Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.4123
Peer-review started: March 22, 2023
First decision: April 19, 2023
Revised: May 2, 2023
Accepted: May 12, 2023
Article in press: May 12, 2023
Published online: June 16, 2023
Processing time: 81 Days and 19.9 Hours
Hereditary multiple exostoses is a rare genetic disorder characterized by the growth of multiple osteochondromas affecting primarily long bones. Chest wall lesions may represent a challenge, particularly in pediatric patients. Pain is a common manifestation. However, life-threatening complications can result from direct involvement of adjacent structures. Surgical resection with appropriate reconstruction is often required.
A 5-year-old male who was diagnosed with hereditary multiple exostoses presented with significant pain from a large growing chest wall exostosis lesion. After appropriate preoperative investigations, he underwent surgical resection with reconstruction of his chest wall using a biologic bovine dermal matrix mesh.
Resection of chest wall lesions in children represents a challenge. Preoperative planning to determine the appropriate reconstruction strategy is essential.
Core Tip: Hereditary multiple exostoses is an uncommon genetic musculoskeletal disorder characterized by multiple skeletal lesions (exostoses). Although most lesions are asymptomatic, pain is a frequent patient complaint. Additionally, life-threatening complications may occur secondary to direct involvement of adjacent structures. Our patient had several skeletal lesions with a large chest wall exostosis causing significant pain and disability. Resection was successfully performed with chest wall reconstruction using a biologic mesh derived from acellular bovine dermis. For similar cases, careful patient selection, proper perioperative planning, and appropriate reconstruction strategy are important factors to achieve the desired outcome.
- Citation: Alshehri A. Chest wall osteochondroma resection with biologic acellular bovine dermal mesh reconstruction in pediatric hereditary multiple exostoses: A case report and review of literature. World J Clin Cases 2023; 11(17): 4123-4132
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/4123.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.4123
Exostosis, also known as osteochondroma, is a benign osseous outgrowth that can occur either sporadically or as a manifestation of a genetic disorder known as hereditary multiple exostoses (HME). The exact etiology of the disease is unknown. However, it is hypothesized that the lesions originate from separation of a portion of the epiphyseal growth plate cartilage from the main epiphysis[1-4]. Although these lesions are usually well tolerated, exostoses might cause significant pain and disabilities. More serious complications have been reported including impingement on neural or vascular structures, joint motion limitation, deformities, limb discrepancy, or malignant transformation[5]. Chest wall osteochondromas can present with life-threatening complications such as pneumothorax, hemothorax, pericardial effusion, or diaphragmatic injury[6-13]. Resection is typically recommended for symptomatic exostoses, and reconstruction may be required depending on several factors[5,14]. A wide range of prosthetic material including biologic meshes have been used to reconstruct the chest wall in pediatric and adult patients[15-23].
In this case report, a 5-year-old male presented with a painful large chest wall exostosis that was resected. Chest wall reconstruction using a biologic mesh derived from acellular bovine dermis was performed as well.
A 5-year-old male presented to our pediatric surgery clinic with a painful mass in the chest wall.
The patient had been diagnosed previously with HME at the age of 1 year. He had multiple bony lesions affecting his chest wall, upper and lower extremities. Despite occasional pain over the bony lesions, his overall functional status was excellent. Since the age of 3 years, his parents noticed a bony lesion over the right side of his chest that was growing. It started to cause significant pain and affected his sleep, right arm movement, and overall ability to attend his school.
Besides diagnosis of HME, the patient had no other illnesses nor any known allergies.
The patient had a strong family history of HME, affecting both his brother and uncle.
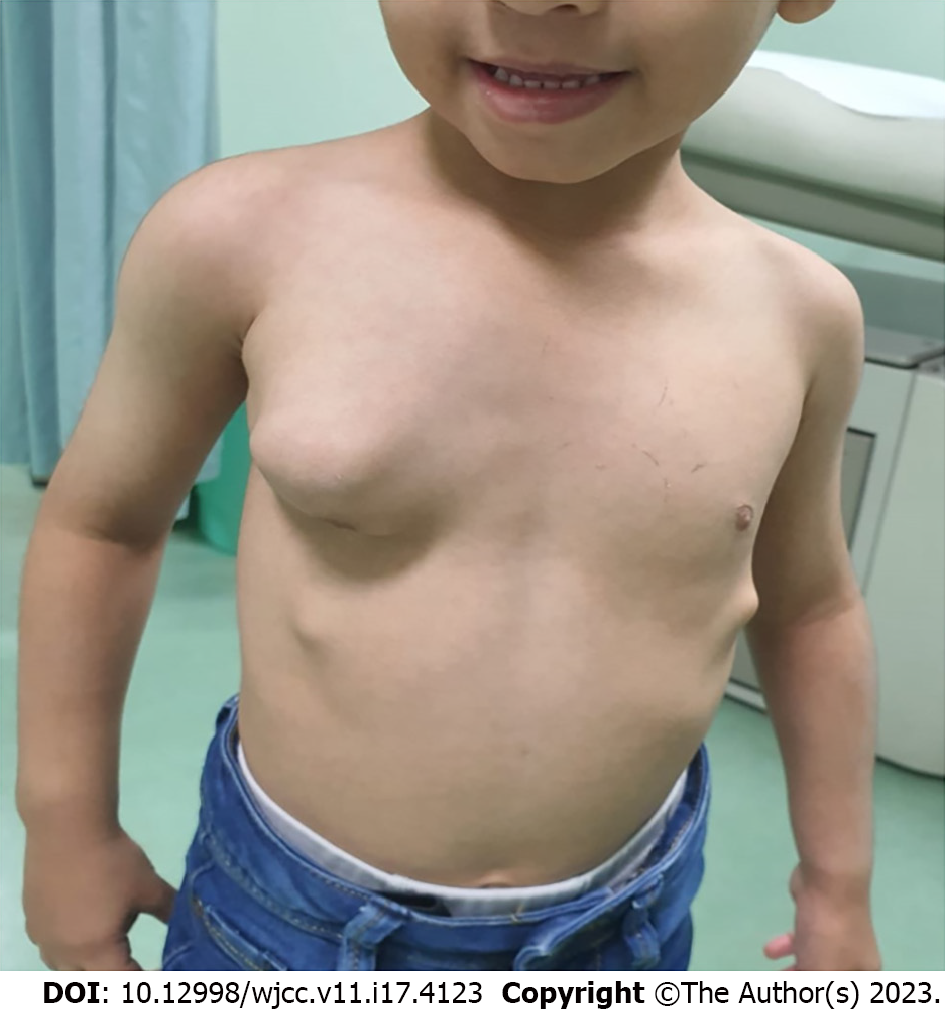
The patient had normal vital signs. His chest examination revealed a 10 cm irregular, hard mass over the right anterior chest centered along the anterior axillary line. The overlying skin was normal (Figure 1). Another 2 cm lesion was felt below the main lesion and close to the right costal margin. A third small lesion was present on the left side of his chest, which was located at the costal margin. Several small bony lesions were felt over his upper and lower extremities close to his elbow and knee joints.
Blood analysis revealed the following: White blood cell count, 12.5 × 109/L (reference range: 5.0-15.5 × 109/L); red blood cell count, 3.5 × 1012/L (reference range: 3.9-5.0 × 1012/L); hemoglobin, 114 g/L (reference range: 110-138 g/L); and platelet count, 284 × 109/L (reference range: 150-350 × 109/L). Biochemical analysis revealed normal levels of total protein (58 g/L), albumin (34 g/L), creatinine (28 µmol/L), and urea nitrogen (4.1 mol/L).
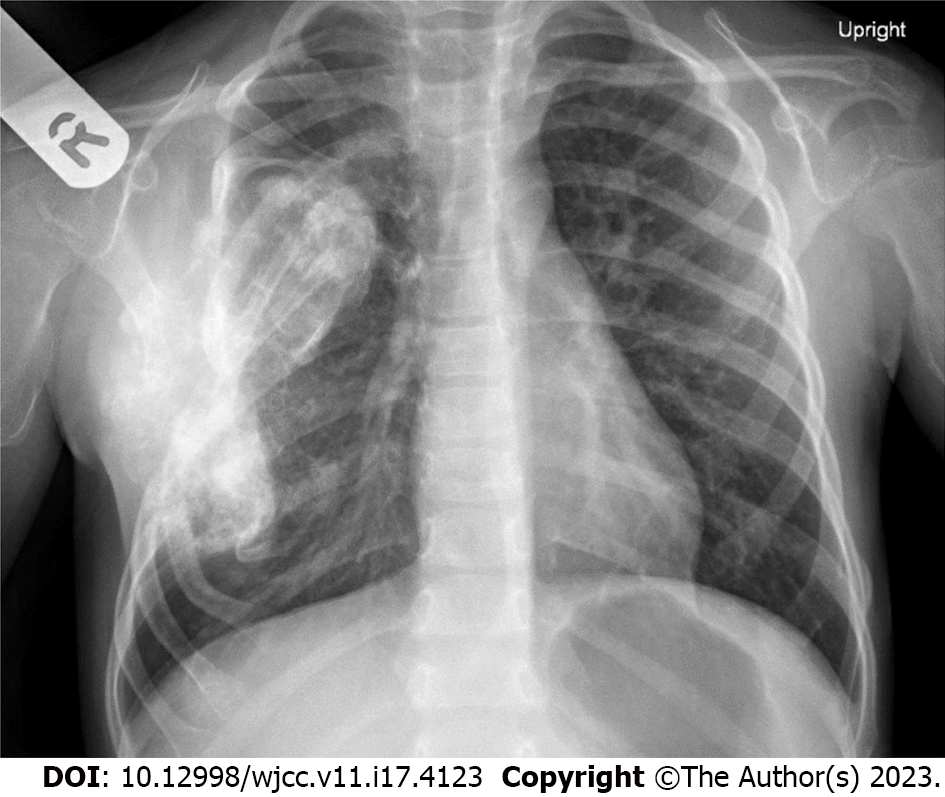
Chest X-ray showed a distorted right middle of the chest. An enlarged osseous/cartilaginous lobulated exophytic lesion compatible with known exostosis demonstrated a dense matrix compatible with cartilaginous matrix. The heart was not enlarged, and the lungs were otherwise clear (Figure 2).
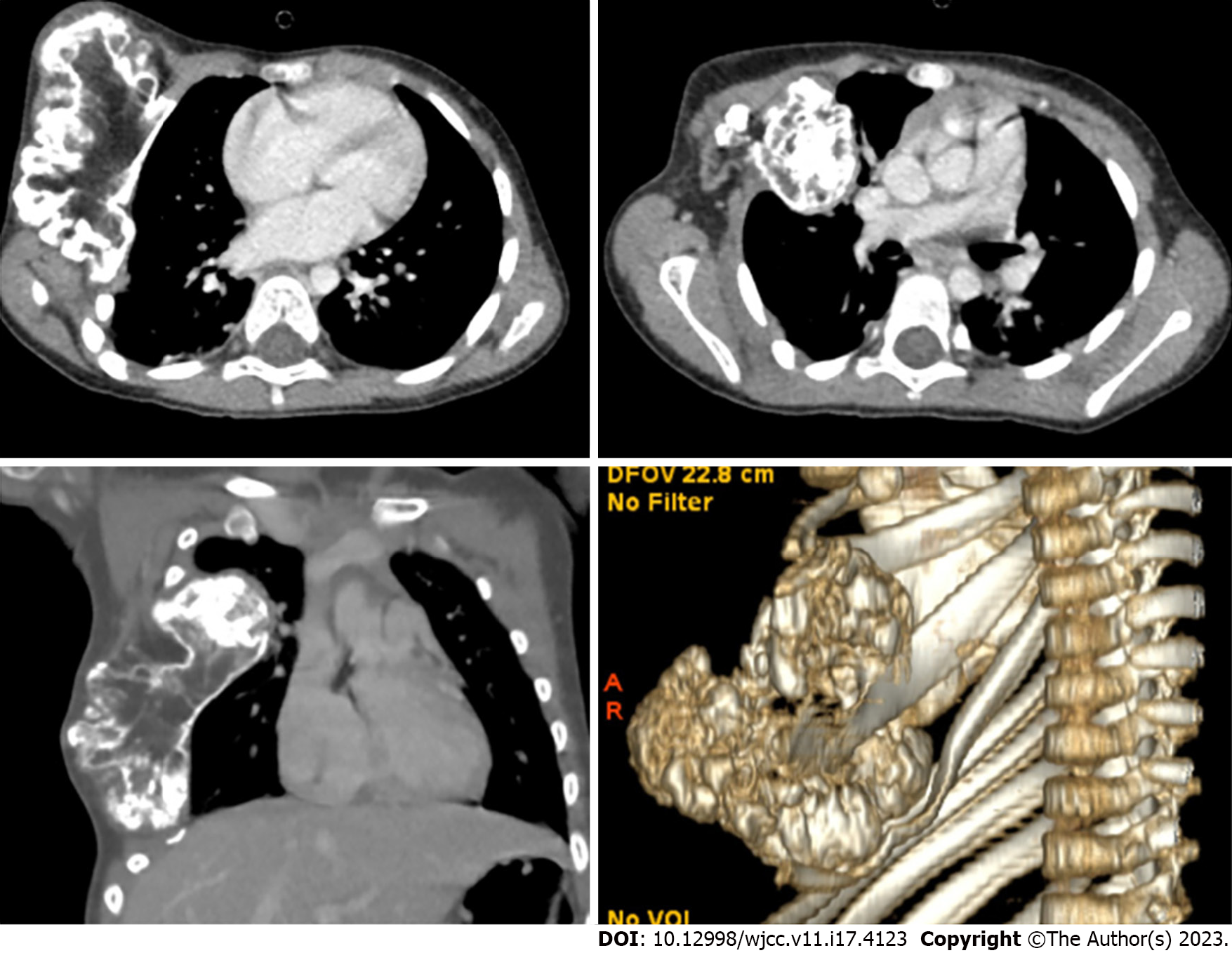
Computed tomography of the chest revealed large exostosis arising from the anterior portion of the right fourth rib extending to the costochondral junction measuring about 8.70 cm × 3.45 cm × 8.60 cm in the craniocaudal, transverse, and anteroposterior dimensions respectively. It had a mass effect on the lower ribs, which caused a chest wall deformity. There was also pseudo-articulation with the third rib. There was extension to the right hemithorax primarily at the level of the upper and middle lobes associated with adjacent middle lobe atelectatic changes. The intrathoracic extension of the exostosis was close to the right pulmonary vasculature. An intrathoracic extension portion measured 0.5 cm × 3.3 cm × 3.7 cm. Scattered exostoses were noted along the third, seventh, and eighth right ribs and the seventh left rib. Three-dimensional reconstruction was created to improve the understanding of the lesion and better guide the surgical operation (Figure 3).
Multiple bony lesions consistent with HME with a dominant large right chest wall lesion that caused significant pain and disability.
Due to the growth of the chest wall lesion and the significant discomfort that the patient experienced, surgical resection of the main chest lesion with chest wall reconstruction using SurgiMend® biologic mesh (Integra LifeSciences Corp., Plainsboro, NJ, United States) was planned. This mesh is composed of acellular dermal matrix derived from fetal and neonatal bovine dermis. This mesh is durable, malleable, and capable of allowing native tissue integration at the defect site. After discussion with the plastic surgeons, a latissimus dorsi muscle flap was planned to cover the mesh, if needed.
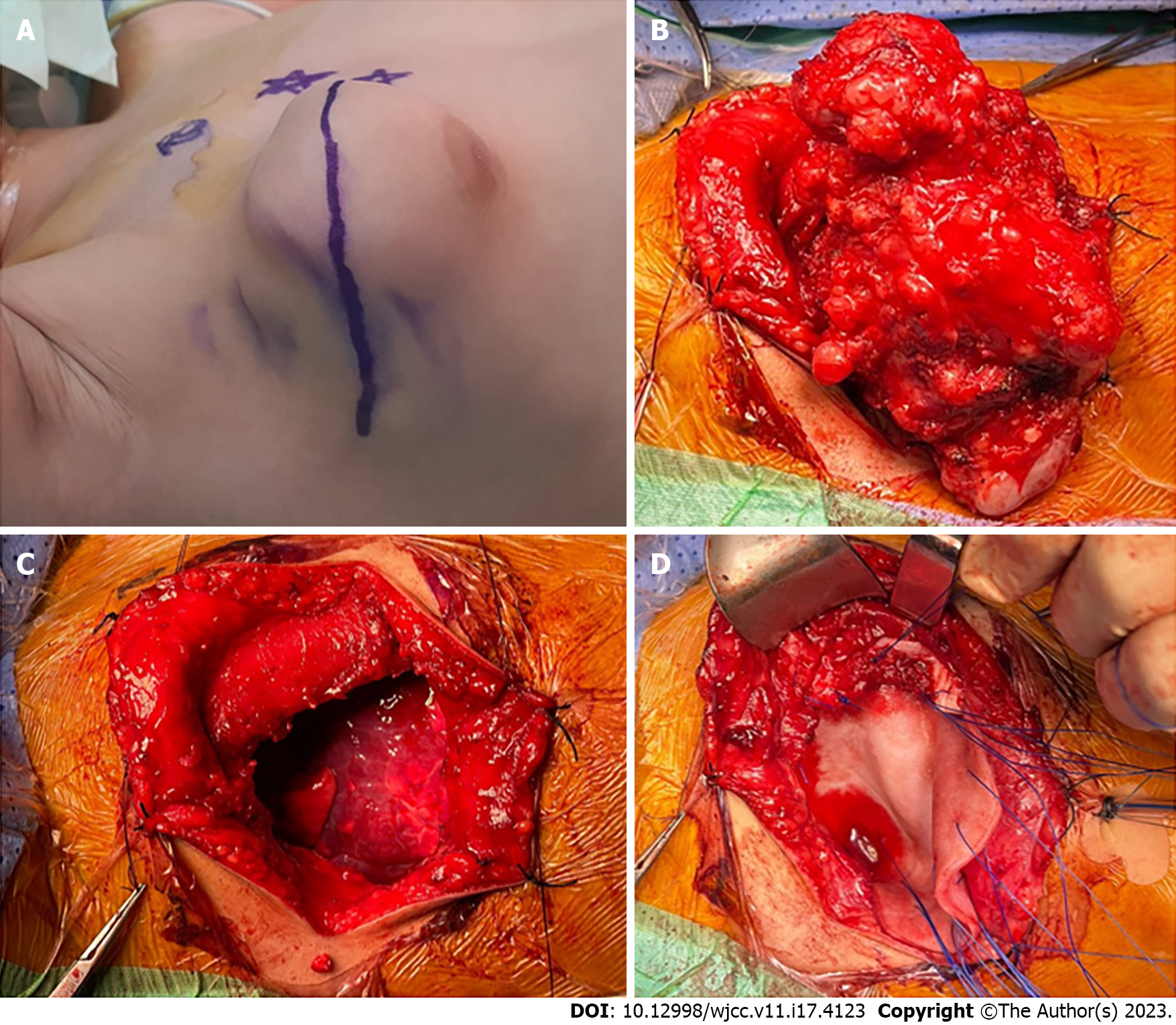
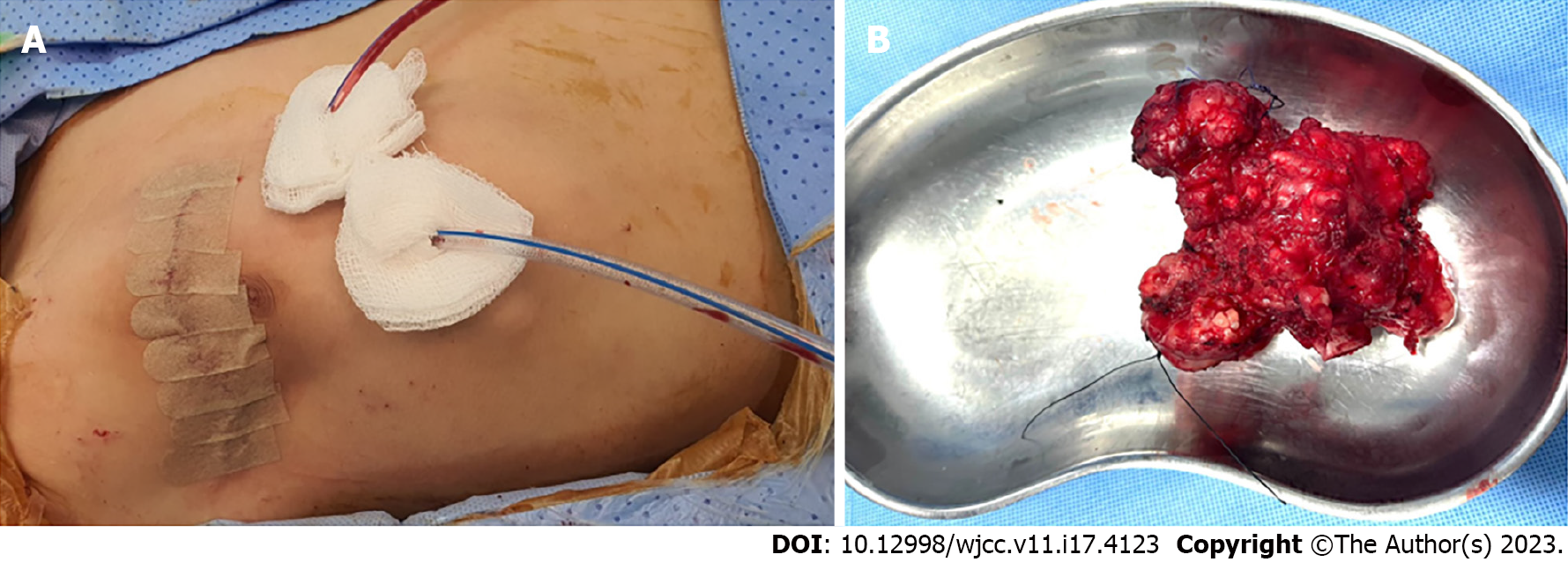
As shown in Figure 4A, the incision was marked over the center of the lesion. The plastic surgeon was present to ensure that the adjacent muscles and their neurovascular bundles were protected. The bony lesion was completely resected with the central portion of the third and fourth ribs (Figure 4B). The intrathoracic part of the lesion was not attached to the lung or mediastinal structures. The resulting chest wall defect was approximately 12 cm × 9 cm (Figure 4C). SurgiMend® mesh was then used to cover the defect in an onlay fashion using 0 prolene interrupted U-shaped sutures involving the surrounding ribs and intercostal muscles (Figure 4D). The reconstruction was satisfactory, and there was adequate soft tissue coverage. Therefore, the latissimus dorsi muscle flap procedure was unnecessary. Two closed suction drains were placed in the chest and in the subcutaneous tissue (Figure 5A). The ex vivo size of the lesion was 10.5 cm × 9.0 cm × 5.5 cm (Figure 5B).
Postoperatively, the patient was admitted to the pediatric intensive care unit and was extubated the next day. The drains were removed on the 3rd postoperative day, and the patient was sent home on the 6th postoperative day. Histopathological assessment of the resected specimen was consistent with osteochondroma without elements of malignancy.
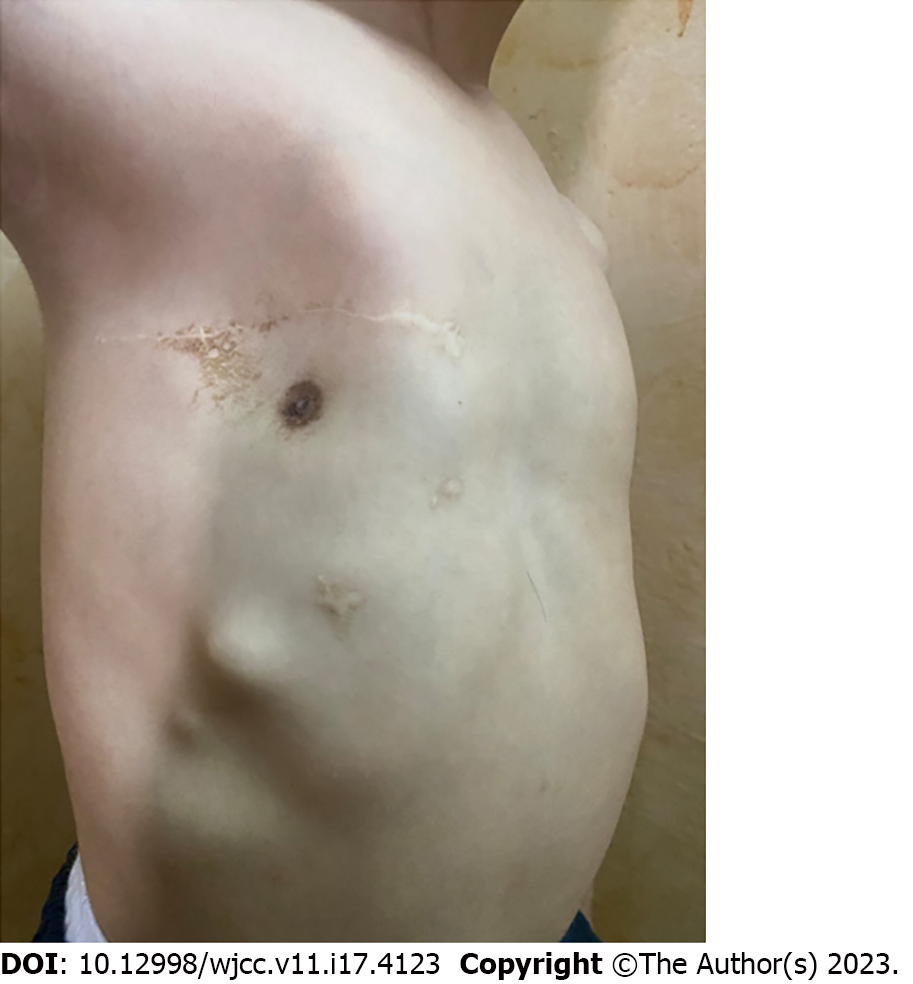
The patient was followed up monthly in the pediatric surgery clinic. He was very cheerful and happy. He has had no complications, and the chest wall reconstruction remained satisfactory at the 1-year follow-up (Figure 6). No recurrence nor chest wall deformities were noted.
HME is a complex musculoskeletal disorder characterized by multiple osteochondromas primarily affecting long bones, ribs, and vertebrae. It is a rare autosomal dominant disorder with an estimated incidence of 1:50000[1,2]. Over 80% of HME cases are associated with loss-of-function mutations in the EXT1 or EXT2 genes. Although exostoses are initially asymptomatic, they can cause significant health problems including pain, limb discrepancy, short stature, nerve or vessel impingement, skeletal deformities, and a restricted range of motion[1,2,5]. In approximately 2% of patients, osteochondromas can undergo malignant transformation to form chondrosarcomas or osteosarcomas[24]. The disease has no medical treatment. However, surgery is recommended for symptomatic exostosis or when malignant transformation is suspected[3,4]. Symptoms that may warrant surgical resection include significant pain or the occurrence of the aforementioned complications[12,25,26]. Several complications of chest wall osteochondromas have been reported in the literature. Some are life-threatening, including erosion into the adjacent structures causing hemothorax, pneumothorax, pericardial effusion, or diaphragmatic injury[6-11,14,26-30]. Rapid growth of the lesion may indicate malignant transformation[3,4,14].
The patient in this case report had significant chest pain due to the mass increasing in size. His pain caused significant problems with attending school and negatively affected his sleeping habits. Additionally, the rapid increase in the size of the dominant chest lesion was concering for malignant transformation. The child and his parents were very interested in surgical treatment, even after discussion of the potential challenges to reconstruct the chest wall and the possible complications.
Surgical resectability of chest wall lesions is determined by several factors including the number of ribs involved, the degree of intrathoracic extension, the involvement of the spinal canal, the reconstructability of the chest wall, and the possible functional outcomes after the resection. Cross-sectional imaging should be carefully studied during surgical planning to accurately define the extent of the tumor, plan the resection technique, and choose the appropriate reconstruction strategy. Three-dimensional reconstruction of the cross-sectional images is a useful preoperative tool to study the surgical field.
In the operating room, the patient should be positioned so that the area to be resected is easily accessible. Access to the latissimus dorsi and pectoralis major muscles is important in cases where reconstruction with the muscle flap is required. In this case, the incision line was marked by the plastic surgeon to ensure that the neurovascular bundles of the major chest wall muscles were not compromised.
After the resection was completed, chest wall reconstruction was composed of two parts: Skeletal reconstruction and soft tissue coverage. Typically, resection involving less than four ribs do not require skeletal reconstruction. However, soft tissue reconstruction is still required in these situations. In contrast, larger defects (involving more than four ribs and/or defect size of > 5 cm) will require skeletal reconstruction with biologic or alloplastic material. The ideal reconstruction should perform the following functions: Restore chest wall rigidity; prevent lung herniation; avoid chest wall contraction; prevent trapping the scapula; protect underlying thoracic and mediastinal structures; and provide acceptable cosmetic results[15,17,20,26,31].
A wide variety of materials are now available for prosthetic chest wall reconstruction, including biologic, alloplastic, and synthetic materials. Table 1 provides a summary of the literature regarding chest wall reconstruction in children. The ideal prosthetic material for chest wall reconstruction should be rigid enough to prevent paradoxical chest wall movement, malleable to allow for contouring, able to grow with or accommodate the growth of the pediatric patient, biologically inert, stimulatory for tissue in-growth, radiolucent, resistant to infection, and relatively inexpensive. Several materials have been used successfully to reconstruct the chest wall of pediatric patients; however, no material has been proven to be superior in chest wall reconstruction in pediatric patients[20,32]. The materials include absorbable polyglactin, non-absorbable polypropylene, and polytetrafluoroethylene. Alternatively, biological meshes derived from cadaveric human dermis, porcine intestinal submucosa, porcine dermis, bovine pericardium, and bovine dermis (SurgiMend®; Integra LifeSciences Corp., Plainsboro, NJ, United States) have been used with acceptable results[14,16,21,22,26,32-35]. For robust reconstruction of very large defects, metallic rib substitutes have been used in pediatric patients[19,36,37]. There have been no comparative studies of the different prosthetic materials, which should be a focus for future studies.
| Ref. | Number of patients | Ages | Diagnoses | Prosthetic material used for reconstruction | Outcomes |
| Grosfeld et al[38], 1988 | 15 | 8 mo-11 yr | ES, RMS, NBM, CS, MS, Wilms’ tumor | Marlex mesh, Gore-Tex® patch, Prolene mesh | Acceptable |
| Dang et al[39], 1999 | 4 | 10 wk-23 yr | ES, PNET, RMS, Askin’s tumor | Marlex mesh, Gore-Tex® patch, Dexon mesh | Acceptable |
| Tuggle et al[40], 2004 | 4 | 6 mo-19 yr | ES, PNET, spindle cell tumor | Bioabsorbable copolymer plates (Lactosorb) | Acceptable |
| Soyer et al[41], 2006 | 9 | 11 mo-14 yr | ES, RMS, OC, NBM | Gore-Tex® patch, Neuro-patch, and Dura | Acceptable (1 minor wound infection after dura reconstruction) |
| Stephenson et al[19], 2011 | 6 | 5-19 yr | ES, OS, Desmoid tumor, congenital thoracic insufficiency (Poland syndrome) | Titanium constructs | Acceptable |
| Jackson et al[42], 2011 | 2 | 3 yr, 4 yr | PPB, infiltrative fibrous hamartoma of infancy | Gore-Tex® patch, STRATOS™ titanium bar | Acceptable |
| Dingemann et al[32], 2012 | 8 | 4-19 yr | ES, OS, PNET, RMS | STRATOS™ titanium bar, Gore-Tex® patch, Vicryl® patch, Tutopatch® | Acceptable |
| Lin et al[21], 2012 | 5 | 9-21 yr | ES, PNET | Biologic mesh (Permacol) | Acceptable |
| Makarawo et al[43], 2015 | 1 | 13 yr | ES | Polylactide bioabsorbable Struts (BioBridge®) | Acceptable |
| Lopez et al[18], 2017 | 22 | 4-18 yr | ES, RMS, CS, OS, SS | Gore-Tex® patch, Marlex mesh, Dexon mesh, SurgiMend® mesh, Vicryl® mesh | 2 wound infections with exposed prosthesis required additional surgeries |
| Miyake et al[44], 2017 | 8 | 8-12 yr | ES | Bioabsorbable copolymer plates (Lactosorb) | Acceptable |
In this patient, SurgiMend®, which is composed of acellular dermal matrix derived from neonatal and fetal bovine dermis, was utilized for chest wall reconstruction. It is known to be durable as well as to conform to the shape of the chest wall. No mesh-related complications were observed within the 1-year follow-up period. The soft tissue coverage in this patient was acceptable without requiring the mobilization of muscle flaps. Based on our experience, we think that acellular dermal matrix mesh is an appropriate prosthesis to be used for chest wall reconstruction in children.
Exostosis, whether sporadic or caused by HME, may require surgical resection if it causes significant symptoms or disability. Moreover, chest wall lesions can be very challenging particularly in pediatric patients. Surgeons must carefully plan the most appropriate chest wall reconstruction strategy and choose from a variety of prosthetic materials. To assess late complications, such as prosthesis complications or acquired chest wall deformities, long-term follow-up is essential, particularly in pediatric patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kahveci R, Turkey; Velikova TV, Bulgaria S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Wicklund CL, Pauli RM, Johnston D, Hecht JT. Natural history study of hereditary multiple exostoses. Am J Med Genet. 1995;55:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 203] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Schmale GA, Conrad EU 3rd, Raskind WH. The natural history of hereditary multiple exostoses. J Bone Joint Surg Am. 1994;76:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 391] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Pacifici M. Hereditary Multiple Exostoses: New Insights into Pathogenesis, Clinical Complications, and Potential Treatments. Curr Osteoporos Rep. 2017;15:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | D'Arienzo A, Andreani L, Sacchetti F, Colangeli S, Capanna R. Hereditary Multiple Exostoses: Current Insights. Orthop Res Rev. 2019;11:199-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Stieber JR, Dormans JP. Manifestations of hereditary multiple exostoses. J Am Acad Orthop Surg. 2005;13:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Matsuno Y, Mori Y, Umeda Y, Imaizumi M, Takiya H. Thoracoscopic resection for costal exostosis presenting with hemothorax in a child. Eur J Pediatr Surg. 2009;19:253-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Kuo SM, Chen KC, Diau GY, Hua YM. Dangerous costal exostosis: hemothorax mimicking empyema in a child. J Pediatr. 2010;156:853, 853.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Simansky DA, Paley M, Werczberger A, Bar Ziv Y, Yellin A. Exostosis of a rib causing laceration of the diaphragm: diagnosis and management. Ann Thorac Surg. 1997;63:856-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Assefa D, Murphy RC, Bergman K, Atlas AB. Three faces of costal exostoses: case series and review of literature. Pediatr Emerg Care. 2011;27:1188-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Chawla JK, Jackson M, Munro FD. Spontaneous pneumothoraces in hereditary multiple exostoses. Arch Dis Child. 2013;98:495-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Pham-Duc ML, Reix P, Mure PY, Pracros JP, Moreux N, Bellon G. Hemothorax: an unusual complication of costal exostosis. J Pediatr Surg. 2005;40:e55-e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Maree G, Rajab S, Almahmod Alkhalil MA. Symptomatic osteochondroma of the chest wall. J Pediatr Surg Case Reports. 2022;81:102288. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Abdullah F, Kanard R, Femino D, Ford H, Stein J. Osteochondroma causing diaphragmatic rupture and bowel obstruction in a 14-year-old boy. Pediatr Surg Int. 2006;22:401-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Bakhshi H, Kushare I, Murphy MO, Gaynor JW, Dormans JP. Chest wall osteochondroma in children: a case series of surgical management. J Pediatr Orthop. 2014;34:733-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Khullar OV, Fernandez FG. Prosthetic Reconstruction of the Chest Wall. Thorac Surg Clin. 2017;27:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Sanna S, Brandolini J, Pardolesi A, Argnani D, Mengozzi M, Dell'Amore A, Solli P. Materials and techniques in chest wall reconstruction: a review. J Vis Surg. 2017;3:95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Sandler G, Hayes-Jordan A. Chest wall reconstruction after tumor resection. Semin Pediatr Surg. 2018;27:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Lopez C, Correa A, Vaporciyan A, Austin M, Rice D, Hayes-Jordan A. Outcomes of chest wall resections in pediatric sarcoma patients. J Pediatr Surg. 2017;52:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Stephenson JT, Song K, Avansino JR, Mesher A, Waldhausen JH. Novel titanium constructs for chest wall reconstruction in children. J Pediatr Surg. 2011;46:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Theodorou CM, Lawrence YS, Brown EG. Chest wall reconstruction in pediatric patients with chest wall tumors: A systematic review. J Pediatr Surg. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 21. | Lin SR, Kastenberg ZJ, Bruzoni M, Albanese CT, Dutta S. Chest wall reconstruction using implantable cross-linked porcine dermal collagen matrix (Permacol). J Pediatr Surg. 2012;47:1472-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Murphy F, Corbally MT. The novel use of small intestinal submucosal matrix for chest wall reconstruction following Ewing's tumour resection. Pediatr Surg Int. 2007;23:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Kane G, Orr D, Pears J, McGuinness J. A Novel Approach to Extensive Chest Wall Reconstruction in a Child. Ann Thorac Surg. 2021;111:e389-e391. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Porter DE, Lonie L, Fraser M, Dobson-Stone C, Porter JR, Monaco AP, Simpson AH. Severity of disease and risk of malignant change in hereditary multiple exostoses. A genotype-phenotype study. J Bone Joint Surg Br. 2004;86:1041-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Yan G, Littlewood A, Latimer MD. Unusual cause of pleuritic chest pain in a child. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Cowles RA, Rowe DH, Arkovitz MS. Hereditary multiple exostoses of the ribs: an unusual cause of hemothorax and pericardial effusion. J Pediatr Surg. 2005;40:1197-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Khosla A, Parry RL. Costal osteochondroma causing pneumothorax in an adolescent: a case report and review of the literature. J Pediatr Surg. 2010;45:2250-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Martino A, Fabrizzi G, Costarelli L, Ilari M, de Benedictis FM. Haemothorax caused by isolated costal exostosis. Eur J Pediatr Surg. 2007;17:129-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Hajjar WM, El-Medany YM, Essa MA, Rafay MA, Ashour MH, Al-Kattan KM. Unusual presentation of rib exostosis. Ann Thorac Surg. 2003;75:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Blondel B, Launay F, Jacopin S, David M, Ungar B, Jouve JL, Bollini G. Siblings with vascular involvement associated with hereditary multiples exostosis. J Pediatr Orthop B. 2013;22:501-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Ferraro P, Cugno S, Liberman M, Danino MA, Harris PG. Principles of chest wall resection and reconstruction. Thorac Surg Clin. 2010;20:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Dingemann C, Linderkamp C, Weidemann J, Bataineh ZA, Ure B, Nustede R. Thoracic wall reconstruction for primary malignancies in children: short-and long-term results. Eur J Pediatr Surg. 2012;22:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Oliveira C, Zamakhshary M, Alfadda T, Alhabshan F, Alshalaan H, Miller S, Kim PC. An innovative method of pediatric chest wall reconstruction using Surgisis and swinging rib technique. J Pediatr Surg. 2012;47:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Russo V, Chiarucci C, Lofiego MF, Fazio C, Bertocci E, Cutaia O, Giacobini G, Lazzeri A, Lamboglia A, Altomonte M, Tunici P, Covre A, Maio M. Fourteenth Meeting of the Network Italiano per la Bioterapia dei Tumori (NIBIT) on Cancer Bio-Immunotherapy, Siena, Italy, October 13-15, 2016. Cancer Immunol Immunother. 2018;67:1023-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | André-Lévigne D, Modarressi A, Karenovics W, Joseph JM, Wilde JCH, Pittet-Cuénod B. Operative Planning of Chest Wall Reconstructions Illustrated by a Large Defect in a Child. Plast Reconstr Surg Glob Open. 2022;10:e4326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 36. | Pelc HJ, Walker J, Davies G. Successful operative management of an asymptomatic chest lesion in hereditary multiple exostosis. J Pediatr Orthop B. 2013;22:505-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Anderson CJ, Spruiell MD, Wylie EF, McGowan CM, Deleyiannis FW, Donaldson NJ, Heare TC. A technique for pediatric chest wall reconstruction using custom-designed titanium implants: description of technique and report of two cases. J Child Orthop. 2016;10:49-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Grosfeld JL, Rescorla FJ, West KW, Vane DW, DeRosa GP, Provisor AJ, Weetman R. Chest wall resection and reconstruction for malignant conditions in childhood. J Pediatr Surg. 1988;23:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Dang NC, Siegel SE, Phillips JD. Malignant chest wall tumors in children and young adults. J Pediatr Surg. 1999;34:1773-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Tuggle DW, Mantor PC, Foley DS, Markley MM, Puffinbarger N. Using a bioabsorbable copolymer plate for chest wall reconstruction. J Pediatr Surg. 2004;39:626-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Soyer T, Karnak I, Ciftci AO, Senocak ME, Tanyel FC, Büyükpamukçu N. The results of surgical treatment of chest wall tumors in childhood. Pediatr Surg Int. 2006;22:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Jackson L, Singh M, Parikh D. A technical innovation in paediatric chest wall reconstruction. Pediatr Surg Int. 2011;27:629-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Makarawo TP, Reynolds RA, Cullen ML. Polylactide bioabsorbable struts for chest wall reconstruction in a pediatric patient. Ann Thorac Surg. 2015;99:689-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Miyake H, Fukumoto K, Yamoto M, Nakajima H, Sekioka A, Yamada Y, Nomura A, Urushihara N. Corrigendum to "Risk factors for recurrence and contralateral inguinal hernia after laparoscopic percutaneous extraperitoneal closure for pediatric inguinal hernia. J Pediatr Surg. 2020;55:780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |