in chronic abdominal pain.
About 20% of adults in the United States are affected by gastroesophageal reflux disease (GERD)[50]. The vasovagal reflex mediates the actions of the lower esophageal sphincter (LES). LES transient relaxations can lead to GERD. Antacid is the mainstay of therapy but cannabis is also shown to provide some symptomatic relief in limited studies. CB1 and CB2 receptors are centrally expressed in the vasovagal nerve pathway and its inhibitory effect can reduce transient relaxations and control GERD symptoms[9]. Studies found that activation of CB receptors inhibits transient LES relaxations in dogs. It was translated to the human model and was shown that THC offered the same effect but only during the first hour post-meal in addition to basal LES pressure reduction. However, half the subjects had episodes of emesis, an undesired side effect[51,52]. It was also demonstrated rimonabant, a selective CB1 receptor antagonist, increased postprandial LES pressure in humans but decreased transient LES relaxations which contrasted a previous animal study. Different dosages, bioavailability and differing cannabis species could explain the discrepancy[52,53]. More human clinical trials are needed to evaluate the effects of cannabis on GERD.
Nausea and vomiting have multiple triggers modulated by the central and peripheral nervous system. As a defense mechanism, these common GI symptoms can be induced by emotional and cognitive stimuli, disturbance in the proprioceptive system, ingestion of harmful contents and toxic drugs such as chemotherapy agents[54]. Serotonin receptor antagonists, steroids and neurokinin-1 inhibitors such as aprepitant are prescribed to manage nausea and vomiting[55]. Different medication classes such as metoclopramide, prochlorperazine or lorazepam may be used for breakthrough or refectory symptoms[56]. Cannabis and related cannabinoids also offer anti-emetic effects and can be utilized for refractory symptoms. They are often the primary or adjuvant therapeutic option depending on the disease state[55]. Literature demonstrates cannabinoids block both acute and delayed emesis and have been used as medicinal purposes for a variety of nausea and vomiting induced causes[57]. CB1 receptors affect the pathogenesis of emesis by its wide expression throughout the brain and in the dorsal vagal complex of the brainstem[1]. It is unclear if CB2 receptors play a role.
The FDA approved dronabinol and nabilone in the 1980s for intractable chemotherapy related nausea and vomiting[55]. Current guidelines recommend cannabinoids as an option for breakthrough or refractory chemotherapy-induced nausea and vomiting in addition to the standard anti-emetic regimen if other therapies failed[58,59]. A meta-analysis of variable quality evidence demonstrated cannabinoids were highly effective to treat chemotherapy-induced nausea and vomiting but differences in symptoms were insignificant when compared with other anti-emetic drugs. Although dizziness was more commonly experienced with cannabinoid use, participants preferred it over traditional anti-emetics[56]. Side effects also included sedation and disorientation[60]. Notably in animal models studying lithium and chemotherapy-induced vomiting, THC suppressed vomiting in a dose dependent manner whereas CBD suppressed vomiting at low doses, yet triggered or enhanced vomiting at high doses[61,62]. Cannabinoid hyperemesis syndrome (CHS) is a well-established and paradoxical adverse effect of chronic cannabis use which can reverse its therapeutic role[54].
Marijuana can aid with appetite stimulation and weight gain. Cannabinoid signaling is involved in appetite stimulation pathways by the expression of CB1 receptors in the forebrain including the hypothalamus[63,64]. Literature suggests that oral cannabis increases ghrelin levels, the hunger hormone that regulates appetite and food intake and modulates insulin sensitivity[65]. Marijuana has been used in disease related anorexia and cachexia such as in acquired immunodeficiency syndrome (AIDS)/human immunodeficiency virus (HIV) and malignancy. In 1985, the FDA approved dronabinol for the treatment of AIDS/HIV related cachexia[63]. In HIV positive patients, smoking cannabis demonstrated increases in body weight and caloric intake[66-68]. However, megestrol acetate, a synthetic progestin, led to greater weight gain than dronabinol in patients with AIDS associated anorexia[69]. In cancer patients, evidence suggests dronabinol can increase appetite and therefore weight gain but it was countered by Brisbois et al[70] and Strasser et al[71] who demonstrated cannabis use exhibited no changes in improved appetite[70,71]. Dronabinol remains inferior to megestrol acetate in weight gain and appetite stimulation for cancer patients with no established benefit in combination therapy[72]. Smoking marijuana has not yet been studied in these patients. A meta-analysis concluded majority of the trials had low quality of evidence with potential reporting bias. Currently, there is no high quality evidence that supports cannabinoids in the treatment of cancer-related or HIV-related anorexia or cachexia[73].
Obesity is a multifactorial disease and major health concern worldwide. Nearly one third of the population is defined as overweight or obese. It is associated with multiple comorbidities including diabetes mellitus and cardiovascular disease[74,75]. Orlistat, an inhibitor of lipase enzymes, is one of the FDA-approved pharmacologic agents to target obesity. The role of cannabis is now being investigated in the management of obesity. Cannabis users generally have lower body mass indexs (BMIs) and are less likely to be obese than nonusers[76-79]. Marijuana is proposed to regulate body weight dependent on BMI and its acute vs chronic use[80]. Underweight individuals who use marijuana acutely may gain weight but individuals with normal or elevated BMIs may lose weight[80]. Moreover, although higher baseline BMI scores led to greater cannabis frequency, the increased cannabis use by adolescents led to greater decreases in BMI overtime[81]. Drugs and food compete for similar reward pathways in the brain which may explain the paradoxical effect of cannabis on BMI[78,80]. Conversely, an observational study found no association between cannabis use and weight change in adolescents, although confounding factors may be present[82]. Withdrawal from cannabis use can also lead to weight loss. Discontinuation of chronic cannabis use downregulates CB receptors, which can therefore lower BMI[83]. An animal study demonstrated mice that lacked CB1 receptors were protected against obesity[84]. Abstinence from cannabis was found to decrease appetite and increase metabolic rates, but these effects were lost when CB1 receptors returned to normal levels[83]. Rimonabant, a CB1 receptor antagonist, was approved in Europe in 2006 for weight loss in obese patients. However its safety concerns, primarily psychiatric side effects, led to its discontinuation shortly after approval[83,85]. The effects of marijuana on BMI is complex and more quality studies are needed to examine its long term use on obesity.
Studies are investigating the impact of cannabis on GI malignancies. It may disrupt tumor signaling pathways through its pro-apoptotic, anti-inflammatory, anti-proliferative and anti-angiogenic effects. Inhibition of endocannabinoid enzymes may also protect against metastasis[2]. Both anti-tumor properties and pro-tumorigenic activity are associated with the over- and under-expression of CB1 and CB2 receptors[86].
Due to CB2 receptor expression in pancreatic cancer cells, CB2 activation is theorized to induce apoptosis in these abnormal cells without affecting healthy cells. Additionally, low CB1 receptor expression demonstrated longer survival in pancreatic cancer[86,87]. Notably, gemcitabine and cannabinoid receptor agonists provide a synergistic effect in pancreatic cancer cells in vitro and in vivo leading to apoptotic and antiproliferative effects[88]. In hepatocellular carcinoma (HCC), higher expression of CB receptors was associated with improved prognosis and disease free survival[89]. Interestingly, a population based study found cannabis users were less likely to have HCC than nonusers. CBD may offer protective effects against HCC by CB2 agonism[90]. Cannabinoids and cannabis extracts are also being investigated in the potential treatment of colorectal cancer[91]. CBD was shown to exert protective effects in vivo through multiple mechanisms and reduce cell proliferation through CB1 and CB2 activation[92,93].
Marijuana may also exert pro-neoplastic effects. It was found to be weakly associated with esophageal cancers[94]. In esophageal squamous cell carcinoma, CB1 receptor overexpression led to poor prognosis, cell proliferation and invasion[95]. One case report suggested chronic cannabis use may be a risk factor for Barrett’s esophagus[96]. Studies of cannabis and its anti-tumor vs pro-tumorigenic activity are conflicting and strong data is lacking. More human trials are necessary to establish its potential protective or harmful properties in GI malignancies.
Cannabis is proposed to play a role in hepatic steatosis and fibrosis, hepatic encephalopathy and alcohol-associated liver disease[1]. CB1 receptor expression can increase lipogenesis, fibrogenesis, decrease fatty acid oxidation and induce hyperphagia while receptor antagonists protect against hepatic steatosis[1]. On the other hand, CB2 receptor activation exerts antifibrogenic effects which can be an important target in cirrhosis treatment[97]. This is supported by animal models which demonstrate CB1 deletion improves hepatic fibrosis and steatosis and CB2 deletion increases inflammation and steatosis[98]. CB2 agonists can reduce oxidative stress and therefore inflammation[1,99]. There was a decreased prevalence of non-alcoholic fatty liver disease in cannabis users proposing a potential benefit[99,100]. There was also a reduced incidence of liver disease in concomitant alcohol and cannabis users likely from the anti-inflammatory properties of cannabis[101]. Evidence is scarce in cannabis’ role in hepatic encephalopathy. Animal models suggest cannabis use improves hepatic encephalopathy due to its anti-inflammatory effects[102,103].
Studies are conflicting in the role of cannabis in viral hepatitis, which may partially be explained by different endpoints and definitions. Cannabis use is thought to be associated with moderate to severe fibrosis in patients with chronic hepatitis C but there is opposing data[97,104]. In HIV and hepatitis C co-infection, marijuana was associated with a decreased risk of steatosis[105,106]. Currently, there is no evidence suggesting the association of cannabis with hepatitis B.
Pancreatitis is an acute inflammatory process of the pancreas that is commonly precipitated by gallstones and heavy alcohol use. Evidence suggests cannabis may lead to an increased risk of pancreatitis. CB1 and CB2 receptors in the pancreas are increased during inflammation but the pathogenesis remains unclear[34]. Several case reports suggest the association of cannabis-induced pancreatitis[107-109]. Multiple systematic reviews found cannabis use was related to acute and recurrent pancreatitis and cannabis cessation evoked no further episodes[110-112]. One study found no impact of cannabis on disease severity or mortality[113].
The risks and benefits of cannabis use and cannabinoids are highly debated in the United States. Significant research has been performed on the risk profile of marijuana and is still underway, although restricted by laws and regulations (Table 2). In some countries where marijuana use is legalized, research guidelines were developed to ensure safe use and guide practitioner prescribing. One such recommendation is the evidence-based Lower-Risk Cannabis Use Guidelines (LRCUG). LRCUG consists of 10 recommendations including choosing a low concentration THC or balanced THC to CBD ratio products and avoidance of risky behaviors such as use under age 16 or daily use, synthetic compounds and driving while impaired[114].
| No. | Risks and side effects |
| 1 | Decreased cognition, learning and memory[118,119] |
| 2 | Schizophrenia and psychosis[116,143] |
| 3 | High tar and carbon monoxide concentrations[120] |
| 4 | Cannabis use disorder/addiction/dependence[131] |
| 5 | Drug addiction[137] |
| 6 | Cannabis withdrawal symptoms[132] |
| 7 | Anxiety[133,135] |
| 8 | Cannabinoid hyperemesis syndrome[139,140] |
| 9 | Chronic bronchitis and chronic cough[121,122] |
| 10 | Increased risk of motor vehicle accidents[125,126] |
| 11 | Unintentional overdose in children[128,129] |
The recommendation to avoid cannabis use before the age of 16 is due to its potential adverse effects on the adolescent brain. The New England Journal of Medicine identified cannabis use can lead to abnormal brain development in the vulnerable adolescent population[115]. Longitudinal functional magnetic resonance imaging studies exhibited cannabis use decreased grey matter in multiple sites within the brain[116,117]. Adolescent onset schizophrenia has also long been theorized to be related to altered grey matter development secondary to marijuana[116]. Multiple studies noted the probable, albeit mixed, evidence that chronic cannabis use decreases cognition, learning and memory[118,119]. This poses significant concerns when experimentation and use of cannabis frequently occurs in the adolescent population[119].
LRCUG also suggests avoidance of inhalation. Inhalation of cannabis has been closely compared to cigarette smoking. Some studies claim cannabis smoking contains approximately 2.8 times as much tar and up to 5 times as much carbon monoxide inhaled in comparison to tobacco smoking[120]. Wu et al[120] noted that cannabis smokers took two thirds greater and one third deeper of an inhale and four times longer of a breath hold resulting in longer exposure, although unaffected by THC concentration[120]. However, there was no comparison of the density between commercial cigarettes and the various methods to smoke cannabis, as well as the purity, frequency and amount of cannabis consumed. Another complicating factor was the co-use of tobacco and cannabis, clouding what scarce research is available[120]. There was strong evidence that cannabis results in chronic cough and mucus production as well as a high risk of developing chronic bronchitis episodes, which likely improves with abstinence[121,122].
The National Academies of Science, Engineering and Medicine (NASEM) conducted a systematic review in 2017 of the available research on cannabis use to also guide policy makers and physicians through evidence-based means. NASEM found no convincing evidence for associations between cannabis and heart disease, stroke, diabetes, COPD, asthma or worsening lung function[121,122]. There was some evidence purporting that smoking cannabis does not increase risk of lung, head and neck cancer in adults[123]. However, cannabis use was associated with a 2.5 times risk of developing non-seminoma testicular cancer[124]. Additionally, cannabis has up to a 30% higher risk of motor vehicle accidents when driving under the influence[125,126]. The risk of occupational injuries in cannabis users was unclear due to confounding variables by individual personal risks[127]. Unfortunately, the risk of childhood accidental exposure has increased. In states that legalized its recreational use, the estimated risk of unintentional overdose in children is as high as 2.8 times compared to states where cannabis is restricted[128,129]. There was a 11%-30% increase in calls to poison centers regarding childhood accidental exposure in states that legalized cannabis[130].
Moreover, cannabis users may develop cannabis use disorder, which is the inability to discontinue use despite physical or psychological harms[131]. Cannabis use disorder affects approximately 10% of regular users and up to 33% of daily users but is likely underdiagnosed[131]. Dependence or addiction can occur in addition to cannabis withdrawal. The DSM-5 defines cannabis withdrawal as any 3 of the 7 following signs or symptoms: Nervousness/anxiety, irritability/anger, sleep difficulty, decreased appetite or weight, depressed mood, restlessness or physical discomfort (abdominal pain, tremors, sweating, fever, chills, and/or headache)[132].
A meta-analysis across 10 countries found a positive association between anxiety and cannabis use after accounting for confounding variables[133]. However, Blanco et al[134] found no association between cannabis and anxiety disorder[134]. This relationship was further explored by Feingold et al[135], who used Blanco’s data set, and demonstrated cannabis users have a higher risk of developing social anxiety disorder specifically[135]. There was no association between cannabis use and the development of mood disorder or major depressive disorder[136]. The type of cannabis can also lead to mixed effects due to differences in composition of varying cannabinoids. Studies demonstrate THC exhibited a pro-anxiety effect whereas CBD displayed an anti-anxiety effect[137].
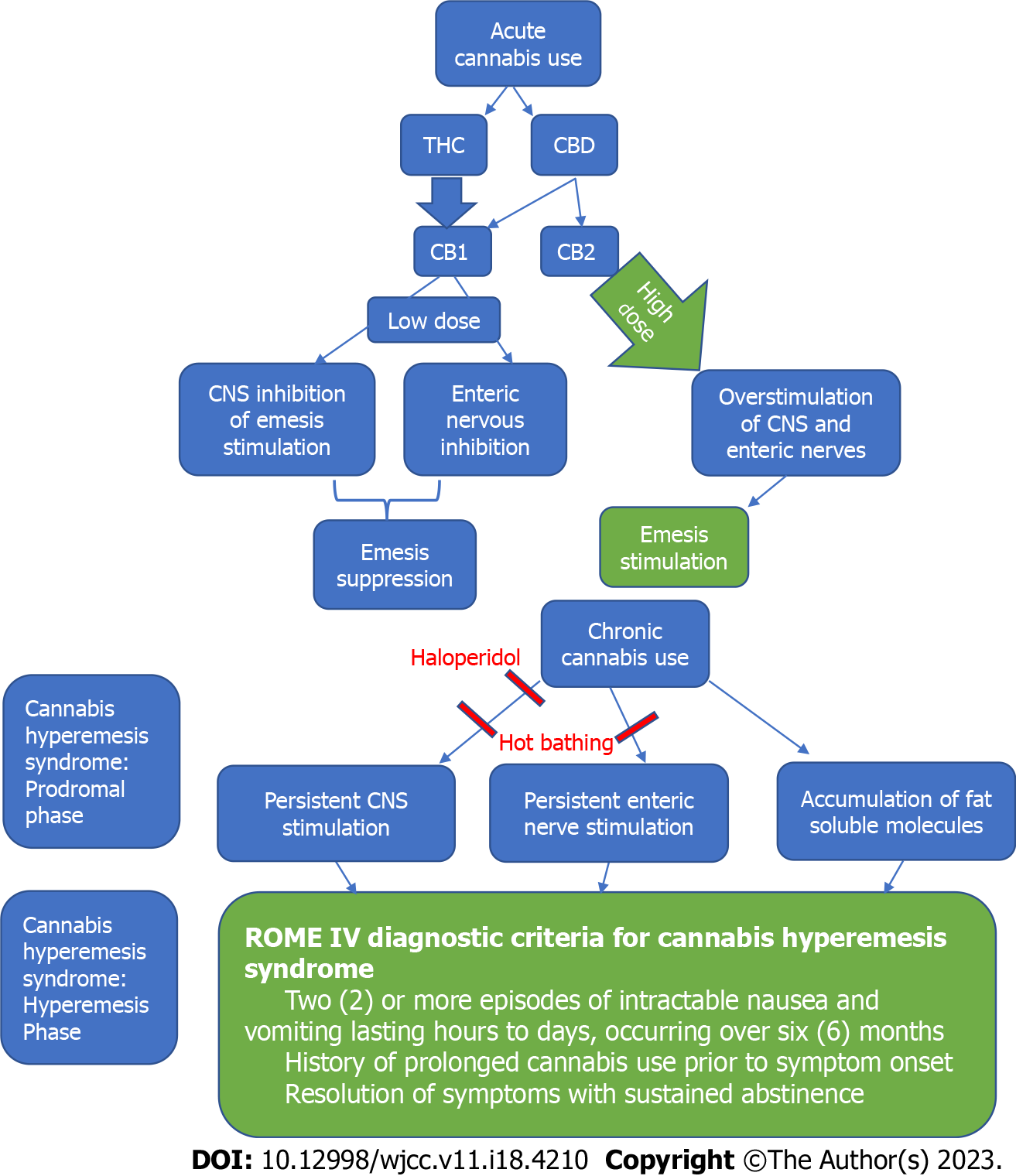
Although cannabis can serve as a potential aid for patients who suffer from cyclic vomiting syndrome, this should not be confused with one its most notorious side effects, CHS (Figure 3)[138]. The exact mechanism of CHS is not fully understood. Cannabis is proposed to have an antiemetic effect at low doses but a pro-emetic effect at higher doses[139]. The Rome IV diagnostic criteria for CHS includes three features: Stereotypical two or more episodes of intractable vomiting lasting hours to days over a period of at least 6 mo, history of prolonged cannabis use prior to symptom onset and relief of episodes from sustained abstinence[140]. A notable, learned behavior is prolonged hot bathing, which offers temporary symptom relief. This is thought to occur via multiple processes including chronic buildup of fat-soluble molecules reaching toxic levels, overstimulation of enteric neurons or hypothalamic disruption of digestion and thermoregulation[139]. Available therapeutics for CHS include serotonin antagonists (ondansetron), antipsychotics (haloperidol), benzodiazepines, aprepitant and topical capsaicin[141]. Haloperidol was found to be superior over ondansetron in managing abdominal pain and nausea in CHS leading to early emergency department discharge[142]. Yet the most effective treatment is absolute abstinence from cannabis[141].
Death from marijuana intoxication is complex. There have been cases of pediatric respiratory depression requiring intubation and cannabis triggered psychosis leading to erratic or dangerous behavior resulting in death[143]. A systematic review of epidemiologic data found that cannabis use and all-cause mortality were not statistically significant[125]. It should be noted that mice studies testing cannabis extracts estimated the lethal dose 50 to be greater than 1000 mg/kg[144]. Many side effects of marijuana have been established but the long-term safety profile needs further investigation.
In the United States, legalization barriers limit cannabis medical research which creates gaps in medical knowledge. However cannabis use has become more increasingly accepted in the public eye as well as for medical use. In the United States, 37 states, the District of Columbia and four United States territories have legalized Cannabis for medical use in different degrees while 21 states and District of Columbia legalized marijuana for recreational use. Some states decriminalized marijuana meaning legal consequences for possession of marijuana are minimal to none[145]. Several states approved medical marijuana for the treatment of a variety of disorders such as IBD, HIV/AIDS-related cachexia and chemotherapy-induced nausea and vomiting. Although states are legalizing marijuana, there is currently no federal legalization for its national use[18]. It is classified as a Schedule 1 substance indicating its high potential for substance abuse without acceptable medical use. This causes clinicians to feel hesitant in offering medical marijuana due to fear of prosecution. State laws designed systems to protect physicians when recommending marijuana use. Physicians require an active state medical license, must maintain a consistent relationship with the patient, and some states require certifications to recommend marijuana[6]. The barriers of legalization are decreasing but investigators continue to face legal and administrative restrictions when attempting to perform research with cannabis and cannabinoids. The lack of knowledge has limited quality clinical research hindering potential novel findings that can inform both clinicians and the community of its therapeutic use and risk profile[18]. As research and patient interest on cannabis continues to grow, physicians must stay well informed on the substance in order to provide patients with appropriate education and guidance of its indications and safety profile.
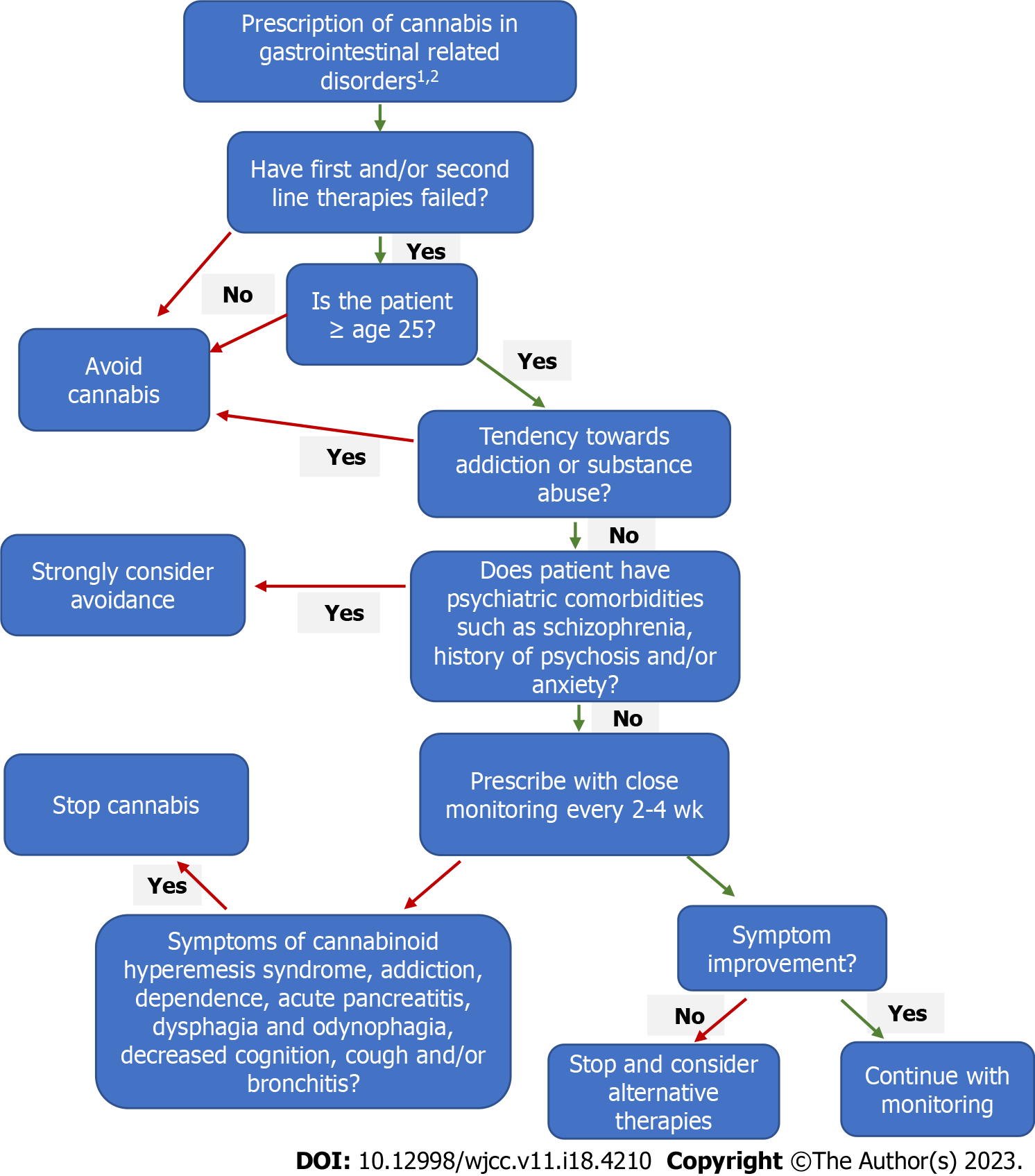
The question of whether gastroenterologists should or should not prescribe cannabis is raised (Figure 4). Although limited data is available, with mixed results, evidence does show beneficial effects of cannabis in a variety of GI symptoms and disorders. It can provide symptomatic control in diseases including IBD and IBS with tolerable side effects. However, research suggests it has no superior role over first line drugs in some disorders. Physicians should prescribe cannabis to patients through an individualized basis and a personalized treatment plan. Similarly to commonly prescribed medications, cannabis may provide therapeutic benefit in some individuals, while offering little to no benefit effect in others. Cannabis can be considered as an alternative agent in adults who failed first or second line therapies or for those who cannot tolerate oral intake, whereas inhalation or sublingual are suitable options. Although the side effects of cannabis are well-established, it is not sufficiently different than the side effects of federally approved drugs including biologic agents, anti-psychotic or anti-depressant medications. However, cannabis should be avoided in pregnancy and those under age 25 such as adolescents and the pediatric population due to its demonstrated effects on brain development. It should also be avoided in patients struggling with addiction or substance abuse due to its addictive properties. Once cannabis is prescribed, there should be initial close monitoring of the usage and side effects, similarly to opioid prescriptions. As evidence becomes more robust, medicinal and recreational legalization of cannabis will likely expand. This will allow for further and more scrutinized research to guide and empower physicians to prescribe cannabis appropriately and safely with evidence-based knowledge of benefits and harms and without concern for legal recourse.
Due to the limited data and risk of side effects, cannabis should be prescribed at the lowest dose. A CBD predominant regimen is encouraged to reduce risk of adverse events including psychotropic effects. CBD should be administered at a low dose of 5 mg once or twice daily. The modified Delphi approach, developed under the guidance of medical experts for chronic pain, recommend incremental increases of CBD 5-10 mg/day every 2-3 d. Once CBD dose reaches greater than 40 mg without achievement of goal, THC can be added. THC dosage of 1 mg/day should be the initial starting dose under the conservative protocol. Weekly incremental increases of THC 1 mg/day is recommended by the modified Delphi approach until goal is achieved or until maximum dose (40 mg) is reached[146]. Slow titration of THC will promote tolerance to the psychoactive properties. A THC predominant regimen can begin with prescribing 1.0-2.5 mg per day dependent on individual risk factors with incremental increases every 3-4 d until 40 mg is reached[147]. Ingestion over inhalation is the preferred route of administration. Another alternative is using equivalent dosing of THC:CBD with 2.5-5.0 mg of each cannabinoid daily and increase by 2.5-5.0 mg every 3 d until THC maximum dose is reached[146]. Caution is warranted when reaching the maximum limit of THC and high doses of CBD as majority of the studies on GI disorders lack data on high doses. Close monitoring every 2-4 wk is recommended to assess efficacy and side effects and need for upward titration or discontinuation of cannabis. Longer periods of follow-up can be done once patient is at a steady dose with tolerable effects[147]. Overall, a conservative approach of starting medical cannabis at a low dose and titrating upwards slowly is preferred due to the existing data available.
Cannabis may offer therapeutic benefit in many GI disorders including inflammatory and neoplastic diseases but appropriate monitoring is essential due to the number of potential harmful side effects. Current evidence demonstrates mixed results due to small sample sizes, different formulations, dosages and routes of cannabis administration and outcome definitions. Its legal barriers cause further investigational obstacles but despite this, ongoing research continues. Further exploration of its safety profile and large randomized clinical trials are warranted to guide gastroenterologists on the effects of cannabis, appropriate indications and dosing as well as proper monitoring for efficacy and adverse side effects.
We thank Michael Waxman, MD, Department of Emergency Medicine and expertise in Addiction Medicine for providing guidance with the revision of the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chiarioni G, Italy; Gad EH, Egypt S-Editor: Fan JR L-Editor: A P-Editor: Zhao S
| 1. | Cohen L, Neuman MG. Cannabis and the Gastrointestinal Tract. J Pharm Pharm Sci. 2020;23:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 2. | Lian J, Casari I, Falasca M. Modulatory role of the endocannabinoidome in the pathophysiology of the gastrointestinal tract. Pharmacol Res. 2022;175:106025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605-613. [PubMed] |
| 4. | Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3564] [Cited by in RCA: 3653] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 5. | Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3980] [Cited by in RCA: 3986] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 6. | Gerich ME, Isfort RW, Brimhall B, Siegel CA. Medical marijuana for digestive disorders: high time to prescribe? Am J Gastroenterol. 2015;110:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2062] [Cited by in RCA: 2060] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 8. | Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, Ward S. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129:437-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Abalo R, Vera G, López-Pérez AE, Martínez-Villaluenga M, Martín-Fontelles MI. The gastrointestinal pharmacology of cannabinoids: focus on motility. Pharmacology. 2012;90:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 594] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 11. | Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 631] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 12. | Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Domino EF. Cannabinoids and the cholinergic system. J Clin Pharmacol. 1981;21:249S-255S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Fezza F, Bari M, Florio R, Talamonti E, Feole M, Maccarrone M. Endocannabinoids, related compounds and their metabolic routes. Molecules. 2014;19:17078-17106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Rankin L, Fowler CJ. The Basal Pharmacology of Palmitoylethanolamide. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 16. | Pacifici R, Zuccaro P, Farré M, Poudevida S, Abanades S, Pichini S, Langohr K, Segura J, de la Torre R. Combined immunomodulating properties of 3,4-methylenedioxymethamphetamine (MDMA) and cannabis in humans. Addiction. 2007;102:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Ihenetu K, Molleman A, Parsons ME, Whelan CJ. Inhibition of interleukin-8 release in the human colonic epithelial cell line HT-29 by cannabinoids. Eur J Pharmacol. 2003;458:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Bogale K, Raup-Konsavage W, Dalessio S, Vrana K, Coates MD. Cannabis and Cannabis Derivatives for Abdominal Pain Management in Inflammatory Bowel Disease. Med Cannabis Cannabinoids. 2021;4:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Doeve BH, van de Meeberg MM, van Schaik FDM, Fidder HH. A Systematic Review With Meta-Analysis of the Efficacy of Cannabis and Cannabinoids for Inflammatory Bowel Disease: What Can We Learn From Randomized and Nonrandomized Studies? J Clin Gastroenterol. 2021;55:798-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Lahat A, Lang A, Ben-Horin S. Impact of cannabis treatment on the quality of life, weight and clinical disease activity in inflammatory bowel disease patients: a pilot prospective study. Digestion. 2012;85:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Naftali T, Lev LB, Yablecovitch D, Half E, Konikoff FM. Treatment of Crohn's disease with cannabis: an observational study. Isr Med Assoc J. 2011;13:455-458. [PubMed] |
| 22. | Dalavaye N, Erridge S, Nicholas M, Pillai M, Bapir L, Holvey C, Coomber R, Rucker JJ, Hoare J, Sodergren MH. The effect of medical cannabis in inflammatory bowel disease: analysis from the UK Medical Cannabis Registry. Expert Rev Gastroenterol Hepatol. 2023;17:85-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 23. | Lal S, Prasad N, Ryan M, Tangri S, Silverberg MS, Gordon A, Steinhart H. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23:891-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn's disease. Inflamm Bowel Dis. 2014;20:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 25. | Naftali T, Bar-Lev Schleider L, Sklerovsky Benjaminov F, Lish I, Konikoff FM, Ringel Y. Medical cannabis for inflammatory bowel disease: real-life experience of mode of consumption and assessment of side-effects. Eur J Gastroenterol Hepatol. 2019;31:1376-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Ravikoff Allegretti J, Courtwright A, Lucci M, Korzenik JR, Levine J. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2809-2814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Kerlin AM, Long M, Kappelman M, Martin C, Sandler RS. Profiles of Patients Who Use Marijuana for Inflammatory Bowel Disease. Dig Dis Sci. 2018;63:1600-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Naftali T, Bar-Lev Schleider L, Dotan I, Lansky EP, Sklerovsky Benjaminov F, Konikoff FM. Cannabis induces a clinical response in patients with Crohn's disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11:1276-1280.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 29. | Naftali T, Mechulam R, Marii A, Gabay G, Stein A, Bronshtain M, Laish I, Benjaminov F, Konikoff FM. Low-Dose Cannabidiol Is Safe but Not Effective in the Treatment for Crohn's Disease, a Randomized Controlled Trial. Dig Dis Sci. 2017;62:1615-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 30. | Desai R, Patel U, Goyal H, Rimu AH, Zalavadia D, Bansal P, Shah N. In-hospital outcomes of inflammatory bowel disease in cannabis users: a nationwide propensity-matched analysis in the United States. Ann Transl Med. 2019;7:252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Naftali T, Bar-Lev Schleider L, Almog S, Meiri D, Konikoff FM. Oral CBD-rich Cannabis Induces Clinical but Not Endoscopic Response in Patients with Crohn's Disease, a Randomised Controlled Trial. J Crohns Colitis. 2021;15:1799-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Irving PM, Iqbal T, Nwokolo C, Subramanian S, Bloom S, Prasad N, Hart A, Murray C, Lindsay JO, Taylor A, Barron R, Wright S. A Randomized, Double-blind, Placebo-controlled, Parallel-group, Pilot Study of Cannabidiol-rich Botanical Extract in the Symptomatic Treatment of Ulcerative Colitis. Inflamm Bowel Dis. 2018;24:714-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 33. | Kafil TS, Nguyen TM, MacDonald JK, Chande N. Cannabis for the Treatment of Crohn's Disease and Ulcerative Colitis: Evidence From Cochrane Reviews. Inflamm Bowel Dis. 2020;26:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 34. | Goyal H, Singla U, Gupta U, May E. Role of cannabis in digestive disorders. Eur J Gastroenterol Hepatol. 2017;29:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 35. | McCallum RW, Soykan I, Sridhar KR, Ricci DA, Lange RC, Plankey MW. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmacol Ther. 1999;13:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Capasso R, Borrelli F, Aviello G, Romano B, Scalisi C, Capasso F, Izzo AA. Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice. Br J Pharmacol. 2008;154:1001-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Bateman DN. Delta-9-tetrahydrocannabinol and gastric emptying. Br J Clin Pharmacol. 1983;15:749-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Li R, Li M, Li B, Chen WH, Liu Z. Cannabis sativa L. alleviates loperamide-induced constipation by modulating the composition of gut microbiota in mice. Front Pharmacol. 2022;13:1033069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Adejumo AC, Flanagan R, Kuo B, Staller K. Relationship Between Recreational Marijuana Use and Bowel Function in a Nationwide Cohort Study. Am J Gastroenterol. 2019;114:1894-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Lin XH, Yuece B, Li YY, Feng YJ, Feng JY, Yu LY, Li K, Li YN, Storr M. A novel CB receptor GPR55 and its ligands are involved in regulation of gut movement in rodents. Neurogastroenterol Motil. 2011;23:862-e342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Lacy BE, Patel NK. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J Clin Med. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 320] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 42. | Desai P, Mbachi C, Vohra I, Salazar M, Mathew M, Randhawa T, Haque Z, Wang Y, Attar B, Paintsil I. Association Between Cannabis Use and Healthcare Utilization in Patients With Irritable Bowel Syndrome: A Retrospective Cohort Study. Cureus. 2020;12:e8008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 43. | Klooker TK, Leliefeld KE, Van Den Wijngaard RM, Boeckxstaens GE. The cannabinoid receptor agonist delta-9-tetrahydrocannabinol does not affect visceral sensitivity to rectal distension in healthy volunteers and IBS patients. Neurogastroenterol Motil. 2011;23:30-35, e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Wong BS, Camilleri M, Eckert D, Carlson P, Ryks M, Burton D, Zinsmeister AR. Randomized pharmacodynamic and pharmacogenetic trial of dronabinol effects on colon transit in irritable bowel syndrome-diarrhea. Neurogastroenterol Motil. 2012;24:358-e169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Wong BS, Camilleri M, Busciglio I, Carlson P, Szarka LA, Burton D, Zinsmeister AR. Pharmacogenetic trial of a cannabinoid agonist shows reduced fasting colonic motility in patients with nonconstipated irritable bowel syndrome. Gastroenterology. 2011;141:1638-47.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Patel RS, Goyal H, Satodiya R, Tankersley WE. Relationship of Cannabis Use Disorder and Irritable Bowel Syndrome (IBS): An Analysis of 6.8 Million Hospitalizations in the United States. Subst Use Misuse. 2020;55:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Sharkey KA, Wiley JW. The Role of the Endocannabinoid System in the Brain-Gut Axis. Gastroenterology. 2016;151:252-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 48. | Parkman HP, Sharkey EP, Nguyen LA, Yates KP, Abell TL, Hasler WL, Snape W, Clarke J, Schey R, Koch KL, Kuo B, McCallum RW, Sarosiek I, Grover M, Farrugia G, Tonascia J, Pasricha PJ; Frank A. Hamilton for the NIH Gastroparesis Consortium. Marijuana Use in Patients with Symptoms of Gastroparesis: Prevalence, Patient Characteristics, and Perceived Benefit. Dig Dis Sci. 2020;65:2311-2320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | de Vries M, van Rijckevorsel DCM, Vissers KCP, Wilder-Smith OHG, van Goor H; Pain and Nociception Neuroscience Research Group. Tetrahydrocannabinol Does Not Reduce Pain in Patients With Chronic Abdominal Pain in a Phase 2 Placebo-controlled Study. Clin Gastroenterol Hepatol. 2017;15:1079-1086.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 50. | Antunes C, Aleem A, Curtis SA. Gastroesophageal Reflux Disease. 2022 Jul 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 51. | Beaumont H, Jensen J, Carlsson A, Ruth M, Lehmann A, Boeckxstaens G. Effect of delta9-tetrahydrocannabinol, a cannabinoid receptor agonist, on the triggering of transient lower oesophageal sphincter relaxations in dogs and humans. Br J Pharmacol. 2009;156:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Lehmann A, Blackshaw LA, Brändén L, Carlsson A, Jensen J, Nygren E, Smid SD. Cannabinoid receptor agonism inhibits transient lower esophageal sphincter relaxations and reflux in dogs. Gastroenterology. 2002;123:1129-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 53. | Scarpellini E, Blondeau K, Boecxstaens V, Vos R, Gasbarrini A, Farré R, Tack J. Effect of rimonabant on oesophageal motor function in man. Aliment Pharmacol Ther. 2011;33:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Zhong W, Shahbaz O, Teskey G, Beever A, Kachour N, Venketaraman V, Darmani NA. Mechanisms of Nausea and Vomiting: Current Knowledge and Recent Advances in Intracellular Emetic Signaling Systems. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 55. | Parker LA, Rock EM, Limebeer CL. Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol. 2011;163:1411-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 56. | Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. 2015;2015:CD009464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 57. | Sharkey KA, Darmani NA, Parker LA. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol. 2014;722:134-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 58. | Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina SB, Eng C, Feyer PC, Jordan K, Noonan K, Sparacio D, Lyman GH. Antiemetics: ASCO Guideline Update. J Clin Oncol. 2020;38:2782-2797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 59. | Ward SJ, Lichtman AH, Piomelli D, Parker LA. Cannabinoids and Cancer Chemotherapy-Associated Adverse Effects. J Natl Cancer Inst Monogr. 2021;2021:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Grimison P, Mersiades A, Kirby A, Lintzeris N, Morton R, Haber P, Olver I, Walsh A, McGregor I, Cheung Y, Tognela A, Hahn C, Briscoe K, Aghmesheh M, Fox P, Abdi E, Clarke S, Della-Fiorentina S, Shannon J, Gedye C, Begbie S, Simes J, Stockler M. Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: a randomised, placebo-controlled, phase II crossover trial. Ann Oncol. 2020;31:1553-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 61. | Kwiatkowska M, Parker LA, Burton P, Mechoulam R. A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew). Psychopharmacology (Berl). 2004;174:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Parker LA, Kwiatkowska M, Burton P, Mechoulam R. Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew). Psychopharmacology (Berl). 2004;171:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | O'Donnell B, Meissner H, Gupta V. Dronabinol. 2022 Sep 5. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 64. | Kirkham TC. Cannabinoids and appetite: food craving and food pleasure. Int Rev Psychiatry. 2009;21:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Farokhnia M, McDiarmid GR, Newmeyer MN, Munjal V, Abulseoud OA, Huestis MA, Leggio L. Effects of oral, smoked, and vaporized cannabis on endocrine pathways related to appetite and metabolism: a randomized, double-blind, placebo-controlled, human laboratory study. Transl Psychiatry. 2020;10:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 66. | Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology (Berl). 2005;181:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J Acquir Immune Defic Syndr. 2007;45:545-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 68. | Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD, Vosburg SK, Haney M. Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study. Psychopharmacology (Berl). 2010;212:675-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Timpone JG, Wright DJ, Li N, Egorin MJ, Enama ME, Mayers J, Galetto G. The safety and pharmacokinetics of single-agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome. The DATRI 004 Study Group. Division of AIDS Treatment Research Initiative. AIDS Res Hum Retroviruses. 1997;13:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 70. | Brisbois TD, de Kock IH, Watanabe SM, Mirhosseini M, Lamoureux DC, Chasen M, MacDonald N, Baracos VE, Wismer WV. Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: results of a randomized, double-blind, placebo-controlled pilot trial. Ann Oncol. 2011;22:2086-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 71. | Cannabis-In-Cachexia-Study-Group; Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, Ko YD, Schnelle M, Reif M, Cerny T. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol. 2006;24:3394-3400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 72. | Jatoi A, Windschitl HE, Loprinzi CL, Sloan JA, Dakhil SR, Mailliard JA, Pundaleeka S, Kardinal CG, Fitch TR, Krook JE, Novotny PJ, Christensen B. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 73. | Mücke M, Weier M, Carter C, Copeland J, Degenhardt L, Cuhls H, Radbruch L, Häuser W, Conrad R. Systematic review and meta-analysis of cannabinoids in palliative medicine. J Cachexia Sarcopenia Muscle. 2018;9:220-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 74. | Panuganti KK, Nguyen M, Kshirsagar RK. Obesity. 2022 Aug 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 75. | Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1743] [Article Influence: 290.5] [Reference Citation Analysis (0)] |
| 76. | Hayatbakhsh MR, O'Callaghan MJ, Mamun AA, Williams GM, Clavarino A, Najman JM. Cannabis use and obesity and young adults. Am J Drug Alcohol Abuse. 2010;36:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Le Strat Y, Le Foll B. Obesity and cannabis use: results from 2 representative national surveys. Am J Epidemiol. 2011;174:929-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 78. | Warren M, Frost-Pineda K, Gold M. Body mass index and marijuana use. J Addict Dis. 2005;24:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Ngueta G, Bélanger RE, Laouan-Sidi EA, Lucas M. Cannabis use in relation to obesity and insulin resistance in the Inuit population. Obesity (Silver Spring). 2015;23:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Sansone RA, Sansone LA. Marijuana and body weight. Innov Clin Neurosci. 2014;11:50-54. [PubMed] |
| 81. | Ross JM, Pacheco-Colón I, Hawes SW, Gonzalez R. Bidirectional Longitudinal Associations Between Cannabis Use and Body Mass Index Among Adolescents. Cannabis Cannabinoid Res. 2020;5:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 82. | Jin LZ, Rangan A, Mehlsen J, Andersen LB, Larsen SC, Heitmann BL. Association Between Use of Cannabis in Adolescence and Weight Change into Midlife. PLoS One. 2017;12:e0168897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Clark TM, Jones JM, Hall AG, Tabner SA, Kmiec RL. Theoretical Explanation for Reduced Body Mass Index and Obesity Rates in Cannabis Users. Cannabis Cannabinoid Res. 2018;3:259-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 84. | Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrié P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 455] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 85. | Samat A, Tomlinson B, Taheri S, Thomas GN. Rimonabant for the treatment of obesity. Recent Pat Cardiovasc Drug Discov. 2008;3:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Sharafi G, He H, Nikfarjam M. Potential Use of Cannabinoids for the Treatment of Pancreatic Cancer. J Pancreat Cancer. 2019;5:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 87. | Michalski CW, Oti FE, Erkan M, Sauliunaite D, Bergmann F, Pacher P, Batkai S, Müller MW, Giese NA, Friess H, Kleeff J. Cannabinoids in pancreatic cancer: correlation with survival and pain. Int J Cancer. 2008;122:742-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 88. | Donadelli M, Dando I, Zaniboni T, Costanzo C, Dalla Pozza E, Scupoli MT, Scarpa A, Zappavigna S, Marra M, Abbruzzese A, Bifulco M, Caraglia M, Palmieri M. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011;2:e152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 89. | Xu X, Liu Y, Huang S, Liu G, Xie C, Zhou J, Fan W, Li Q, Wang Q, Zhong D, Miao X. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet Cytogenet. 2006;171:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 90. | ElTelbany A, Khoudari G, Al-Khadra Y, McCullough A, Alkhouri N. Lower Rates of Hepatocellular Carcinoma Observed Among Cannabis Users: A Population-Based Study. Cureus. 2022;14:e24576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 91. | Zaiachuk M, Pryimak N, Kovalchuk O, Kovalchuk I. Cannabinoids, Medical Cannabis, and Colorectal Cancer Immunotherapy. Front Med (Lausanne). 2021;8:713153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 92. | Aviello G, Romano B, Borrelli F, Capasso R, Gallo L, Piscitelli F, Di Marzo V, Izzo AA. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J Mol Med (Berl). 2012;90:925-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 93. | Romano B, Borrelli F, Pagano E, Cascio MG, Pertwee RG, Izzo AA. Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine. 2014;21:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 94. | Hashibe M, Morgenstern H, Cui Y, Tashkin DP, Zhang ZF, Cozen W, Mack TM, Greenland S. Marijuana use and the risk of lung and upper aerodigestive tract cancers: results of a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2006;15:1829-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 95. | Hijiya N, Shibata T, Daa T, Hamanaka R, Uchida T, Matsuura K, Tsukamoto Y, Nakada C, Iha H, Inomata M, Moriyama M. Overexpression of cannabinoid receptor 1 in esophageal squamous cell carcinoma is correlated with metastasis to lymph nodes and distant organs, and poor prognosis. Pathol Int. 2017;67:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Levy J, Buhl K, Fernandez C, Kumaraswamy J. Does Smoking Cannabis Increase the Risk of Barrett's Esophagus? Cureus. 2020;12:e6913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 97. | Ishida JH, Peters MG, Jin C, Louie K, Tan V, Bacchetti P, Terrault NA. Influence of cannabis use on severity of hepatitis C disease. Clin Gastroenterol Hepatol. 2008;6:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 98. | Dibba P, Li AA, Cholankeril G, Iqbal U, Gadiparthi C, Khan MA, Kim D, Ahmed A. The Role of Cannabinoids in the Setting of Cirrhosis. Medicines (Basel). 2018;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 99. | Adejumo AC, Alliu S, Ajayi TO, Adejumo KL, Adegbala OM, Onyeakusi NE, Akinjero AM, Durojaiye M, Bukong TN. Cannabis use is associated with reduced prevalence of non-alcoholic fatty liver disease: A cross-sectional study. PLoS One. 2017;12:e0176416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 100. | Vázquez-Bourgon J, Ortiz-García de la Foz V, Suarez-Pereira I, Iruzubieta P, Arias-Loste MT, Setién-Suero E, Ayesa-Arriola R, Gómez-Revuelta M, Crespo J, Crespo Facorro B. Cannabis consumption and non-alcoholic fatty liver disease. A three years longitudinal study in first episode non-affective psychosis patients. Prog Neuropsychopharmacol Biol Psychiatry. 2019;95:109677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 101. | Adejumo AC, Ajayi TO, Adegbala OM, Adejumo KL, Alliu S, Akinjero AM, Onyeakusi NE, Ojelabi O, Bukong TN. Cannabis use is associated with reduced prevalence of progressive stages of alcoholic liver disease. Liver Int. 2018;38:1475-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 102. | Zhu J, Peltekian KM. Cannabis and the liver: Things you wanted to know but were afraid to ask. Can Liver J. 2019;2:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Avraham Y, Grigoriadis N, Poutahidis T, Vorobiev L, Magen I, Ilan Y, Mechoulam R, Berry E. Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br J Pharmacol. 2011;162:1650-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 104. | Liu T, Howell GT, Turner L, Corace K, Garber G, Cooper C. Marijuana use in hepatitis C infection does not affect liver biopsy histology or treatment outcomes. Can J Gastroenterol Hepatol. 2014;28:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 105. | Barré T, Rojas Rojas T, Lacombe K, Protopopescu C, Poizot-Martin I, Nishimwe ML, Zucman D, Esterle L, Billaud E, Aumaitre H, Bouchaud O, Rey D, Piroth L, Salmon-Ceron D, Wittkop L, Sogni P, Carrieri MP, Serfaty L, Marcellin F. Cannabis use and reduced risk of elevated fatty liver index in HIV-HCV co-infected patients: a longitudinal analysis (ANRS CO13 HEPAVIH). Expert Rev Anti Infect Ther. 2021;19:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | Nordmann S, Vilotitch A, Roux P, Esterle L, Spire B, Marcellin F, Salmon-Ceron D, Dabis F, Chas J, Rey D, Wittkop L, Sogni P, Carrieri P; ANRS CO13 HEPAVIH Study Group. Daily cannabis and reduced risk of steatosis in human immunodeficiency virus and hepatitis C virus-co-infected patients (ANRS CO13-HEPAVIH). J Viral Hepat. 2018;25:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 107. | Pagliari D, Saviano A, Brizi MG, Mancarella FA, Cannone F, Musso M, Franza L, Attili F, Gasbarrini A. Cannabis-induced acute pancreatitis: a case report with comprehensive literature review. Eur Rev Med Pharmacol Sci. 2019;23:8625-8629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 108. | Lubega F, Lwanga A. An Unexpected Case of Cannabis-Induced Pancreatitis. Cureus. 2021;13:e13253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 109. | Ghazaleh S, Alqahtani A, Nehme C, Abugharbyeh A, Said Ahmed TS. A Rare Case of Cannabis-induced Acute Pancreatitis. Cureus. 2019;11:e4878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 110. | Barkin JA, Nemeth Z, Saluja AK, Barkin JS. Cannabis-Induced Acute Pancreatitis: A Systematic Review. Pancreas. 2017;46:1035-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 111. | Azam C, Buscail L, Culetto A, Lapeyre-Mestre M. Cannabinoid-Related Acute Pancreatitis: An Update from International Literature and Individual Case Safety Reports. Drug Saf. 2022;45:215-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 112. | Jaiswal V, Mukherjee D, Batra N, Ruchika F, Susheela AT, Chia JE, Naz S, Victor AA, Pokhrel NB, Song D, Seen T, Almas T, Saleh MA, Bansrao AS, Mansoor E. Acute pancreatitis as a rare adverse event among cannabis users: A systematic review. Medicine (Baltimore). 2022;101:e29822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 113. | Simons-Linares CR, Barkin JA, Wang Y, Jaiswal P, Trick W, Bartel MJ, Barkin JS. Is There an Effect of Cannabis Consumption on Acute Pancreatitis? Dig Dis Sci. 2018;63:2786-2791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 114. | Fischer B, Russell C, Sabioni P, van den Brink W, Le Foll B, Hall W, Rehm J, Room R. Lower-Risk Cannabis Use Guidelines: A Comprehensive Update of Evidence and Recommendations. Am J Public Health. 2017;107:e1-e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 115. | Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1573] [Cited by in RCA: 1763] [Article Influence: 160.3] [Reference Citation Analysis (0)] |
| 116. | James A, Hough M, James S, Winmill L, Burge L, Nijhawan S, Matthews PM, Zarei M. Greater white and grey matter changes associated with early cannabis use in adolescent-onset schizophrenia (AOS). Schizophr Res. 2011;128:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 117. | Koenders L, Cousijn J, Vingerhoets WA, van den Brink W, Wiers RW, Meijer CJ, Machielsen MW, Veltman DJ, Goudriaan AE, de Haan L. Grey Matter Changes Associated with Heavy Cannabis Use: A Longitudinal sMRI Study. PLoS One. 2016;11:e0152482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 118. | Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N. Acute and Chronic Effects of Cannabinoids on Human Cognition-A Systematic Review. Biol Psychiatry. 2016;79:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 437] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 119. | Lawn W, Fernandez-Vinson N, Mokrysz C, Hogg G, Lees R, Trinci K, Petrilli K, Borissova A, Ofori S, Waters S, Michór P, Wall MB, Freeman TP, Curran HV. The CannTeen study: verbal episodic memory, spatial working memory, and response inhibition in adolescent and adult cannabis users and age-matched controls. Psychopharmacology (Berl). 2022;239:1629-1641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Wu TC, Tashkin DP, Djahed B, Rose JE. Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med. 1988;318:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 314] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 121. | National Academies of Sciences E and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC): National Academies Press (US), 2017. |
| 122. | Hancox RJ, Shin HH, Gray AR, Poulton R, Sears MR. Effects of quitting cannabis on respiratory symptoms. Eur Respir J. 2015;46:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 123. | de Carvalho MF, Dourado MR, Fernandes IB, Araújo CT, Mesquita AT, Ramos-Jorge ML. Head and neck cancer among marijuana users: a meta-analysis of matched case-control studies. Arch Oral Biol. 2015;60:1750-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 124. | Gurney J, Shaw C, Stanley J, Signal V, Sarfati D. Cannabis exposure and risk of testicular cancer: a systematic review and meta-analysis. BMC Cancer. 2015;15:897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 125. | Calabria B, Degenhardt L, Hall W, Lynskey M. Does cannabis use increase the risk of death? Drug Alcohol Rev. 2010;29:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 126. | Rogeberg O, Elvik R. The effects of cannabis intoxication on motor vehicle collision revisited and revised. Addiction. 2016;111:1348-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 204] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 127. | Wadsworth EJ, Moss SC, Simpson SA, Smith AP. A community based investigation of the association between cannabis use, injuries and accidents. J Psychopharmacol. 2006;20:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 128. | Onders B, Casavant MJ, Spiller HA, Chounthirath T, Smith GA. Marijuana Exposure Among Children Younger Than Six Years in the United States. Clin Pediatr (Phila). 2016;55:428-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 129. | Wang GS, Le Lait MC, Deakyne SJ, Bronstein AC, Bajaj L, Roosevelt G. Unintentional Pediatric Exposures to Marijuana in Colorado, 2009-2015. JAMA Pediatr. 2016;170:e160971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 130. | Wang GS, Roosevelt G, Le Lait MC, Martinez EM, Bucher-Bartelson B, Bronstein AC, Heard K. Association of unintentional pediatric exposures with decriminalization of marijuana in the United States. Ann Emerg Med. 2014;63:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 131. | Connor JP, Stjepanović D, Le Foll B, Hoch E, Budney AJ, Hall WD. Cannabis use and cannabis use disorder. Nat Rev Dis Primers. 2021;7:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 240] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 132. | Assocation AP. DSM-5-TR: Diagnostic and Statistical Manual of Mental Disorders 5th, text revision ed. Washington (DC): American Psychiatric Association Publishing, 2022. |
| 133. | Kedzior KK, Laeber LT. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population--a meta-analysis of 31 studies. BMC Psychiatry. 2014;14:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 134. | Blanco C, Hasin DS, Wall MM, Flórez-Salamanca L, Hoertel N, Wang S, Kerridge BT, Olfson M. Cannabis Use and Risk of Psychiatric Disorders: Prospective Evidence From a US National Longitudinal Study. JAMA Psychiatry. 2016;73:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 135. | Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and anxiety disorders: Results from a population-based representative sample. Eur Neuropsychopharmacol. 2016;26:493-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 136. | Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and mood disorders: A longitudinal study. J Affect Disord. 2015;172:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 137. | Prud'homme M, Cata R, Jutras-Aswad D. Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence. Subst Abuse. 2015;9:33-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 138. | Venkatesan T, Levinthal DJ, Tarbell SE, Jaradeh SS, Hasler WL, Issenman RM, Adams KA, Sarosiek I, Stave CD, Sharaf RN, Sultan S, Li BUK. Guidelines on management of cyclic vomiting syndrome in adults by the American Neurogastroenterology and Motility Society and the Cyclic Vomiting Syndrome Association. Neurogastroenterol Motil. 2019;31 Suppl 2:e13604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 139. | Ruffle JK, Bajgoric S, Samra K, Chandrapalan S, Aziz Q, Farmer AD. Cannabinoid hyperemesis syndrome: an important differential diagnosis of persistent unexplained vomiting. Eur J Gastroenterol Hepatol. 2015;27:1403-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 140. | Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150:1257-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1036] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 141. | Senderovich H, Patel P, Jimenez Lopez B, Waicus S. A Systematic Review on Cannabis Hyperemesis Syndrome and Its Management Options. Med Princ Pract. 2022;31:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 142. | Ruberto AJ, Sivilotti MLA, Forrester S, Hall AK, Crawford FM, Day AG. Intravenous Haloperidol Versus Ondansetron for Cannabis Hyperemesis Syndrome (HaVOC): A Randomized, Controlled Trial. Ann Emerg Med. 2021;77:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 143. | Hancock-Allen JB, Barker L, VanDyke M, Holmes DB. Notes from the Field: Death Following Ingestion of an Edible Marijuana Product--Colorado, March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:771-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 144. | Lima KSB, Silva MEGDC, Araújo TCL, Silva CPDF, Santos BL, Ribeiro LAA, Menezes PMN, Silva MG, Lavor ÉM, Silva FS, Nunes XP, Rolim LA. Cannabis roots: Pharmacological and toxicological studies in mice. J Ethnopharmacol. 2021;271:113868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 145. | Avery D. Election Day 2022: These 5 States Could Legalize Marijuana: CNET; 2022. Available from: https://www.cnet.com/culture/election-day-2022-these-5-states-could-legalize-marijuana/. |
| 146. | Bhaskar A, Bell A, Boivin M, Briques W, Brown M, Clarke H, Cyr C, Eisenberg E, de Oliveira Silva RF, Frohlich E, Georgius P, Hogg M, Horsted TI, MacCallum CA, Müller-Vahl KR, O'Connell C, Sealey R, Seibolt M, Sihota A, Smith BK, Sulak D, Vigano A, Moulin DE. Consensus recommendations on dosing and administration of medical cannabis to treat chronic pain: results of a modified Delphi process. J Cannabis Res. 2021;3:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 147. | MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 374] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 148. | Naftali T, Bar Lev Schlieder L, Sklerovsky Benjaminov F, Lish I, Hirsch J, Konikoff FM. P398 Cannabis induces clinical and endoscopic improvement in moderately active ulcerative colitis (UC). J Crohns Colitis. 2018;12 Suppl 1:S306. [DOI] [Full Text] |
| 149. | Hoffenberg EJ, McWilliams S, Mikulich-Gilbertson S, Murphy B, Hoffenberg A, Hopfer CJ. Cannabis Oil Use by Adolescents and Young Adults With Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2019;68:348-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 150. | Choi C, Abougergi M, Peluso H, Weiss SH, Nasir U, Pyrsopoulos N. Cannabis Use is Associated With Reduced 30-Day All-cause Readmission Among Hospitalized Patients With Irritable Bowel Syndrome: A Nationwide Analysis. J Clin Gastroenterol. 2022;56:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 151. | Foltin RW, Fischman MW, Byrne MF. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite. 1988;11:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 175] [Article Influence: 4.7] [Reference Citation Analysis (0)] |