Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.4035
Peer-review started: March 6, 2023
First decision: April 10, 2023
Revised: April 24, 2023
Accepted: May 16, 2023
Article in press: May 16, 2023
Published online: June 16, 2023
Processing time: 98 Days and 5.8 Hours
Diabetic foot ulcer (DFU) is a serious health issue of diabetes mellitus that affects innumerable people worldwide. Management and treatment of this complication are challenging, especially for those whose immune system is weak.
To discuss the plants and their parts used to heal DFU, along with the mode of their administration in diabetic patients.
The original articles on “the plants for the treatment of DFU” studied in clinical cases only were obtained from various bibliographic databases using different keywords.
The search resulted in 22 clinical cases records with 20 medicinal plants belonging to 17 families on 1553 subjects. The fruits and leaves were the most preferentially used parts for DFU treatment, regardless of whether they were being administered orally or applied topically. Of the 20 medicinal plants, 19 reported their effectiveness in increasing angiogenesis, epithelialization, and granulation, thus hastening the wound-healing process. The efficacy of these botanicals might be attributed to their major bioactive compounds, such as actinidin and ascorbic acid (in Actinidia deliciosa), 7-O-(β-D-glucopyranosyl)-galactin (in Ageratina pichinchensis), omega-3-fatty acid (in Linum usitatissimum), isoquercetin (in Melilotus officinalis), anthocyanins (in Myrtus communis), and plantamajoside (in Plantago major).
The validation of mechanisms of action underlying these phytocompounds contributing to the management of DFU can aid in our better understanding of creating efficient treatment options for DFU and its associated problems.
Core Tip: Due to the fact that diabetic foot ulcer (DFU) may result in osteomyelitis and lower limb amputation in diabetic patients, this exhaustive systematic review can offer clinically relevant treatments for DFU using natural remedies. This review focuses on metabolites from 19 medicinal plants that could contribute to DFU healing. The recovery time for DFU, the route of administration of medicinal plants, and a comparison of the treated group to the positive and negative control groups were also included in this study to better understand the beneficial effects of using botanicals in the management of DFU.
- Citation: Narzary I, Swarnakar A, Kalita M, Middha SK, Usha T, Babu D, Mochahary B, Brahma S, Basumatary J, Goyal AK. Acknowledging the use of botanicals to treat diabetic foot ulcer during the 21st century: A systematic review. World J Clin Cases 2023; 11(17): 4035-4059
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/4035.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.4035
The present scenario of human civilization has become prone to several forms of disharmony and the development of different forms of diseases responsible for the curtailment of life in every possible way. Among those varieties of disease conditions, diabetes mellitus (DM), a state of elevated blood sugar level in the body, in the long run of disease format, has paved the path for living in anguish and suffering both physically and mentally. Several aetiological factors are responsible for the development of DM. Anxiety and stress are only two of the numerous issues that have become a matter of concern in the medical field today. In the modern world, the younger generation has abandoned physical exercise in favour of social media and other technologies for entertainment and other purposes, resulting in obesity and a sedentary lifestyle. Many Western countries have witnessed the early onset of DM (type 2) in children between 1990 and 2000[1]. Remarkably, between 1998 and 2005, there was an eight-fold rise in prescriptions for reducing glucose levels in the United Kingdom[1]. Nearly 200 million people worldwide have DM, with type 2 being the most frequent. It is expected to rise exponentially to 300 million people in the next two decades[2] or 439 million by 2030[3]. The predicted increase in the number of diabetic cases by Shaw et al[3] has been reached in just eight years, which was actually expected to occur over a span of 20 years[4]. Additionally, every year, nearly 400 million people suffer from DM and around 1.6 million people die from it; the vast majority of them are from low-income families[5,6].
Among all the consequences, diabetic foot ulcer (DFU) is one of the most significant complications that DM patients face. Some of the significant causes of DFU include poor glycemic management, repetitive trauma, underlying neuropathy, peripheral vascular disease, and poor foot care. The prevalence of DFU is increasing globally, and more people are suffering due to inadequate information and precarious economic conditions. The global DFU incidence was 8.5%[7], with North America (13%) having the highest incidence and Europe (3%) and Africa (7.2%) having the lowest[8]. DFU has been reported to be more prevalent in males than females[9]. According to DFU statistics in India, 25% acquire DFU, 50% become infected, and around 20% require amputation. Notably, DFU contributes to nearly 80% of amputations in India each year[10].
Neuropathy is the underlying cause of DFU. The polyol pathway converts hexose sugar into polyols, which is an important element during nerve injury. In a normal physiological state, glucose is metabolized through the hexokinase route. However, in a hyperglycemic condition, the hexokinase pathway becomes saturated, allowing the polyol pathway to take over. The enzyme aldose reductase converts glucose to sorbitol. Sorbitol is difficult to break down, resulting in increased cellular osmolarity and cell damage. The activation of the polyol pathway, which also participates in numerous metabolic processes, reduces the concentration of pyridine nucleotides. This results in a drop in glutathione, nitric oxide, myoinositol and taurine concentrations[11]. The chemical conversion of sugar affects the reserves of nicotinamide adenine dinucleotide phosphate (NADP), which are responsible for the vasodilator nitric oxide. Vasoconstriction and ischemia are caused by NADP disruption, resulting in nerve cell injury and cell death. These factors cause a chain reaction that affects the body’s motor, autonomic, and sensory systems, as well as the foot muscles, resulting in the deformity in the flexion and extension of the affected foot, the development of broken skin, and the creation of ulceration. The changes in autonomic nervous system function affect sweat and oil production; this causes the skin on the feet to dry out, leading to recurrent tears and infections. The loss of sensation induced by peripheral neuropathy worsens the development of ulcers. The repeated damage to the afflicted area goes undetected by the patient and, in the long term, becomes painful, necessitating hospitalization and, in many cases, amputation and death[12]. Plants and their secondary metabolites have been demonstrated to be beneficial in treating and controlling diabetes[13]. Particularly, Vaccinium arctostaphylos L[14], Securigera securidaca L[15], Gymnema sylvestre L[16], Atriplex halimus L[17], Allium cepa L[18], Citrullus colocynthis (L.) Schrad[19], Silybum marianum (L.) Gaertn[20], Trigonella foenum-graecum L[21] and Allium sativum L[22] have traditionally been related to an antioxidant mechanism and the treatment of diabetes by lowering blood glucose levels.
This review aims to clarify the scientific data supporting the ethnobotanical use of plant parts as treatment options for DFU. Therefore, this systematic review intends to explore the many therapeutic cases addressing this significant issue with a special emphasis on the potential routes of administration of the compounds derived from plants for treating DFU.
This systematic review of the clinical cases on the use of plants to treat DFU was performed as per the PRISMA guidelines[23].
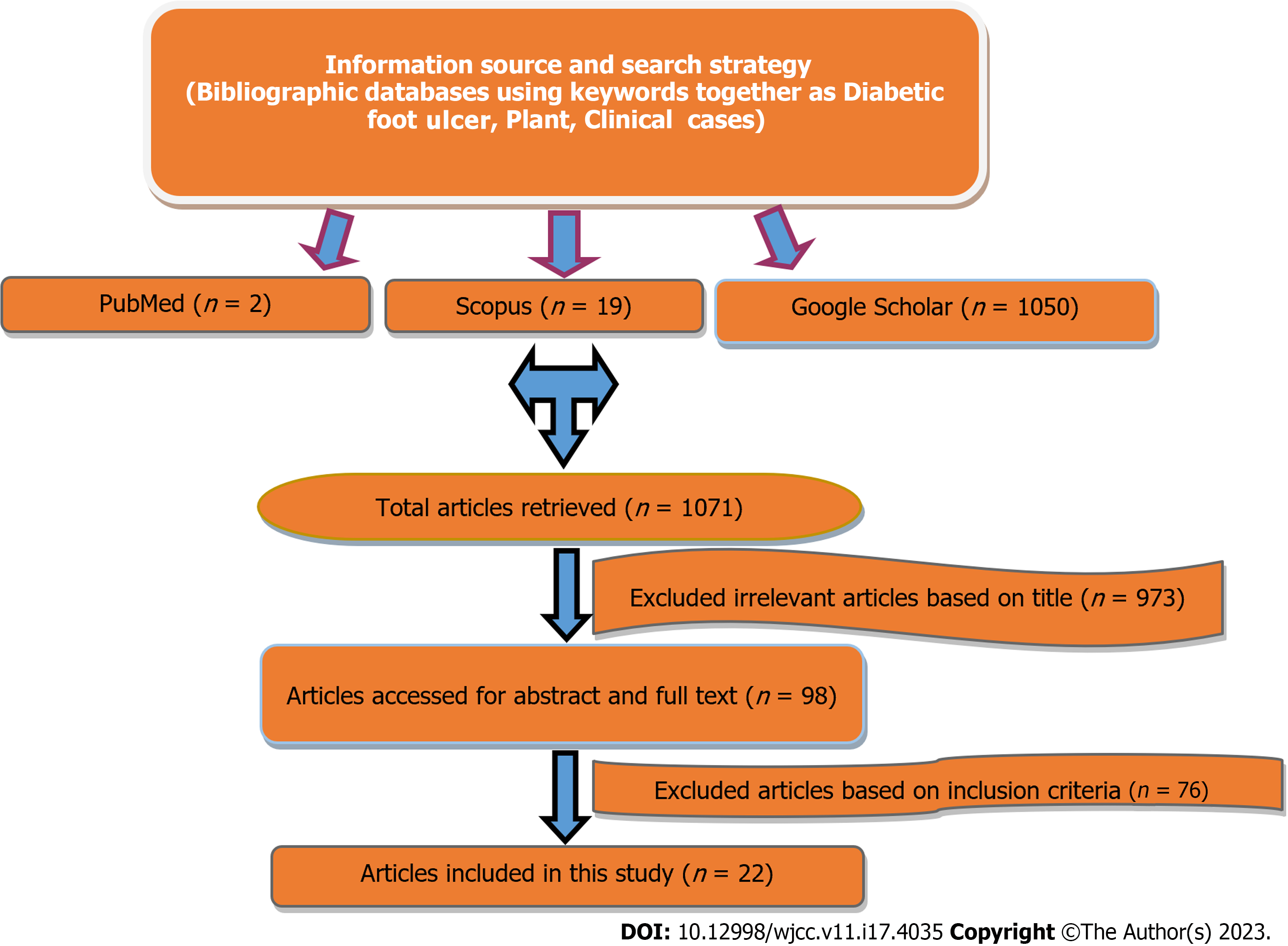
Original research articles were searched in various bibliographical databases like PubMed, Scopus, and Google Scholar, using the various keywords (Figure 1) listed below:
(“diabetic foot”[MeSH Terms] OR (“diabetic”[All Fields] AND “foot”[All Fields]) OR “diabetic foot”[All Fields] OR (“diabetic”[All Fields] AND “foot”[All Fields] AND “ulcer”[All Fields]) OR “diabetic foot ulcer”[All Fields]) AND (“plants”[All Fields] OR “planted”[All Fields] OR “planting”[All Fields] OR “plantings”[All Fields] OR “plants”[MeSH Terms] OR “plants”[All Fields] OR “plant”[All Fields]) AND “clinic”[All Fields] OR “clinics”[All Fields] OR “clinical”[All Fields] OR “clinically”[All Fields] OR “clinical”[All Fields] OR “clinics”[All Fields]) AND (“cases public health commun mark”[Journal] OR “cases”[All Fields]).
TITLE-ABS-KEY (diabetic AND foot AND ulcer AND clinical AND cases) AND PUBYEAR >2000 AND PUBYEAR >2000; TITLE-ABS-KEY (diabetic AND foot AND ulcer AND clinical AND cases) AND PUBYEAR >2000 AND PUBYEAR >2000; TITLE-ABS-KEY (diabetic AND foot AND ulcer AND plant AND clinical AND cases) AND PUBYEAR >2000 AND PUBYEAR >2000; TITLE-ABS-KEY (dfu AND plant AND clinical AND cases) AND PUBYEAR >2000 AND PUBYEAR >2000.
“Diabetic foot ulcer”, “plant” and “clinical cases”.
The publications of the last decade (published between 1st January 2000 to 7th December 2022) that focused on the clinical cases carried out to validate the effects of plants on DFU were set as the inclusion criteria for selecting the relevant reports in this study. Only the abstracts and full-text articles that met the aforementioned requirements were taken into account for interpretation.
The research studies not published in English were not included. In addition, any information about injuries other than DFU and information not involving human subjects were categorically omitted.
The information relevant to the investigation, including metabolites, sample size, length of therapy, country of treatment, parts used, and manner of administration, retrieved from full-text papers, was entered into an Excel sheet from full-text articles (Microsoft Office Version 2018 for Windows, Washington, United States). The Excel sheet was used as a means of gathering data.
After preliminary screening, a total of 1071 abstracts/citations were obtained from several bibliographic databases, including 2 from PubMed, 19 from Scopus, and 1050 from Google Scholar. Ninety-eight (98) papers were considered full-text examination studies after removing duplicates, and the titles and abstracts were screened for relevance. Finally, 22 clinical case-related articles fulfilled both the inclusion and exclusion criteria. A flowchart showing the selection strategy of the research studies and publication number at each stage is shown in Figure 1.
There were 1553 subjects enrolled in total among the 22 investigations. The countries that attracted the most interest were Iran (45.45%, n = 10), Brazil (13.64%, n = 3), Nigeria and India (9.09%, n = 2 each), Mexico, Thailand, Taiwan, Indonesia, and the West Indies (4.55%, n = 1 each), and other countries. The majority (13.64%, n = 3 each) of the research publications were reported in 2020 and 2021. The most often used mode of administration of the herbal preparation in the chosen cases was by topical delivery (45.45%, n = 10), followed by oral (18.18%, n = 4) and intravenous (13.64%, n = 3) routes. The herbal therapies to treat DFU were given orally in five trials. One of the five trials failed to show a meaningful effect on DFU compared to the control group. Three studies involving oral treatment (Bolajoko et al[24], Soleimani et al[25], and Bolajoko[26]) combined herbal and conventional therapy to improve the impact of conventional therapy on DFU. The most often used plant parts utilized in those studies were the leaf (22.7%, n = 5), followed by the fruit (18.18%, n = 4), the seed, the bloom, and the aerial part (9.09%, n = 2 each). Except for one experiment, all investigations concluded well, with recovery times ranging from 9 to 112 d (Table 1).
| Scientific name | Family | Sanskrit name | Country | Parts used | Sample size | Other ingredients | Mode of administration | Metabolites or nutraceuticals along with result | Duration | Inference of the study | Ref. |
| Actindia deliciosa | Actinidiaceae | Kiwi | Iran | Fruit | 37 | NR | Injection (3 mm thick layer) every 12 h | Actinidin and ascorbic acid/angiogenesis (increased), epithelialization, vascularization and granulation. Ulcer size (decreased) | 11.24 ± 3.66 d (experimental), 17.76 ± 4.88 d (control) | Complete wound healing and effective | Mohajeri et al[35] |
| Actindia deliciosa | Actinidiaceae | Kiwi | Iran | Fruit | 18 | Eucerin cream | Topical (wound was covered with the mixture) every 24 h | Actinidin, wound size (decreased) (0.94 ± 0.58 cm2), experimental vs control (% change in wound size: -1.25 ± 0.21 vs 0.21 ± 0.05) | 28 d | Reduction in wound size and effective | Kardoust et al[36] |
| Ageratina pichinchensis | Asteraceae | - | Mexico | Aerial part | 36 | Cold cream | Topical one a week | 7-O-(β-D-glucopyranosyl)-galactin/wound size (decreased) | 65.47 ± 47.08 d experimental); 77.46 ± 50.80 d (control) | Complete wound healing and effective | Romero-Cerecero et al[37] |
| Calendula officinalis | Asteraceae | Zergul | Brazil | Flowers | 32 | NR | Topical (5 mL) once a day | Calendula oil/wound healing (increased), pain (decreased) | 30 d | Reduction in wound size and effective | Carvalho et al[73] |
| Calendula officinalis | Asteraceae | Zergul | Brazil | Flowers | 84 | NR | Spray twice daily | Wound healing (increased), 78% had complete healing after 30 wk | 15.5 ± 6.7 wk | Reduction in wound size and effective | Buzzi et al[74] |
| Garcinia kola | Clusiaceae | Balya | Nigeria | Seed | 120 | NR | Oral (capsule) 250-500 mg | Wound healing (increased), Reduction in wound size by 63.30% in experimental group | 63-84 d | Reduction in wound size and effective | Bolajoko et al[24] |
| Kalanchoe pinnata | Crassulaceae | Pranabijah | West Indies | Leaves | 478 | NR | Plaster (boiled leaf) and poultice once to thrice daily | Wound healing (increased) | 9 d | Effective | Cawich et al[99] |
| Linum usitatissimum | Linaceae | Atasi | Iran | Seed oil | 60 | NR | Supplement (1000 mg/d omega-3 fatty acid from flaxseed oil) | Omega-3- fatty acid/ulcer length (decreased), width and depth, experimental vs control % change in (ulcer length: -2.0 ± 2.3 vs -1.0 ± 1.1 cm; width: -1.8 ± 1.7 vs -1.0 ± 1.0 cm; and depth: -0.8 ± 0.6 vs -0.5 ± 0.5 cm) | 84 d | Reduction in wound size and effective | Soleimani et al[25] |
| Melilotus officinalis | Fabaceae | Vanamethika | Iran | NR | 10 | NR | Intravenous infusion | Melilotus/wound (decreased) size (reduced by 50%), experimental vs control (wound size: 6.69 ± 60 vs 12.32 ± 11 cm2) | 56 d | Reduction in wound size and effective | Bagheri et al[113] |
| Melilotus officinalis | Fabaceae | Vanamethika | Iran | NR | 40 | NR | Orally twice a day | Melilotus/wound healing (increased), experimental vs control (completely healed: 90.0% vs 70.0%) | 84 d | Complete wound healing and effective | Bahrami et al[114] |
| Momordica charantia | Cucurbitaceae | Karavella | Indonesia | Leaf | 30 | NR | Oral (6 g) per day | Wound healing (increased), experimental vs control: [TNF-α serum levels (pg/mL): -29.50 ± 8.6 vs -202.47 ± 610.2; PEDIS degree (decreased): 1.9 ± 0.6 vs 2.2 ± 0.8] | 28 d | Not effective | Rosyid et al[122] |
| Olea europaea | Oleaceae | Jaitun | Iran | Fruit | 60 | NR | Topically (oil) once a day | Wound healing (increased), experimental vs control (completely healed: 76.60% vs 0.00%) | 28 d | Complete wound healing and effective | Abdoli et al[130] |
| Olea europaea | Oleaceae | Jaitun | Iran | Fruit | 34 | NR | Topical (oil) once a day | Wound healing (increased). Ulcer area (decreased) and depth, experimental vs control (completely healed: 73.33% vs 13.30%; change in ulcer (area: -54.7% ± 28.8% vs +2.7% ± 47.2%), depth (area: -60.1% ± 13.8% vs -29.6% ± 12.6%) | 28 d | Complete wound healing and effective | Nasiri et al[131] |
| Plantago major | Plantaginaceae | Asvagola | Iran | Leaves | 94 | Gel | Topically (oil) once a day | Plantamajoside/wound healing (increased), experimental vs control (completely healed: 64.00% vs 20.50%) | 14 d | Complete wound healing and effective | Ghanadian et al[125] |
| Plantago major and Aloe vera | Plantaginaceae, Asphodelaceae | Asvagola and Ghrit kumara | Iran | NR | 40 | NR | Intervention twice a day | Ulcer surface decreased, ulcer depth decreased | 28 d | Reduction in wound size and effective | Najafian et al[55] |
| Plectranthus amboinicus and Centella asiatica | Lamiaceae and Apiaceae | Yavani, Mandukaparni | Taiwan | NR | 24 | Cream (composed of cetostearyl alcohol, ireine, liquid petrolatum, methyl paraben propyl paraben, Span 60, Tween 60, white petrolatum, water, and pigments) | Topically (2 mm thickness) twice a day | Wound healing (increased), experimental vs control [improved Wagner grade: 90.9% vs 70.0% (% change in wound size: -27.18 vs -22.64)] | 14 d | Reduction in wound size and effective but not significant compared to controlled group | Kuo et al[84] |
| Quercus infectoria | Fagaceae | Mayakku | Thailand | Nutgalls | 51 | Ethanol | Topical once a day | Wound healing (increased), experimental vs control (80.7% vs 20.0%) | 96 d | Complete wound healing and effective | Chokpaisarn et al[152] |
| Sesamum radiatum and Azadirachta indica | Pedaliaceae and Meliaceae | Tila, Nimba | India | Seed and leaves | 15 | Ghee and honey | Topically (3 mm thick) once a day | Wound size and exudates (decreased), no granulation, reduction in wound size by 88.0% in the experimental group | 45 d | Complete wound healing and effective | Tripathy et al[160] |
| Teucrium polium | Lamiaceae | - | Iran | Aerial part | 70 | Eucerin | Topically twice daily | Wound healing (increased), experimental vs control; ulcer area (decreased): 0.717 ± 0.19 cm2vs 1.63 ± 0.72 cm2 | 28 d | Reduction in wound size and effective | Fallah Huseini et al[169] |
| Tinospora cordifolia | Menispermaceae | Guduchi | India | NR | 50 | NR | Intervention | Wound healing (increased), experimental vs control (change in ulcer area: 0.15 cm2/d vs 0.07 cm2/d; ulcer perimeter: 0.09 cm2/d vs 0.07 cm2/d; ulcer depth: 2.20 cm2/d vs 1.40 cm2/d; wound score: 14.40 cm2/d vs 10.60 cm2/d; no. of debridements: 1.90 cm2/d vs 2.50 cm2/d) | 28 d | Reduction in wound size and effective | Purandare and Supe[176] |
| Vasconcellea cundinamarcensis | Caricaceae | - | Brazil | Latex | 50 | NR | Intervention thrice a week | Wound healing (increased), experimental vs control. Completely healed: 68.7% vs 31.3%) | 112 d | Complete wound healing and effective | Tonaco et al[182] |
| Vernonia amygdalina | Asteraceae | - | Nigeria | Leaves | 120 | NR | Oral (capsule) 250-500 mg/kg body weight | Wound healing (increased), reduction in wound size by 60.0% in experimental group | 63-84 d | Complete wound healing and effective | Bolajoko[26] |
Diabetes-related foot ulceration is common and incapacitating, frequently necessitating limb amputation[27]. It is associated with worsening the patient’s condition and a significant negative economic impact[28]. There are several ways to treat diabetic wounds, but one option that has attracted attention internationally in the recent two decades is the use of medicinal plants[29]. Twenty medicinal plant species from 17 different families have been reported in the current review, with the majority of the plants coming from the Asteraceae family.
Actinidia deliciosa (A. deliciosa) is a Chinese native plant, later made available by cultivation in New Zealand and the United States[30]. In an experiment by Masoudpour et al[31], third-degree burn wounds were generated on rats that were infected by Pseudomonas aeruginosa. The first group received topical silver sulfadiazine, the second a slice of fresh A. deliciosa fruit, and the third did not receive any treatment. In terms of wound healing and contraction, the study found that A. deliciosa fruit was much more successful than silver sulfadiazine. In another animal experiment in 2010, A. deliciosa was reported to heal wounds faster with ulcer debridement, angiogenesis and disinfection compared to a group treated with Vaseline sterile gauze and silver sulfadiazine cream[32]. The major compound present in A. deliciosa is actinidin which is responsible for the fruit’s well-known proteolytic and meat-tenderizing actions, making it a good choice for herbal debridement[33]. The inclusion of key ingredients such as lutein, beta-carotene, fisetin, and vitamins C, E, and K supports that A. deliciosa might be used as an herbal debridement agent[34]. Mohajeri et al[35] studied the effect of topical A. deliciosa treatment on neuropathic DFU. When comparing the A. deliciosa-treated group to the control group, the authors observed a mean reduction in the surface area of foot ulcers. They also detected enhanced angiogenesis and vascularization, as well as an increase in collagen and granulation tissue. Actinidin has been reported to be responsible for the wound-healing process. Kardoust et al[36] conducted a study with 18 DFU patients who were randomly allocated to either the control or experimental groups. The control group was given Eucerin as a basic ointment, whereas a conventional wound dressing added with A. deliciosa extract was applied to the experimental group. The mean wound area of the experimental group was significantly less than that of the control group after 4 wk of treatment. The difference in average wound size between the experimental and control groups showed that A. deliciosa could help with wound healing. Thus, a wound dressing with A. deliciosa extract may help reduce the treatment time and be used in place of surgical debridement in DFU patients.
Ageratina pichinchensis (A. pichinchensis) is a wild plant native to Mexico[37]. In the Mexican state of Morelos, this plant is used in traditional medicine to prepare a remedy for skin lesions[38]. A chemical examination of this plant’s extract indicated the presence of chromones and compounds from the benzofurane group[39,40]. The antifungal efficacy of A. pichinchensis extracts against Trichophyton rubrum and Trichophyton menthagrophytes bacterial strains have also been reported[41]. In an in-vivo animal study, the A. pichinchensis aqueous extract completely cured the lesions in all cases at the end of the dosing period, forming a large number of fibroblasts, collagen fibers, elastin, hair follicles, vascular neoplasms, and a bridging cell-by-cell regeneration process, compared to the control group treated with fibrinolysin (Fibrase SA®)[42]. The aqueous and hexane-ethyl acetate (EA) extract of A. pichinchensis were investigated for wound healing activity in a DFU rat model. The results showed that 100% of animals treated with A. pichinchensis extracts healed the wounds between days 4 and 11; however, the positive and negative control groups exhibited only 70% and 40% of wound healing, respectively, at day 11. 7-O-(β-d-glucopyranosyl)-galactin molecule has been found to be responsible for the healing effect of A. pichinchensis[43]. Romero-Cerecero et al[44] studied the effectiveness and tolerability of a phytopharmaceutical made from a standardized extract (5% cream formulation) of A. pichinchensis in DFU patients, where micronized silver sulfadiazine (1%) was employed as a control. All the subjects who finished the study had their ulcers totally cured. The patients in the experimental group had a wound-healing rate of 77.5% after six weeks of treatment, compared to 69.8% in the control group. The average time required for complete wound healing for patients in the experimental group was 65.47 ± 47.08 d, whereas patients in the control group needed 77.46 ± 50.80 d. Thus, A. pichinchensis extract has shown the ability to enhance wound healing in DFU patients.
Aloe vera (A. vera) is a native of North Africa and Spain, but it is currently grown throughout hot, arid parts of Asia, Europe, and America[45]. Cancer, free radical activity, diabetes, inflammation, microbiological infections, tyrosinase activity, spermatogenic activity, β-secretase inhibitory effect, and proliferative activity have all been reported to be reduced by anthraquinones and glycosides contained in A. vera sap[46]. Thirty patients with multi-drug-resistant organisms infected leg ulcers were treated with topical A. vera gel and thirty with topical antibiotics. Bacterial growth in the study group was reduced from 100% (30 instances) to 6.7% (2 cases) by day 11, but bacterial growth in the control group remained unchanged by day 11. A. vera gel is less expensive and more effective against multi-drug resistant pathogens when compared to commonly used topical antimicrobial medicines[47]. In an interesting study, ultraviolet (UV)-induced polymerization was used to create polyvinylpyrrolidone-based hydrogels containing A. vera juice and L-ascorbic acid. It was observed that the polymer structure became more wrinkled as the amount of crosslinker increased. Following that, it was demonstrated that as the amount of crosslinker grew, the crosslinking density of hydrogels rose, and therefore their swelling ratio dropped. The hydrogels generated with a higher average molecular weight crosslinking agent demonstrated greater swelling ability than materials prepared with a lower average molecular weight crosslinking agent. Furthermore, as the amount of crosslinking agent grew, so did the tensile strength and % elongation of the hydrogels[48]. The experiments were carried out on three types of diabetic rats: Non-diabetic (ND), type I (IDDM), and type II (NIDDM). A. vera leaf pulp or gel extracts did not affect the blood sugar levels of ND rats. In IDDM and NIDDM rats, A. vera leaf pulp extract demonstrated hypoglycemic effectiveness, with type II diabetes benefiting more than glibenclamide. On the other hand, A. vera leaf gel extract revealed hyperglycemic activity in NIDDM rats. Consequently, it was concluded that non-gel A. vera leaf pulps might be beneficial in treating non-insulin-dependent DM[49]. In another study, regular post-treatment with A. vera for 21 d indicated possible hypoglycemic action in oral glucose tolerance tests and normoglycemic rats, as well as the antidiabetic effect in alloxanized rats[50]. One study demonstrated that aloe emodin (AE), an anthraquinone in the A. vera plant, inhibits hemoglobin (Hb) aggregation. To confirm this finding, UV-visible spectroscopy, intrinsic fluorescence, thioflavin T, Congo red test, and transmission electron microscopy were utilized. Data from Fourier transform infrared (IR) spectroscopy and circular dichroism showed that sheet structure has been removed and helices have been formed[51]. In one investigation, in-vitro cell proliferation and migration assays were performed on normal primary human skin fibroblasts and keratinocytes in the growth medium containing A. vera solution and preservatives at varied doses. The findings imply that A. vera promotes wound healing by boosting fibroblast and keratinocyte proliferation and migration, as well as shielding keratinocytes against preservative-induced mortality[52]. The prophylactic effectiveness of A. vera gel ethanolic extract in Wistar rats was examined using a DFU paradigm. The results provide scientific support for the use of A. vera gel ethanolic extract, revealing that the gel decreased diabetic foot lesions in rats[53]. The A. vera cream was compared to normal saline dressings (NSDs) for infected diabetic leg ulcers. Sixty patients with infected lower leg ulcers were placed into two groups: One received daily A. vera dressings, while the other received daily saline-soaked dressings. The efficacy of A. vera cream dressing was recorded to be higher than that of NSD[54]. Najafian et al[55] evaluated the efficacy of Aloe vera/Plantago major gel (Plantavera gel) in treating DFU, which was a randomized clinical trial with 40 DFU patients. The patients in the intervention group were randomly assigned to receive topical Plantavera gel in addition to standard care, whereas the patients in the control group received topical Placebo gel in addition to standard care. At the end of the study, a significant difference between the two groups in terms of total ulcer score was observed, and Plantavera gel significantly reduced the ulcer surface compared to the control group with no adverse effects.
Azadirachta indica (A. indica), sometimes known as neem, is an Indian native that has been naturalized in most tropical and subtropical areas[56]. A phytochemical screening study revealed the presence of steroids, triterpenoids, reducing sugars, alkaloids, phenolic compounds, flavonoids, and tannins[57,58]. The anti-inflammatory and antipyretic effects of A. indica extract were investigated; the extract showed strong anti-inflammatory and antipyretic properties in rabbits[59]. An aqueous extract of A. indica leaves was tested for anthelmintic efficacy against Pheretima posthuma, Raillietina spiralis and Ascaridia galli. The extract exhibited antihelmintic action, showing that the aqueous extract possesses vermicidal activity and is an efficient anthelmintic[60]. A. indica leaf extract inhibited Plasmodium falciparum in vitro and Plasmodium vivax in vivo. As a result, using A. indica to treat various medical conditions, particularly infectious disorders, is strongly warranted[61]. When compared to the control group of adult male Wistar rats, A. indica extract significantly accelerated the day of complete wound closure in the experimental group. The aqueous leaf extract of A. indica promoted wound healing by boosting inflammatory response and neovascularisation[62]. When diabetes was adequately compensated at the Wound Care Units of Pisa, Ragusa and Modena - Italy, 16 DFUs from a study were managed at home with a blend of Hypericum perforatum and Azadirachta indica (HyperoilTM). DFUs were recovered in all patients between 2 and 10 mo after beginning HyperoilTM therapy. During this period, all patients’ HbA1c levels declined, DFU discomfort was reduced, and hypertension was well compensated. These preliminary data showed that utilizing HyperoilTM in conjunction with strict diabetes control might be a low-cost and effective new home care option for severe DFUs[63]. Following the successful use of a Hypericum flower extract (Hypericum perforatum) and nimh oil (Azadirachta indica; Hyperoil) in foot wounds with exposed bone in a patient with bilateral advanced diabetic ulcers, it was hypothesized that the improvement was due to improved glycemic control and peripheral microvascular circulation. A case study recounts the extraordinarily positive outcome of another patient who utilized Hyperoil for an infection-damaged diabetic foot without prior surgical intervention. DFU healing was associated with decreased local infection and better glycemic control. This patient’s outcome illustrates that the efficacy of a low-cost Hyperoil therapy for the diabetic foot is connected not only with the presence of significant microvascular dysfunction but also with appropriate local treatment[64].
Calendula officinalis (C. officinalis) is indigenous to the region stretching from Macaronesia East across the Mediterranean region to Iran[65]. The phytochemical investigation of C. officinalis revealed the presence of carbohydrates, amino acids, lipids, volatile oils, fatty acids, carotenoids, terpenoids, flavonoids, quinones, and coumarins[66-68]. The C. officinalis flower extracts inhibited the proliferation of the human immunodeficiency virus-type 1 (HIV-1). The organic extract demonstrated significant anti-HIV efficacy in an in-vitro MTT/tetrazolium-based test. The organic extract was also demonstrated to inhibit HIV-1 reverse transcription activity in a dose- and time-dependent manner. These studies have shown that an organic extract of C. officinalis flowers had anti-HIV properties with therapeutic potential[69]. In traditional medicine, C. officinalis is used to treat wounds, ulcers, herpes, scars, skin damage, frostbite, and blood purification. According to pharmacological studies, calendula extracts have antiviral and antigenotoxic properties in vitro[70]. In another research study, biologically active compounds were discovered and measured in C. officinalis flowers. The UV-visible (UV-VIS) spectroscopy enabled the identification and characterization of the whole range of phenolic and flavonoid acids, while high-performance liquid chromatography (HPLC) was used to detect and quantify phenolic compounds. In methanolic extracts, the polyphenol content was shown to be related to antioxidant activity. The UV-VIS spectra of assimilator pigments (e.g., chlorophylls), polyphenols, and flavonoids extracted from C. officinalis flowers were a quantitative evaluation of compounds that absorb at wavelengths greater than 360 nm[71]. A study looked at the molecular mechanism underlying the wound healing properties of Calendula extracts. The effects of three Calendula flower extracts (n-hexanic, ethanolic, and aqueous) on the inflammatory phase of wound healing in immortalized human keratinocytes and human dermal fibroblasts were studied. Calendula flower n-hexanic and ethanolic extracts influence the inflammatory phase in human immortalized keratinocytes by activating the transcription factor nuclear factor-kappa B and increasing the amount of the chemokine interleukin (IL)-8 at both the transcriptional and protein levels. The ethanolic extract inhibited collagenase activity in vitro while increasing the amount of collagen in the supernatant of human dermal fibroblasts, indicating that the granulation tissue was affected[72]. Carvalho et al[73] evaluated the effects of low-level laser therapy on DFUs alone and in conjunction with C. officinalis oil. The research study involved a group of 32 diabetics of both sexes. At random, the participants were divided into four groups. Low-level laser treatment and low-level laser therapy combined with essential fatty acids groups had reduced pain. All groups had the same ankle-brachial index and Doppler ultrasound. The study indicated that low-level laser therapy, either alone or in conjunction with C. officinalis oil, was effective in lowering pain and accelerating tissue repair in the diabetic foot. Buzzi et al[74] performed a prospective, descriptive pilot study to determine the therapeutic effectiveness of C. officinalis hydroglycolic extract in the treatment of DFUs. The ulcers were treated twice daily with a spray solution of C. officinalis hydroglycolic extract and coated with sterile, saline-moistened gauze and bandages, followed by foot unloading with suitable protective footwear. 54%, 68%, and 78% of patients had complete wound closure after 11, 20, and 30 wk of treatment, respectively. The quantity of exudate, fibrin slough, and necrotic tissue was significantly reduced after treatment with C. officinalis hydroglycolic extract, with no adverse reactions.
Centella asiatica has been used as a medicinal plant in India for thousands of years, and it is mentioned in the well-known ‘Sushruta Samhita’, an ancient Indian medical text[75]. Among the active compounds found in it include pentacyclic triterpenes such as asiaticoside, madecassoside, asiatic acid, and madecassic acid[76,77]. An HPLC technique employing an octadecyl silylated silica column quantified the amount of such bioactive terpene acids[78]. Its chemical elements have several medicinal uses with antibacterial, anti-inflammatory, anticancer, neuroprotective, antioxidant, and wound healing properties[79]. Keloids, leg ulcers, phlebitis, slow-healing wounds, leprosy, surgical lesions, striae distensae, and cellulitis are all well treated with Centella asiatica extract[80]. Animal studies have revealed that C. asiatica improves memory, increases response time, and aids in wound healing. C. asiatica aqueous extract in dose-dependent amounts decreased the appearance of aging skin, collagen, and topical scars[81]. In vivo and in vitro studies of the methanolic fraction of C. asiatica exhibited great polyvalent activity, indicating that it has the potential to be an efficient wound healer[82]. In a pre-clinical study, the wounds of 64 male Spraque-Dawley rats were treated with a placebo, C. asiatica Linn., Garcinia mangostana Linn., and neomycin. The rats were divided into two groups: Normal control and diabetes-induced, with each group divided into four groups. The results demonstrated that the ‘wounds of the treated group epithelialized and contracted faster than the non-treated groups, proving the validity of traditional usage of C. asiatica Linn. and Garcinia mangostana Linn.[83]. A clinical study by Kuo et al[84] demonstrated the treatment of a topical cream containing P. amboinicus (Lour.) Spreng. (Lamiaceae) and C. asiatica (L.) Urban (Umbelliferae) resulted in the reduction of a greater portion of the wound size when compared to the hydrocolloid fiber dressing group.
Garcinia kola’s (G. kola) trade name is bitter cola, which is highly regarded in African ethnomedicine, making the plant an important component in folk medicine[85]. According to one study, the most prevalent medical uses of G. kola were for treating cough, mouth infection, liver problems, diarrhea, and dysentery[86]. The pharmacologic studies on this plant’s seed, leaf, and root demonstrated antibacterial, antiviral, antiulcer, anti-inflammatory, antihepatotoxic, antidiabetic, antihypertensive, adaptogenic, aphrodisiac, and anti-asthmatic activities[87]. The phytochemical analysis of the plant extract revealed the presence of flavonoids, tannins, cardiac glycosides, saponins, steroids, reducing sugars, phlobatannins, polyphenols, pydroxymethyl anthraquinones, glucides and alkaloids[88,89]. The saponin extract from G. kola root showed substantial antioxidant and free radical scavenging activity in vitro and in vivo experiments on alloxan-induced diabetic rats, indicating it might be employed as a natural antioxidant[90]. Using the excision wound and dead wound space experimental models, researchers investigated the effect of Garcinia hydroxyl biflavanonol (GB1) on wound healing in streptozotocin-induced diabetic rats. Topical GB1 treatment reduced excision wound dimensions and epithelialization time in a concentration-dependent manner. Furthermore, GB1 dramatically reduced malondialdehyde (MDA) levels compared to the negative control, suggesting that GB1 activities may aid in diabetic wound healing[91]. The antioxidant and antidiabetic effects of G. kola ethanolic seed extract on alloxan-induced diabetic albino rats were investigated. Standard procedures were used for the in-vitro antioxidant test. In the in-vivo investigation, 36 albino rats were fasted for 16-18 h before being injected intraperitoneally with 150 mg/kg body weight of alloxan monohydrate to induce diabetes. The animals were placed into six groups: Normal, positive, negative, and groups that received 500, 250, and 125 mg/kg of the extract, respectively. The 500 mg/kg extract-treated group had lower blood glucose levels than the positive control. The antioxidant studies revealed a considerable dose-dependent improvement in free radical scavenging activity[92]. Bolajoko et al[24] compared glycemic control and oxidative stress levels in type 2 diabetics with or without chronic foot/leg ulcer to NDs with or without chronic foot/leg ulcer after an 8-wk freeze-dried G. kola dosage. All participants were given conventional treatment (metformin, glibenclamide, or insulin alone, or metformin with glibenclamide or metformin with insulin). The study participants included 30 diabetics with foot/leg ulcers (DFU), 30 diabetics without ulcers (T2DM), 30 NDs with chronic foot/leg ulcers, and 30 NDs without ulcers were divided into three groups: Subgroup-1 (250 mg G. kola), subgroup-2 (500 mg G. kola), and subgroup-3 (no-supplementation). The individuals that took 250 or 500 mg G. kola for 8 wk had lower total plasma peroxides (TPP), oxidative stress index and plasma glucose, as well as improved wound healing, higher total antioxidant status (TAS) and antioxidant-micronutrients. The findings showed that G. kola supplementation might be utilized as an adjuvant in the prevention and treatment of type 2 diabetes with or without foot/leg ulcers.
Kalanchoe pinnata (K. pinnata) is a succulent plant from Madagascar[93]. K. pinnata include anthocyanins and flavonoids, which have antibacterial, antioxidant, cytotoxic, anticancer, antiparasitic, antiallergic, and hepatoprotective properties[94]. An excision wound model was used to assess the wound-healing activities of K. pinnata extract in rats. When compared to the control and the mupirocin-treated standard, animals given with the ethanolic leaf extract exhibited less wound area and higher hydroxyproline concentration. K. pinnata leaf extract showed high wound-healing capability, enhanced wound contraction rate, and hydroxyproline content in extract-treated mice that were validated by histological studies[95]. According to one research, hydroethanolic K. pinnata leaf extracts suppressed phospholipase activity, demonstrating antiophidic action against the effects of B. jararaca snake venom, implying that they might be exploited as a novel source of bioactive molecules against bothropic venom[96]. A chloroform/aqueous extract of K. pinnata leaves was evaluated in vitro against MTCC 78pBR322 Escherichia coli, MTCC 227 Candida albicans, MTCC 265 Rhodococcus rhodochrous, and MTCC 2682 Arthrobacter protophormial. When compared to the standard antibiotic used as a control, the aqueous extract considerably suppressed the zone of bacterial growth, suggesting its antibacterial properties[97]. A research study developed and compared two creams comprising an aqueous leaf extract of K. pinnata and its major flavonoid. When administered topically to a rat excision model, both creams healed the wound by increasing re-epithelialization and collagen fibers. According to HPLC-ESI-MS/MS investigation, the primary phenolics in K. pinnata leaf extract were identified to be flavonol glycosides. The wound-healing capacity of K. pinnata was demonstrated by the outcomes of treatment following both creams[98]. In a research study, Cawich et al[99] divided individuals with diabetic foot infections into two groups: The study group, which utilized topical K. pinnata, and the medical therapy group, which used conventional therapy. The medical therapy group had 382 patients, whereas the study group had 96. The study and medical therapy groups had an identical incidence of all amputations and death. The study showed that using topical K. pinnata to treat diabetic foot infections might be beneficial.
Linum usitatissimum or linseed is a native crop of West Asia and the Mediterranean coastal nations, Asia Minor, Egypt, Algeria, Tunis, Spain, Italy, and Greece[100]. According to phytochemical screening studies, the principal chemical components of L. usitatissimum, include secoisolariciresinol diglucoside (major bioactive compound), omega-3 fatty acids, phenols, flavonoids, sterols, proteins, antioxidants, and numerous soluble and insoluble fibers[101,102]. The fatty acid components, particularly polyunsaturated fatty acids like linoleic acid, are thought to play an important role in wound healing[103]. A study investigated the antibacterial and antibiofilm properties of L. usitatissimum crude extract (Lu. Cr). The bacterial strains found on diabetic foot bandages were investigated and the Gram-positive bacteria were identified to be the most common in all the studied groups. Lu. Cr has the most bactericidal activity against Staphylococcus aureus (S. aureus) compared to other bacteria in the study. This research revealed that linseed had antibacterial properties that might be utilized to treat diabetic foot[104]. L. usitatissimum oil and mucilage were investigated for antiulcer activity in a rat model of ethanol-induced stomach ulcer. Pre-treatment of rats with flaxseed oil and mucilage significantly reduced the incidence and duration of ethanol-induced stomach ulcers. An oral dose of flaxseed oil (5 mL/kg) reduced ulcer severity more than ranitidine (50 mg/kg). According to this study, both flaxseed oil and flaxseed mucilage can provide cytoprotection against ethanol-induced stomach ulcers in rats[105]. Soleimani et al[25] conducted a study on 60 people with grade 3 DFU. All the subjects received conventional treatment [ciprofloxacin (Cipro) 400 mg IV plus clindamycin 900 mg IV]. The subjects were randomly grouped into one of two groups for 12 wk: Those receiving 1000 mg omega-3 fatty acids from flaxseed oil supplements or those receiving a placebo. After a 12-wk intervention, omega-3 fatty acid supplementation resulted in a significant decrease in ulcer length, width and depth, and found substantial reductions in blood insulin levels compared to the placebo. Overall, omega-3 fatty acid supplementation improved ulcer size measurements, insulin metabolic markers, serum high-sensitivity C-reactive protein, plasma total antioxidant capacity, and glutathione levels in DFU patients. Furthermore, the effects of omega-3 fatty acids in flaxseed oil on improving metabolic profiles may have indirectly impacted wound healing.
Melilotus officianalis (M. officinalis) is native to the United States and Canada[106]. Melilotus officinalis contains coumarins, melilotin, phenolic acids, flavonoids, steroids, saponins, volatile oils, lipids, triterpenes, carbohydrates, sugar, anthraquinone glycosides, mucilage, tannin, bishydroxycoumarin, choline, alcohols, and uric acid[107]. The antimicrobial, antioxidant, anticancer, antiinflammatory, neural, protective, sedative, anxiolytic, smooth muscle relaxant and hypotensive activities are among the many pharmacological effects of this plant[108]. The effects of 0.25% coumarin M. officinalis extract on acute inflammation produced by the oil of turpentine in male rabbits were investigated. In this in vivo study, M. officinalis inhibited circulating phagocyte activation and citrulline production, indicating its anti-inflammatory property[109]. A three-layer electrospun nanofiber wound dressing was developed in a study, with the scaffold’s external, middle, and inner layers made of polycaprolactone (PCL), PCL/collagen, and collagen nanofibers, respectively. Various amounts of M. officinalis extract were also added to the collagen nanofibers as a physiologically active component. The effectiveness of the produced dressings as wound-healing agents were tested in streptozotocin-induced diabetic rats. The histopathological and histomorphometry evaluations demonstrated that herbal extract-loaded electrospun dressings (especially those containing 0.08 g of extract) are promising in promoting diabetic ulcer healing[110]. A study examined the phytochemicals responsible for the antifungal activity of M. officinalis, which is frequently grown in Iraq. The agar diffusion method was used to study the antifungal activity of the water-soluble fraction and pinpoint the key ingredient. At 1% and higher dosages, Iraqi M. officinalis demonstrated significant antifungal effectiveness against five diagnostic fungi. When compared to a standard, thin-layer chromatography of the acid-hydrolyzed glycoside shows the presence of kaempferol flavonoid, which was supported by high-performance thin-layer chromatography, UV, and IR spectroscopy. The major phytochemical in the extract’s polar component, kaempferol glycoside, is responsible for its potent antifungal effect[111]. In Iran, M. officinalis dry extract is marketed under the trade name Semelil (ANGIPARSTM) and is used to treat chronic wounds, particularly DFUs[112]. Bagheri et al[113] investigated the safety and healing rates of DFUs in people who were given ANGIPARSTM. Ten diabetic patients participated in this single-arm trial before-after clinical research. The wound area was calculated using planimetry. According to this research study, the medication may reduce wound size by at least 50% over the course of eight weeks with no discernible side effects. In a study by Bahrami et al[114], 40 patients with DFUs that had been present for at least 4 wk were randomly assigned to receive either oral ANGIPARS or a placebo twice a day for a maximum of 6 wk, and they were observed for up to 12 wk. The results showed a significant reduction in wound surface area in both groups. In the ANGIPARS group, the mean improvement ratio was 95.8%, compared to 79.2% in the placebo group. 90% of the ANGIPARS group and 70% of the placebo group had fully healed wounds after 12 wk. At weekly evaluations, the former group had a larger mean percent of wound area decrease than the placebo group.
Momordica charanthia (M. charantia), a medicinal plant, is native to India and is widely grown in Asian nations[115]. The primary metabolites of M. charantia include common sugars, proteins and chlorophyll, whereas secondary metabolites include alkaloids, flavonoids, tannins, saponins, diosgenin, proteins, calcium, and copper[116]. A qualitative phytochemical study on M. charantia indicates the presence of phytochemicals such as flavonoids, saponins, terpenoids, coumarins, emodins, alkaloids, proteins, cardiac glycosides, anthraquinones, anthocyanins, and steroids[117]. The aqueous and ethanolic extracts of M. charantia inhibited the growth of S. aureus, Bacillus subtilis, Escherichia coli (E. coli) and Pseudomonas aeruginosa. The findings of this study backed up the use of M. charantia in herbal medicine and suggested that it might be utilized as a source of antibacterial agents for treating disorders caused by the pathogenic bacteria investigated[118]. The wound-healing efficacy of M. charantia fruit powder was studied in rats utilizing an excision, incision, and dead space wound model. The powder ointment showed a statistically significant response in wound-contracting ability, wound closure time, length of epithelization, wound tensile strength, and wound tissue regeneration compared to the control group[119]. A study evaluated the alterations in transforming growth factor (TGF) expression in diabetic lesions treated with topical M. charantia fruit extract. Fifty-six male Sprague-Dawley rats were divided into five diabetes groups of 10 rats each, as well as a normal control group. Diabetes was induced in diabetic groups by intravenous injection of 50 mg/kg streptozotocin. Full-thickness excision wounds were created on the thoracodorsal region of the animals; these wounds were then treated for 10 d with vehicle, M. charantia powder, M. charantia ointment, and povidone ointment or ointment base. The wound closure was substantially quicker in the M. charantia ointment group than in the untreated diabetes group. The study demonstrated that M. charantia ointment has good potential for use as an alternative topical therapy for diabetic wounds that accelerates wound healing by increasing TGF expression[120]. In another study, the impact of bitter melon leaves extract supplementation on glycemic status in DFU patients was investigated. Thirty DFU patients with PEDIS 1-8 scores who satisfied the criteria were split into two groups: 15 as a treatment group receiving bitter melon leaves extract and 15 as a control group receiving placebo. After 4 wk of therapy, the baseline value of glycated albumin in the treatment group increased, whereas it decreased in the control group. However, the examination of the effect of bitter melon leaves extract supplementation on the value of glycated albumin yielded no significant findings[121]. The impact of bitter melon leaves extract on blood tumor necrosis factor (TNF)-α levels and DFU improvement was studied by Rosyid et al[122]. Thirty DFU patients took part in the experiment and were separated into two groups based on PEDIS scores, with 15 patients in the treatment group receiving bitter melon leaves extract at a dose of 6 g/d and the remaining 15 patients in the control group receiving a placebo. The baseline blood TNF-α levels in both the treatment and control groups decreased after four weeks of therapy. PEDIS degrees decreased in the extract treatment and control groups from baseline, weeks 2, 3, and 4, but there was no effect on DFU improvement.
Olea europaea leaves have long been used in traditional medicine in Greece, Spain, Italy, France, Turkey, Israel, Morocco, and Tunisia[123]. O. europaea known as the olive, has traditionally been used as a diuretic, hypotensive, emollient, laxative, febrifuge, skin cleanser, cholagogue, and treatment of urinary infections, gallstones, bronchial asthma, and diarrhea, as they have antioxidant, anticarcinogenic, antiinflammatory, antimicrobial, antihypertensive, antidyslipidemic, cardiotonic, and antiplatelet properties[124,125]. The antimicrobial activity of O. europaea leaves extract against Campylobacter jejuni, Helicobacter pylori, and S. aureus [including methicillin-resistant S. aureus (MRSA)] was discovered[126]. In an in-vivo investigation, wound contraction and healing activity were best observed in normal and diabetic experimental rats treated with a newly developed ointment of O. europaea leaf extracts combined with Shea butter[127]. When compared to a regularly used amorphous hydrogel, the unique O. europaea leaf extract hydrogel (EHO-85) was tested for its capacity to expedite wound healing. Compared to a control amorphous hydrogel, EHO-85 significantly accelerated wound healing regardless of ulcer etiology (pressure, venous leg, or diabetic foot) or prognosis, doubling the median wound area decrease. An intention-to-treat analysis was performed on 195 patients. This novel medication modulates the ulcer microenvironment by altering reactive oxygen species and pH, which explains the demonstrated granulation formation and pain-relieving powers of EHO-85[128]. In one case study, a 45-year-old lady with T2DM had a big toe wound and was given propolis and olive oil. The patient had a 2 cm2 wound on her foot and was told to wash it with serum, apply topical admixture to the lesion, and change it every 12 h. The ulcer healed entirely within a week after therapy, proving that propolis with olive oil may cure DFU[129]. Abdoli et al[130] wanted to see how topical olive oil dressing combined with standard therapy compared to standard care alone for treating grade 1 and 2 DFUs in T2DM patients. The research study used 60 T2DM patients. For four weeks, the intervention group got standard treatment, including wound irrigation with normal saline, oral antibiotics, and daily topical olive oil dressing, whereas the control group received just standard care. At weeks one, two, three, and four, the olive oil group had significantly higher scores for ulcer degree, colour, drainage, and surrounding tissue healing than the control group. As a result, topical olive oil dressing aided in the healing of DFU. Nasiri et al[131] conducted a double-blind, randomized clinical trial on 34 patients with Wagner’s ulcer DFU grade 1 or 2. The patients in the intervention group got topical olive oil in addition to routine treatment, whereas the patients in the control group only received normal care. At the conclusion of the fourth week, there were significant variations between the two groups in three ulcer parameters: Degree, colour, and surrounding tissues, as well as total ulcer status. When compared to the control group, olive oil dramatically reduced ulcer area and depth and completed higher ulcer healing at the end of the study. The findings showed that using olive oil in conjunction with routine care is more beneficial than using routine care alone to treat DFU.
According to academics, Plantago major (P. major) has been present for over 4000 years, especially in Europe, America, and Asia[132]. Based on phytochemical investigations, P. major contains volatile compounds, triterpenoids, phenolic acids, and flavonoids[132]. Traditional Persian medicine describes the benefits of P. major on wound healing, treatment of intestinal ulcers, hematemesis, upper and lower gastrointestinal bleeding, dyspepsia, hemorrhoids, stomachache, and dysentery[133]. A case study discovered that decoction of P. major alleviated patient symptoms within a few days of starting therapy, indicating the potential benefits of P. major for treating pancolitis[134]. Supplementation with two capsules of P. major seed (500 mg per capsule) decreased blood levels of alanine aminotransferase, aspartate aminotransferase, and triglyceride in non-alcoholic fatty liver disease patients in a clinical trial research[135]. A pilot study on wound healing in a rat model discovered that P. major’s leaf extract might accelerate wound healing[136]. The therapeutic benefits of P. major topical formulation on stage 1 pressure ulcers in 130 patients were studied. The study’s findings revealed a substantial difference in damage resolution between the test and control groups. HPLC was used to standardize the topical formulation based on the amount of quercetin, one of the phytochemicals in P. major and no adverse effect was observed[137]. A research study evaluated the therapeutic impact of P. major extract with 1% sulfadiazine on the healing of second-degree burn wounds in a control group. The investigation and control groups received 10% P. major ointment and 1% silver sulfadiazine ointment, respectively. The average complete healing time in the P. major and control groups was 11.73 d and 13 d, respectively. The study discovered that P. major ointment is a safe and efficient herbal product for the treatment of second-degree burn wounds since it not only cures the lesion but also serves as an analgesic and antibacterial agent[138]. Ghanadian et al[125] conducted a clinical study where patients with DFU or pressure ulcers were randomly assigned to medication (P. major) or control groups. In the medicine treatment group, a 10% Plantago extract topical gel was used on the wound once daily for two weeks, whereas the control group utilized an acceptable fresh dressing. Plantago extract gel significantly decreased wound size when compared to the control. Furthermore, the treatment group had significantly more patients who healed completely than the control group. According to the findings of the study, using P. major topical gel expedited the healing of DFUs and pressure ulcers.
Plectranthus amboinicus (P. amboinicus) is a medicinal herb that has been used in traditional medicines for therapeutic purposes in the form of syrup[139]. It is mainly found on the Maluku Islands, close to Indonesia[140]. According to a phytochemical research investigation, P. amboinicus contains flavonoids such as apigenin, luteolin, and salvigenin[141]. Swamy et al[141] indicated that the therapeutic properties of this plant are due to the existence of secondary metabolites such as flavonoids, glycosides, phenols, tannins, and steroids. The capacity of P. amboinicus (Lour.) Spreng. to resist clinical isolates of MRSA was examined. Using broth microdilution and bioautography, the in vitro efficacy of the hydroalcoholic extract (HE), EA fraction, and its subfractions against MRSA clinical isolates were assessed. Bacterial suspensions were combined with Citodex and administered subcutaneously into male Swiss mice to stimulate abscess formation. Two doses of HE, the EA fraction, or vancomycin were delivered intraperitoneally to mice at 3 and 12 h after infection. The lowest minimum inhibitory concentrations (MIC, 0.25 to 0.5 mg/mL) were found in the EA fraction and its subfractions. The plant samples were tested bacteriostatic at 2 × and 4 × MIC and bactericidal at 100 mg/mL. The abscess volume, bacterial cell counts in abscess slurries, and inflammatory ratings decreased significantly in the HE and EA fraction-treated groups. The samples were effective in treating the animals in a dose-dependent manner, confirming the efficiency of P. amboinicus fractions against MRSA both in vitro and in vivo[142]. Kuo et al[84] examined and compared the effects of a topical cream comprising P. amboinicus (Lour.) Spreng. (Lamiaceae) and C. asiatica (L.) Urban (Umbelliferae) on DFUs in comparison to the effects of hydrocolloid fiber wound dressing. The study comprised of 24 type 1 or type 2 diabetes patients, aged 20 and above, with Wagner grade 3 foot ulcers following surgical debridement. Twelve patients were randomly assigned to receive WH-1 cream containing P. amboinicus and C. asiatica for two weeks, and another 12 patients were treated with hydrocolloid fiber dressings. Wagner grade improvement was noted in a relatively greater proportion of patients in the WH-1 cream group (10 of 12; 90.9%) than in the hydrocolloid fiber dressing group.
Quercus infectoria (Q. infectoria) is a tiny tree or shrub endemic to Turkey, Greece, Iran, and Syria[143]. Pharmacological studies on Q. infectoria revealed that acetone-treated methanolic extract was an active analgesic and reduced blood sugar levels in rats, but chloroform-treated methanolic extract worked as a central nervous system (CNS) depressant and an efficient local anesthetic[144]. The presence of three compounds, syringic, gallic, and ellagic acids, as well as CNS activity, were reported in an alcoholic extract of Q. infectoria galls[145]. The alcoholic extract of Q. infectoria galls also demonstrated anti-inflammatory activity both in vitro and in vivo. In a research study, oral gall extract therapy inhibited carrageenan, histamine, serotonin, and prostaglandin E2 (PGE2)-induced paw oedemas, whereas local application prevented phorbol myristate acetate (PMA)-induced ear irritation. In vitro exposure of rat peritoneal macrophages to gall extract increased lipopolysaccharide-stimulated PGE2 and NO production as well as PMA-stimulated superoxide formation[146]. By determining the MIC and minimum bactericidal concentration values, the aqueous and acetone extracts of galls of Q. infectoria demonstrated to have antimicrobial activity against Gram-negative and Gram-positive bacterial species, indicating that the galls of Q. infectoria are a potentially good source of antimicrobial agents[147]. The effects of an ethanolic extract of shade-dried Q. infectoria leaves on wound healing in rats were studied using incision, excision, and dead-space wound models. The plant increased the levels of the antioxidant enzymes, superoxide dismutase and catalase, in the granuloma tissue, showing a clear, beneficial effect on wound healing[148]. A study was carried out to look at the use of Q. infectoria as a topical medicine for the treatment of chronic wounds. Twenty Q. infectoria formulations (QiFs) were created pharmaceutically and evaluated for antibacterial effectiveness. The wound-healing activities in streptozotocin-induced diabetic rats and control rats were investigated. When compared to citrate injection controls, streptozotocin administration (50 mg/kg) caused substantial hyperglycemia. Three days after wounding, QiF was applied topically to one of each animal’s identical wounds, while physiological saline (control) was applied to the other. QiF10 exhibited antibacterial and antioxidant activities, which may aid in wound healing in diabetic mice[149]. An alcoholic extract of the plant Q. infectoria was studied for its antibacterial, cytotoxic, and antioxidant activities. A crude extract inhibited C. albicans growth the most, followed by S. aureus and E. coli. Treatment with alcoholic extract also inhibited cell growth of the human breast cancer MCF-7 cell line in a concentration-dependent manner. The findings indicate that the plant Q. infectoria is an excellent source of antiproliferative and cytotoxic chemicals. The DPPH experiment also revealed that the extract had higher antioxidant activity[150]. In one study, the wound-healing effectiveness of topical co-administration of hydroethanolic extracts of Pistacia atlantica (P. atlantica) hulls and Q. infectoria galls in streptozotocin-induced diabetic mice was assessed using an excision wound model. The diabetic mouse model was divided into four groups: Control (soft yellow paraffin), P. atlantica 5%, Q. infectoria 5%, and Q. infectoria 5% + P. atlantica 5% mixed soft yellow paraffin. On the back of each mouse, two circular, full-thickness skin incisions were made. According to the findings, topical treatment of each hydroethanolic extract of P. atlantica and Quercus infectoria extract alone or in combination improves the wound-healing activity in diabetic mice by decreasing inflammatory stages, edema, and immune cell migration scores and increasing new vascular creation, fibroblast infiltration, collagen production scores, and GLUT-1- and GPC3-positive cells[151]. Chokpaisarn et al[152] assessed the clinical effectiveness of a pharmaceutically prepared Q. infectoria solution for the treatment of chronic diabetic ulcers in patients. The diabetic patients with ulcers (Wagner Ulcer Classification grade 1-3) were randomized to receive traditional wound treatment (normal saline solution) plus Q. infectoria solution or the standard solution alone for three months. At week 12, the decrease in wound size in the study and control groups were 91.51% and 76%, respectively, with the study group having more patients with complete wound closure than the control group. The solution of Q. infectoria has been clinically proven to have tremendous potential as an alternate therapy for DFUs.
Despite being native to Africa, Sesamum radiatum (S. radiatum) is extensively dispersed throughout Asia’s tropical and subtropical climates[153]. The nutritional properties of S. radiatum leaves revealed a significant amount of protein, high values of macronutrients and micronutrients, phenolic compounds, and a good antioxidant capacity[154]. During the phytochemical examination, quinones, tannins, alkaloids, sterols, terpenes, polyphenols, saponosides, and reducing compounds were detected in S. radiatum leaf extract[155]. Sesamum gum was discovered to include glucuronic acid, mannose, galactose, and xylose, as well as trace levels of glucose, rhamnose, and arabinose[156]. The essential oil isolated from the dried leaves of S. radiatum indicated that the predominant ingredient was n-hexadecanoic acid, followed by 9,12,15-octadecanoic acid-(Z,Z,Z), dodecanoic acid, and tetradecanoic acid. The findings suggest its usefulness for cardiovascular and estrogenic activity, male infertility, constipation, fungal and bacterial infections, and bruising[157]. Using the agar diffusion technique, ethanolic, methanolic, and aqueous extracts of S. radiatum leaves were tested for antibacterial activity against Gram-positive and Gram-negative bacteria and yeast. The presence of carboxylic acids and phenolic groups in essential oils, and antioxidants such as sesamol, sesamolin, and sesamin, were shown by gas chromatography-mass spectrometry (GC-MS) phytochemical screening of methanolic extract. Both the methanolic and ethanolic extracts exhibit wide antibacterial activity against all the microorganisms supporting folkloric assertions of antibacterial efficacy[158]. Using the agar diffusion technique, ethanolic and aqueous extracts of S. radiatum leaves were tested for antibacterial activity in vitro. The phytochemical screening using GC-MS revealed the presence of essential oils, namely the phenolic and carboxylic acid groups. The ethanolic extract modestly inhibited Streptococcus pneumoniae and C. albicans, whereas S. aureus, Pseudomonas aurogenosa, and E. coli were not. The aqueous extract, on the other hand, had no inhibitory impact in any of the tested microorganisms[159]. Tripathy et al[160] studied the wound-healing potential of Nimbadi Kalka, an Ayurvedic paste in which S. radiatum is the primary major ingredient, on 15 DFU patients with baseline HbA1c levels. S. radiatum was administered daily to the wound site for 45 d, showing a significant decrease in wound size and exudates. In the case of granulation tissue, there was a peak rise on the 15th d of therapy, showing the creation of granulation tissue. As a result, Nimbadi Kalka enhanced wound healing by lowering ulcer size in DFU patients.
Teucrium polium (T. polium) is a flowering plant native to Europe, North Africa, and Southwest Asia[161]. T. polium has been used to treat diabetes, rheumatologic diseases, inflammation, and gastrointestinal disorders[162,163]. T. polium L. methanolic extract (rutin and apigenin) was also discovered to have antioxidant effects[164]. T. polium extracts showed in vitro antibacterial activity against Bacillus anthracis, Bordetella bronchiseptica, and Salmonella typhi, indicating that it might be used as a source of antiseptic compounds for medicinal applications[165]. T. polium honey-treated animals improved wound contraction, closure time, and tensile strength, as well as epithelium proliferation, angiogenesis granulation, and fibrous connective tissue in an in vivo rat skin wound healing study[166]. A test was done on diabetic rats to see how well T. polium extract ointment treated wounds. 64 male Wistar rats were made diabetic by inducing alloxan and wounds made in their bodies for the experimental purpose. Eight groups of eight rats each were formed using the following treatments: Control, eucerin, phenytoin, 2%, 3%, 4%, 5%, and 10% T. polium. The ointment was administered to the wound twice daily. T. polium extract ointment with a 10% ointment accelerates wound healing in diabetic rats and is equivalent to the phenytoin group, according to the wound healing process of T. polium ointments with base ointment (eucerin) and phenytoin ointment[167]. A study looked at the effect of the co-administration of ointments produced from T. polium hydroethanolic extract (TPEO) and Aloe vera gel (AVGO) on excisional wound healing in a diabetic mice model. The mice were divided into six groups after diabetes induction and a circular excisional wound: Control mice given mupirocin (a traditional drug), mice given 5 and 10% TPEO, mice given 5 and 10% AVGO, and mice given a combination of 5% TPEO and 5% AVGO (TPEO + AVGO). The results showed that using the ointments, especially in combination, shortened the inflammatory phase and reduced tissue MDA, TNF-α, and IL-1 levels compared to the mupirocin group[168]. Fallah Huseini et al[169] performed an experiment with 70 diabetic patients with foot ulcers, scoring 1 or 2 on the Wagner’s scale to investigate the safety and efficacy of topical T. polium ointment in addition to conventional care. The patients were randomly divided into two groups and both groups of patients got normal therapy for DFUs. Furthermore, for four weeks, group one received topical T. polium ointment, whereas group two received topical placebo ointment. The result showed reduced mean ulcer surface area in the T. polium group compared to the placebo group, and the T. polium group had a higher percentage of patients who healed completely at the end of the study. The inclusion of T. polium ointment in normal therapy sped up the healing period of diabetic non-infected foot ulcers.
Tinospora cordifolia (T. cordifolia), often known as Gulbel or Indian Tinospora in English and Guduchi in Sanskrit, is an Indian plant[170]. The leaf extract has been proven to be effective against infections caused by E. coli, S. aureus, S. pyrogens, B. subtilis and P. vulgaris, as well as helping in cell repair and rejuvenation[171]. Plant phytochemicals such as alkaloids, terpenoids, lignans, and steroids contributed to the pharmacological activities; also, its chloroform fraction contains pharmacologically active substances like rutin and quercetin, which have anticancer activity in MDA-MB-231 and MCF 7 breast cancer cells[172,173]. In vitro and in vivo studies demonstrated that the alkaloid present in T. cordifolia contributes to the antihyperglycemic effect via insulin-releasing and insulin-mimicking pathways, hence reducing postprandial hyperglycemia[174]. The excision wounds, resutured incision wounds, and dead space wounds were inflicted on male Wistar rats (n = 6, in each group) under mild thiopentone anesthesia to investigate the effect of T. cordifolia on wound healing. Planimetry was used to investigate the effects of a 250 mg/kg methanol extract of T. cordifolia stem delivered orally once a day for 10 d in the resutured incision, dead space, and excision wounds. In all three models, T. cordifolia promotes wound healing, including increased wound contraction and shorter days for complete epithelization in excision wounds, increased breaking strength in resutured incision wounds, and increased granuloma dry weight and cellular infiltration in granulation tissue[175]. Purandare and Supe[176] conducted a double-blind, randomized, controlled research on 50 patients to evaluate the role of T. cordifolia as an adjuvant for the treatment of DFU. The mean ulcer size, depth, and perimeter were assessed, and swabs were collected for culture. Blood was drawn to determine the percentage of polymorphonuclear phagocytosis. Medical therapy, glycemic management, wound care, and debridement were all optimized. Net improvement was reported in 17 patients in the trial group and 13 patients in the control group. Thus, diabetic patients with foot ulcers who received T. cordifolia as adjuvant treatment had a considerably superior end result, with enhanced wound healing, decreased debridements and increased phagocytosis.
Vasconcellea cundinamarcensisis native to tropical America[177]. The in vivo investigation revealed that the proteolytic fraction (P1G10) is composed of 14 proteolytic isoforms that protect and repair the stomach by raising mucus content, lowering gastrin levels to avoid hyperacidity, improving excisional wound healing, and boosting PGE2 production[178]. P1G10 was investigated as a natural antifungal and shown to be capable of inhibiting mycelium development and cell adhesion in B. cinerea by inducing membrane integrity and cell wall degradation[179]. In a cutaneous wound excision model, P1G10 treatment accelerated the onset of the early inflammatory phase and reduced the negative effects generated by residual TGF- or MMPs, therefore enhancing scar quality[180]. The corneal healing capability of P1G10 was investigated using an ethanol-chemical burn in rabbit eyes. Except when administered at 10 g/mL, P1G10 is safe for ocular administration. P1G10 at 1 g/mL stimulates corneal re-epithelization, resulting in full wound healing 72 h after the chemical burn. P1G10 also influenced the arrangement of collagen fibers in the stroma and modulated the inflammatory response, indicating its potential corneal healing characteristics[181]. Tonaco et al[182] engaged 50 patients in a prospective, randomized, double-blind study to assess the efficacy and safety of a wound healing topical dressing containing 0.1% P1G10 to a hydrogel (control) regimen. The results showed that 5 patients in the control group had 100% ulcer healing, 3 had ≥ 80% healing, 11 had ≤ 80% ulcer changes, and the remainder had no changes or their wounds became worse. Meanwhile, 11 patients in the P1G10 group had complete healing, 4 had ≥ 80% healing, 5 had ulcer alterations of less than 80%, and the remainder had no changes or their wounds deteriorated. Thus, the P1G10 group outperformed the control group in wound healing.
Vernonia amygdalina (V. amygdalina) sometimes known as the bitter leaf, is found across tropical Africa[183]. Traditional healers utilize the plant as an antihelminth, antimalarial, laxative, digestive tonic, appetizer, febrifuge, and for wound healing[184]. According to phytochemical research, this plant is high in proteins, lipids, fibers, amino acids, minerals, vitamins and carbohydrates[185]. In an in vitro study, the methanol extract of V. amygdalina leaves was shown to be effective against bacterial isolates[186]. An in-vivo testing of extracts from the leaves and root bark of V. amygdalina for antimalarial efficacy against drug-sensitive Plasmodium berghei in mice resulted in a reduction of parasitaemia[187]. The antibacterial and antifungal activity of vernolide and vernodalol, two known sesquiterpene lactones produced from Vernonia amygdalina leaves, was investigated. Both are bactericidal to Gram-positive bacteria and fungal species supporting the ethnomedical uses in the treatment of infectious diseases[188]. V. amygdalina leaves juice improved cutaneous wound healing in rats. Compared to the negative control, there was an increase in fibroblast recruitment, epithelial cell migration, neovascularization, and a decrease in polymorphonuclear leukocyte infiltration[189]. The impact of a cod liver oil (CLO)-enriched V. amygdalina leaf-based diet (CLVA) on wound healing in type 2 diabetic rats was studied. Thirty-six albino rats were divided into six groups at random: Control (C), diabetic untreated control (DC), reference drug control (RD), 10% CLVA, 20% CLVA, and 30% CLVA. Except for C, all groups were diabetic rats with wounds, while C group comprised of ND rats with wounds. The diets of groups C, DC, and RD were devoid of CLO and V. amygdalina leaves. The final three groups were provided 10, 20, and 30% V. amygdalina leaves and CLO in their diet. When compared to DC, the wound contraction rate rose considerably, with wound area pictures suggesting progressive wound closure in CLVA-fed animals[190]. Bolajoko[26] examined the effects of freeze-dried V. amygdalina leaves on glycemic indices and oxidative stress indicators in type 2 diabetics with or without chronic foot/leg ulcers to NDs with or without chronic foot/leg ulcers. All participants were given conventional treatment (metformin, glibenclamide, or insulin alone, or metformin with glibenclamide or metformin with insulin). One hundred and twenty people were recruited and divided into four groups. Each group was separated into three subgroups: Subgroup-1 (250 mg V. amygdalina), subgroup-2 (500 mg V. amygdalina), and subgroup-3 (500 mg V. amygdalina) (no supplementation). Decrease in fasting plasma glucose and HbA1c, TPP and OSI, as well as substantial increase in TAS, vitamin A, vitamin C, and vitamin E were seen in the subgroups with herbal supplements. Increases in formamidopyrimidine DNA glycosylase, HbA1c, and exacerbation of wounds, TPP, OSI, reductions in TAS and antioxidant micronutrients were identified in no supplementation subgroups.
The absence of blood glucose monitoring during therapy is the first possible constraint. Except for three research, none of the others mentioned managing blood glucose levels. The second identified barrier is a scarcity of clinical case records. The remaining plant species, with the exception of five, have only undergone one clinical study. It would be difficult to extrapolate the statistical outcomes of one therapy study to a larger cohort. None of the experiments dealing with the oral administration of the medicinal plants on DFU could shed light on the influence of herbal medication on circulatory, cardiovascular or neurological activities. Aspects to consider in the future is the monitoring of blood glucose levels for each plant species. Conducting clinical cases with more patients for each species to improve research accuracy.
Twenty different medicinal plants were tested in clinical research for their capacity to treat DFUs by fostering wound healing. All 19 plant species, except one, have led to wound healing. Ten plant species demonstrated total wound healing, while the others demonstrated significant healing. These plant species have a great potential for skin lesion healing, with substantial changes compared to various controls. With significant differences compared to controls, these plant species have a high potential for healing skin lesions. According to this review, the medicinal plants that were investigated and their derivatives can be used to create effective oral and topically applied treatments for DFUs with dermatological effects, providing dermatologists with a new tool in their toolbox.
Diabetes has been a major health issue for centuries. Though the basic understanding regarding the incidence of diabetes is still at a rudimentary level, it is associated with a number of factors, which, if not monitored, may lead to severe consequences. Diabetic foot ulcer (DFU) is one of the most significant complications faced by diabetic patients worldwide. Poor glycemic management, repetitive trauma, underlying neuropathy, peripheral vascular disease, and poor foot care are the major causes leading to DFU.
Currently, a number of drugs (like insulin, sulphonylureas, biguanides, etc.) are used as antihyperglycemic medications to regulate blood glucose levels. Although synthetic oral antihyperglycemic drugs are effective, they are still accompanied by undesirable side effects; so, in recent years, interest has gradually shifted towards herbal medications. Since time immemorial, plants and plant-derived products have been used to prevent or treat a host of illnesses, including diabetes, and thus could be of potential benefit for DFU therapy.
This systematic review aims to summarize the therapeutic cases addressing the use of botanicals for treating DFU.
The original articles on “the plants for the treatment of DFU” studied in clinical cases only were obtained from various bibliographic databases like PubMed, Scopus, and Google Scholar using different keywords.
A total of 19 clinical cases were recorded employing the use of 20 medicinal plants belonging to 17 families on 1347 subjects. The fruits and leaves were the most preferred plant parts used to treat DFU, regardless of their oral or topical routes of administration.
Of the 20 different medicinal plants, 19 plant species showed potential benefits toward wound healing. Fifty percent of the plant species demonstrated total wound healing, while the others demonstrated significant partial healing. The enlisted medicinal plants can be used to create effective oral and topically applied treatments for DFU.
The effect of these plants on the sugar levels might be studied along with DFU treatment. The effect of these plants on the circulatory, cardiovascular and neurological systems can also be studied in the future to understand their mechanisms of action and develop effective treatments for DFU.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Leung PC, China; Mostafavinia A, Iran S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Wilmot E, Idris I. Early onset type 2 diabetes: risk factors, clinical impact and management. Ther Adv Chronic Dis. 2014;5:234-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 2. | Goyal AK, Middha SK, Usha T, Sen A. Analysis of toxic, antidiabetic and antioxidant potential of Bambusa balcooa Roxb. leaf extracts in alloxan-induced diabetic rats. 3 Biotech. 2017;7:120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4438] [Cited by in RCA: 4372] [Article Influence: 291.5] [Reference Citation Analysis (4)] |
| 4. | Brahma S, Goyal AK, Dhamodhar P, Kumari MR, Jayashree S, Usha T, Middha SK. Can Polyherbal Medicine be used for the Treatment of Diabetes? - A Review of Historical Classics, Research Evidence and Current Prevention Programs. Curr Diabetes Rev. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Rajput DS, Basha SM, Xin Q, Gadekallu TR, Kaluri R, Lakshmanna K, Maddikunta PK. Providing diagnosis on diabetes using cloud computing environment to the people living in rural areas of India. J Ambient Intell Human Comput. 2022;13:2829-2840. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Mahesh TR, Kumar D, Vinoth Kumar V, Asghar J, Mekcha Bazezew B, Natarajan R, Vivek V. Blended Ensemble Learning Prediction Model for Strengthening Diagnosis and Treatment of Chronic Diabetes Disease. Comput Intell Neurosci. 2022;2022:4451792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Lo ZJ, Surendra NK, Saxena A, Car J. Clinical and economic burden of diabetic foot ulcers: A 5-year longitudinal multi-ethnic cohort study from the tropics. Int Wound J. 2021;18:375-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Krause F. The Diabetic Foot, an Issue of Foot and Ankle Clinics of North America. 1st ed. USA: Academic Press, 2022: 529. |
| 9. | Shi L, Xue J, Zhao W, Wei X, Zhang M, Li L, Xu Z, Wang A. The Prognosis of Diabetic Foot Ulcer is Independent of age? A Comparative Analysis of the Characteristics of Patients with Diabetic Foot Ulcer in Different age Groups: A Cross-Sectional Study from China. Int J Low Extrem Wounds. 2022;15347346221125844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (1)] |
| 10. | Ghosh P, Valia R. Burden of diabetic foot ulcers in India: evidence landscape from published literature. Value Health. 2017;20:A485. [DOI] [Full Text] |
| 11. | Potash JB, Buervenich S, Cox NJ, Zandi PP, Akula N, Steele J, Rathe JA, Avramopoulos D, Detera-Wadleigh SD, Gershon ES, DePaulo JR Jr, Feinberg AP, McMahon FJ; NIMH Genetics Initiative Bipolar Disorder Consortium. Gene-based SNP mapping of a psychotic bipolar affective disorder linkage region on 22q12.3: association with HMG2L1 and TOM1. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:59-67. [PubMed] [DOI] [Full Text] |
| 12. | Clayton Jr W, Elasy TA. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin Diabetes. 2009;27:52-58. [DOI] [Full Text] |
| 13. | Bahmani M, Golshahi H, Saki K, Rafieian-Kopaei M, Delfan B, Mohammadi T. Medicinal plants and secondary metabolites for diabetes mellitus control. Asian Pac J Trop Dis. 2014;4:S687-S692. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Chu WK, Cheung SCM, Lau RAW, Benzie IFF. Bilberry (Vaccinium myrtillus L.). In: Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. Boca Raton (FL): CRC Press/Taylor & Francis; 2011. Chapter 4. [PubMed] |
| 15. | Hosseinzadeh H, Ramezani M, Danaei AR. Antihyperglycaemic effect and acute toxicity of Securigera Securidaca L. seed extracts in mice. Phytother Res. 2002;16:745-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Shanmugasundaram ER, Rajeswari G, Baskaran K, Rajesh Kumar BR, Radha Shanmugasundaram K, Kizar Ahmath B. Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin-dependent diabetes mellitus. J Ethnopharmacol. 1990;30:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 107] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Baskaran K, Kizar Ahamath B, Radha Shanmugasundaram K, Shanmugasundaram ER. Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. J Ethnopharmacol. 1990;30:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 146] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Bever BO, Zahnd GR. Plants with oral hypoglycaemic action. Int J Crude Drug Res. 1979;17:139-196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Augusti KT, Sheela CG. Antiperoxide effect of S-allyl cysteine sulfoxide, an insulin secretagogue, in diabetic rats. Experientia. 1996;52:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 120] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Rahman MA, Mossa JS, Al-Said MS, Al-Yahya MA. Medicinal plant diversity in the flora of Saudi Arabia 1: a report on seven plant families. Fitoterapia. 2004;75:149-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Lirussi F, Beccarello A, Zanette G, De Monte A, Donadon V, Velussi M, Crepaldi G. Silybin-beta-cyclodextrin in the treatment of patients with diabetes mellitus and alcoholic liver disease. Efficacy study of a new preparation of an anti-oxidant agent. Diabetes Nutr Metab. 2002;15:222-231. [PubMed] |
| 22. | Ribes G, Sauvaire Y, Baccou JC, Valette G, Chenon D, Trimble ER, Loubatières-Mariani MM. Effects of fenugreek seeds on endocrine pancreatic secretions in dogs. Ann Nutr Metab. 1984;28:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47091] [Article Influence: 2943.2] [Reference Citation Analysis (0)] |
| 24. | Bolajoko EB, Onyeaghala AA, Akinosun OM, Attah AF, Adewumi OJ, Adeoti AT, Iyun AO, Fasanmade AA, Moody JO, Khine AA. Hypoglycaemic and Antioxidative Properties of Freeze-Dried Garcinia Kola Seeds in Type 2 Diabetics and Non-Diabetics with Chronic Foot/Leg Ulcer in Ibadan, Nigeria: A case-control clinical study. Afr Health Sci. 2021;34:342-363. |
| 25. | Soleimani Z, Hashemdokht F, Bahmani F, Taghizadeh M, Memarzadeh MR, Asemi Z. Clinical and metabolic response to flaxseed oil omega-3 fatty acids supplementation in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. J Diabetes Complications. 2017;31:1394-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Bolajoko EB. Effect of freeze-dried Vernonia amygdalina Del. leaves on glycemia, oxidative stress biomarkers and selected metals in Type 2 diabetics with and without foot/Leg ulcer. Arch Basic Appl Med. 2020;8:69-83. |
| 27. | Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361:1545-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 615] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 28. | Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther. 2012;3:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 29. | Oguntibeju OO. Medicinal plants and their effects on diabetic wound healing. Vet World. 2019;12:653-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Huang H, Ferguson AR. Kiwifruit in China. New Zeal J Crop Hort. 2001;29:1-14. [DOI] [Full Text] |
| 31. | Masoudpour H, Mohajeri G, Khademi EF, Adibi S. Comparison between dressing with kiwifruit and silver sulfadiazine ointment in treatment of Pseudomonas infections in third-degree burns. J Am Coll Surg. 2009;209:S80-S81. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Mohajeri G, Masoudpour H, Heidarpour M, Khademi EF, Ghafghazi S, Adibi S, Akbari M. The effect of dressing with fresh kiwifruit on burn wound healing. Surgery. 2010;148:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Nieuwenhuizen NJ, Maddumage R, Tsang GK, Fraser LG, Cooney JM, De Silva HN, Green S, Richardson KA, Atkinson RG. Mapping, complementation, and targets of the cysteine protease actinidin in kiwifruit. Plant Physiol. 2012;158:376-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Low C, Webb CJ, Thomas L, Ramos E, Panarese A, Clarke RW, Raynor T, Goodwin Jr WJ. The efficacy of fruit juices in disimpacting meat bolus obstruction. Otolaryngol. 2004;131:P166. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Mohajeri G, Safaee M, Sanei MH. Effects of topical Kiwifruit on healing of neuropathic diabetic foot ulcer. J Res Med Sci. 2014;19:520-524. [PubMed] |
| 36. | Kardoust M, Salehi H, Taghipour Z, Sayadi A. The Effect of Kiwifruit Therapeutics in the Treatment of Diabetic Foot Ulcer. Int J Low Extrem Wounds. 2021;20:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Romero-Cerecero O, Zamilpa A, Tortoriello J. Pilot study that evaluated the clinical effectiveness and safety of a phytopharmaceutical elaborated with an extract of Ageratina pichinchensis in patients with minor recurrent aphthous stomatitis. J Ethnopharmacol. 2015;173:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Sánchez-Ramos M, Marquina-Bahena S, Alvarez L, Román-Guerrero A, Bernabé-Antonio A, Cruz-Sosa F. Phytochemical, Pharmacological, and Biotechnological Study of Ageratina pichinchensis: A Native Species of Mexico. Plants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Gómez F, Quijano L, Calderón JS, Perales A, Ríos T. 2, 2-Dimethylchromenes from Eupatorium aschembornianum. Phytochemistry. 1982;21:2095-2097. [DOI] [Full Text] |
| 40. | Rios MY, Aguilar-Guadarrama AB, Navarro V. Two new benzofuranes from Eupatorium aschenbornianum and their antimicrobial activity. Planta Med. 2003;69:967-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Navarro García VM, Gonzalez A, Fuentes M, Aviles M, Rios MY, Zepeda G, Rojas MG. Antifungal activities of nine traditional Mexican medicinal plants. J Ethnopharmacol. 2003;87:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Romero-Cerecero O, Zamilpa-Álvarez A, Ramos-Mora A, Alonso-Cortés D, Jiménez-Ferrer JE, Huerta-Reyes ME, Tortoriello J. Effect on the wound healing process and in vitro cell proliferation by the medicinal Mexican plant Ageratina pichinchensis. Planta Med. 2011;77:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Romero-Cerecero O, Zamilpa A, Díaz-García ER, Tortoriello J. Pharmacological effect of Ageratina pichinchensis on wound healing in diabetic rats and genotoxicity evaluation. J Ethnopharmacol. 2014;156:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Romero-Cerecero O, Zamilpa A, Tortoriello J. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis in patients with diabetic foot ulcer: a randomized, controlled pilot study. Planta Med. 2015;81:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Manvitha K, Bidya B. Aloe vera: a wonder plant its history, cultivation and medicinal uses. J Pharmacogn Phytochem. 2014;2:85-88. |
| 46. | Baruah A, Bordoloi M, Baruah HP. Aloe vera: A multipurpose industrial crop. Ind Crops Prod. 2016;94:951-963. [DOI] [Full Text] |
| 47. | Banu A, Sathyanarayana B, Chattannavar G. Efficacy of fresh Aloe vera gel against multi-drug resistant bacteria in infected leg ulcers. Australas Med J. 2012;5:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Kędzierska M, Jamroży M, Kudłacik-Kramarczyk S, Drabczyk A, Bańkosz M, Potemski P, Tyliszczak B. Physicochemical Evaluation of L-Ascorbic Acid and Aloe vera-Containing Polymer Materials Designed as Dressings for Diabetic Foot Ulcers. Materials (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Okyar A, Can A, Akev N, Baktir G, Sütlüpinar N. Effect of Aloe vera leaves on blood glucose level in type I and type II diabetic rat models. Phytother Res. 2001;15:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Lanjhiyana S, Garabadu D, Ahirwar D, Bigoniya P, Rana AC, Patra KC, Lanjhiyana SK, Karuppaih M. Antihyperglycemic potential of Aloe vera gel in experimental animal model. Ann Biol Res. 2011;2:17-31. |
| 51. | Furkan M, Alam MT, Rizvi A, Khan K, Ali A, Shamsuzzaman, Naeem A. Aloe emodin, an anthroquinone from Aloe vera acts as an anti aggregatory agent to the thermally aggregated hemoglobin. Spectrochim Acta A Mol Biomol Spectrosc. 2017;179:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Teplicki E, Ma Q, Castillo DE, Zarei M, Hustad AP, Chen J, Li J. The Effects of Aloe vera on Wound Healing in Cell Proliferation, Migration, and Viability. Wounds. 2018;30:263-268. [PubMed] |
| 53. | Daburkar M, Lohar V, Rathore AS, Bhutada P, Tangadpaliwar S. An in vivo and in vitro investigation of the effect of Aloe vera gel ethanolic extract using animal model with diabetic foot ulcer. J Pharm Bioallied Sci. 2014;6:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Aqsa T, Shafique MS, Qureshi U, Changeez M, Farooqi JS, Khan JS. Efficacy of Aloe vera cream for healing diabetic foot ulcers. J Rawalpindi Med Coll. 2019;23:113-115. |
| 55. | Najafian Y, Khorasani ZM, Najafi MN, Hamedi SS, Mahjour M, Feyzabadi Z. Efficacy of Aloe vera/ Plantago Major Gel in Diabetic Foot Ulcer: A Randomized Double-Blind Clinical Trial. Curr Drug Discov Technol. 2019;16:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 56. | Hashmat I, Azad H, Ahmed A. Neem (Azadirachta indica A. Juss)-A nature’s drugstore: an overview. Int Res J Biol Sci. 2012;1:76-79. |
| 57. | Vinoth B, Manivasagaperumal R, Rajaravindran M. Phytochemical analysis and antibacterial activity of Azadirachta indica A. Juss. Int J Plant Sci. 2012;2:50-55. |
| 58. | Prashanth GK, Krishnaiah GM. Chemical composition of the leaves of Azadirachta indica Linn (Neem). 2014. |
| 59. | Okpanyi SN, Ezeukwu GC. Anti-inflammatory and antipyretic activities of Azadirachta indica. Planta Med. 1981;41:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Rabiu H, Subhasish M. Investigation of in vitro anthelmintic activity of Azadirachta indica leaves. Int J Drug Dev Res. 2011;3:94-100. |
| 61. | Deshpande PK. Phytochemical analysis and evaluation of antimalarial activity of Azadirachta indica. 2014. |
| 62. | Osunwoke Emeka A, OlotuEmamoke J, Allison Theodore A, Onyekwere Julius C. The wound healing effects of aqueous leave extracts of Azadirachta indica on wistar rats. J Nat Sci Res. 2013;3:181-186. |
| 63. | Iabichella ML. The use of a mixture of Hypericum perforatum and Azadirachta indica for the management of diabetic foot ulcers: a case series. J Diabetes Metab. 2015;6:2. [DOI] [Full Text] |
| 64. | Iabichella ML, Caruso C, Lugli M. The use of an extract of Hypericum perforatum and Azadirachta indica in a neuropathic patient with advanced diabetic foot. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Sharrif MM, Hamed HK. Pot marigold (Calendula officinalis) medicinal usage and cultivation. J Sci Res Essay. 2012;7:1468-1472. [DOI] [Full Text] |
| 66. | Jan N, Andrabi KI, John R. Calendula officinalis-an important medicinal plant with potential biological properties. Proc Indian Natn Sci Acad. 2017;83:769-7870. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Muley BP, Khadabadi SS, Banarase NB. Phytochemical constituents and pharmacological activities of Calendula officinalis Linn (Asteraceae): a review. Trop J Pharm Res. 2009;8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Kemper KJ. Calendula (Calendula officinalis). Longwood Herbal Task Force. 1999;1. |
| 69. | Kalvatchev Z, Walder R, Garzaro D. Anti-HIV activity of extracts from Calendula officinalis flowers. Biomed Pharmacother. 1997;51:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Ashwlayan VD KA, Verma M, Garg VK, Gupta SK. Therapeutic potential of Calendula officinalis. Pharm Pharmacol Int J. 2018;6:149-155. [DOI] [Full Text] |
| 71. | Butnariu M, Coradini CZ. Evaluation of Biologically Active Compounds from Calendula officinalis Flowers using Spectrophotometry. Chem Cent J. 2012;6:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 72. | Nicolaus C, Junghanns S, Hartmann A, Murillo R, Ganzera M, Merfort I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J Ethnopharmacol. 2017;196:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 73. | Carvalho AF, Feitosa MC, Coelho NP, Rebêlo VC, Castro JG, Sousa PR, Feitosa VC, Arisawa EA. Low-level laser therapy and Calendula officinalis in repairing diabetic foot ulcers. Rev Esc Enferm USP. 2016;50:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 74. | Buzzi M, de Freitas F, Winter M. A Prospective, Descriptive Study to Assess the Clinical Benefits of Using Calendula officinalis Hydroglycolic Extract for the Topical Treatment of Diabetic Foot Ulcers. Ostomy Wound Manage. 2016;62:8-24. [PubMed] |
| 75. | Gohil KJ, Patel JA, Gajjar AK. Pharmacological Review on Centella asiatica: A Potential Herbal Cure-all. Indian J Pharm Sci. 2010;72:546-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 76. | Bylka W, Znajdek-Awiżeń P, Studzińska-Sroka E, Dańczak-Pazdrowska A, Brzezińska M. Centella asiatica in dermatology: an overview. Phytother Res. 2014;28:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 77. | Seevaratnam V, Banumathi P, Premalatha MR, Sundaram SP, Arumugam T. Functional properties of Centella asiatica (L.): a review. Int J Pharm Pharm Sci. 2012;4:8-14. |
| 78. | Inamdar PK, Yeole RD, Ghogare AB, de Souza NJ. Determination of biologically active constituents in Centella asiatica. J Chromatogr A. 1996;742:127-130. [RCA] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Prakash V, Jaiswal NI, Srivastava MR. A review on medicinal properties of Centella asiatica. Asian J Pharm Clin Res. 2017;10:69-74. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 80. | Hausen BM. Centella asiatica (Indian pennywort), an effective therapeutic but a weak sensitizer. Contact Dermatitis. 1993;29:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Hamidpour R. Medicinal property of Gotu kola (Centella asiatica) from the selection of traditional applications to the novel phytotherapy. Inter J Cancer Res. 2015;3. [DOI] [Full Text] |
| 82. | Azis HA, Taher M, Ahmed AS, Sulaiman WM, Susanti D, Chowdhury SR, Zakaria ZA. In vitro and In vivo wound healing studies of methanolic fraction of Centella asiatica extract. S Afr J Bot. 2017;108:163-174. [DOI] [Full Text] |
| 83. | Nganlasom J, Suttitum T, Jirakulsomchok D, Puapairoj A. Effects of Centella Asiatica Linn. and Garcinia Mangostana Linn. on the healing of dermal wounds in diabetic rats. Srinagarind Med J. 2008;. |
| 84. | Kuo YS, Chien HF, Lu W. Plectranthus amboinicus and Centella asiatica Cream for the Treatment of Diabetic Foot Ulcers. Evid Based Complement Alternat Med. 2012;2012:418679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 85. | Adesuyi AO, Elumm IK, Adaramola FB, Nwokocha AG. Nutritional and phytochemical screening of Garcinia kola. Adv J Food Sci Technol. 2012;4. |
| 86. | Iwu M, Igboko O. Flavonoids of Garcinia kola seeds. J Nat Prod. 2004;45. [DOI] [Full Text] |
| 87. | Buba CI, Okhale SE. Garcinia kola: The phytochemistry, pharmacology and therapeutic applications. Int J Pharmacognosy. 2016;3:67-81. [DOI] [Full Text] |
| 88. | Adegboye MF, Akinpelu DA, Okoh AI. The bioactive and phytochemical properties of Garcinia kola (Heckel) seed extract on some pathogens. Afr J Biotechnol. 2008;7. |
| 89. | Ebana RU, Madunagu BE, Ekpe ED, Otung IN. Microbiological exploitation of cardiac glycosides and alkaloids from Garcinia kola, Borreria ocymoides, Kola nitida and Citrus aurantifolia. J Appl Bacteriol. 1991;71:398-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Smith YA, Adanlawo IG. In vitro and in vivo antioxidant activity of saponin extracted from the root of Garcinia kola (bitter Kola) on alloxan-induced diabetic rats. World J Pharm Pharm Sci. 2014;3:8-26. |
| 91. | Nwaehujor CO, Nwinyi FC, Igile GO. The wound healing activities of Garcinia hydroxybiflavanonol (GB1) from Garcinia kola in streptozotocin-induced diabetic rats. Int J Biochem Photon. 2013;114:173-180. |
| 92. | Omodamiro OD, Ajah O, Ewa-Ibe C. Evaluation of antioxidant potential and anti-diabetic effect of ethanol seed extract of Garcinia kola (bitter kola) in albino rat. J Med Herbs Ethnomed. 2020;. |
| 93. | Pattewar SV. Kalanchoe pinnata: phytochemical and pharmacological profile. Int J Pharm. 2012;2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Alves CV, da Silva Santiago SR, Soares ER, de Almeida RA, de Lima BR, de Carvalho CS, Santiago PA. Determination of the chemical profile extracts obtained from Kalanchoe pinnata (Lam.) Pers native of municipality Tabatinga-AM. Res Society Develop. 2022;11:e1411427103. [DOI] [Full Text] |
| 95. | Nayak BS, Marshall JR, Isitor G. Wound healing potential of ethanolic extract of Kalanchoe pinnata Lam. leaf--a preliminary study. Indian J Exp Biol. 2010;48:572-576. [PubMed] |
| 96. | Fernandes JM, Félix-Silva J, da Cunha LM, Gomes JA, Siqueira EM, Gimenes LP, Lopes NP, Soares LA, Fernandes-Pedrosa MF, Zucolotto SM. Inhibitory Effects of Hydroethanolic Leaf Extracts of Kalanchoe brasiliensis and Kalanchoe pinnata (Crassulaceae) against Local Effects Induced by Bothrops jararaca Snake Venom. PLoS One. 2016;11:e0168658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 97. | Raj V, Kumar A, Singh V, Kumar P, Kumar V. In vitro antimicrobial activity of Kalanchoe pinnata leaf. Int J Curr Pharm Res. 2012;2:3-5. |
| 98. | Coutinho MAS, Casanova LM, Nascimento LBDS, Leal D, Palmero C, Toma HK, Dos Santos EP, Nasciutti LE, Costa SS. Wound healing cream formulated with Kalanchoe pinnata major flavonoid is as effective as the aqueous leaf extract cream in a rat model of excisional wound. Nat Prod Res. 2021;35:6034-6039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 99. | Cawich SO, Harnarayan P, Budhooram S, Bobb NJ, Islam S, Naraynsingh V. Wonder of Life (kalanchoe pinnata) leaves to treat diabetic foot infections in Trinidad & Tobago: a case control study. Trop Doct. 2014;44:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 100. | Badole SL, Zanwar AA, Bodhankar SL. Antihyperglycemic potential of secoisolaricinol diglucoside. In: Watson RR, Preedy VR. Bioactive Food as Dietary Interventions for Diabetes. 1st ed. USA: Academic Press, 2013: 53-57. |
| 101. | Akter Y, Junaid M, Afrose SS, Nahrin A, Alam MS, Sharmin T, Akter R, Hosen SMZ. A Comprehensive Review on Linum usitatissimum Medicinal Plant: Its Phytochemistry, Pharmacology, and Ethnomedicinal Uses. Mini Rev Med Chem. 2021;21:2801-2834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 102. | Hussain MS, Kaur G, Mohapatra C. Nutritional composition and functions of flaxseed (Linum usitatissimum linn.). Food Ther Health Care. 2021;3:88-91. [DOI] [Full Text] |
| 103. | Poljšak N, Kreft S, Kočevar Glavač N. Vegetable butters and oils in skin wound healing: Scientific evidence for new opportunities in dermatology. Phytother Res. 2020;34:254-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 104. | Haroon M, Iqbal MJ, Hassan W, Ali S, Ahmed H, Hassan SU. Evaluation of methanolic crude extract of Linum usitatissimum for the removal of biofilm in diabetic foot isolates. Braz J Biol. 2021;83:e245807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 105. | Dugani A, Auzzi A, Naas F, Megwez S. Effects of the oil and mucilage from flaxseed (linum usitatissimum) on gastric lesions induced by ethanol in rats. Libyan J Med. 2008;3:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 106. | Hooser SB, Wilson CR. Comparative Hepatotoxicology. In: McQueen CA. Comprehensive Toxicology. 2nd ed. USA: Academic Press, 2010: 403-419. |
| 107. | Al-Snafi AE. Chemical constituents and pharmacological effects of Melilotus Officinalis-A review. 2020. |
| 108. | Anwer MS, Mohtasheem M, Azhar I, Hasan M, Bano H. Chemical constituents from Melilotus officinalis. 2008. |
| 109. | Pleşca-Manea L, Pârvu AE, Pârvu M, Taămaş M, Buia R, Puia M. Effects of Melilotus officinalis on acute inflammation. Phytother Res. 2002;16:316-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 110. | Derakhshan MA, Nazeri N, Khoshnevisan K, Heshmat R, Omidfar K. Three-layered PCL-collagen nanofibers containing melilotus officinalis extract for diabetic ulcer healing in a rat model. J Diabetes Metab Disord. 2022;21:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 111. | Al-ani WM. Antifungal activity of Mellilotus officinalis of Iraq. 2014. |
| 112. | Al-Ani WM. Isolation of coumarin from Mellilotus officinalisof Iraq. 2014. |
| 113. | Bagheri MH, Borhani Haghighi A, Novitsky YA, Ranjbar Omrani G. Effect of ANGIPARSTM, a new herbal drug on diabetic foot ulcer: A phase 2 clinical study. DARU J Pharm Sci. 2007;16 Suppl 1. |
| 114. | Bahrami A, Aliasgarzadeh A, Sarabchian M, Mobasseri M. Efficacy of oral ANGIPARS in chronic diabetes foot ulcer: a double blind placebo controlled study. Iran J Endocrinol Metab. 2009;11:647-655. |
| 115. | Samadov BS. The chemical composition of the medicinal plant Momordica Charantia L used in folk medicine. Themat J Chem. 2022;6:36-51. |
| 116. | Daniel P, Supe U, Roymon MG. A review on phytochemical analysis of Momordica charantia. Int J Adv Pharm Biol Chem. 2014;3:214-220. |
| 117. | Okunowo W. Nutritional and chemical evaluation of Momordica charantia. J Med Plants Res. 2010;4:2189-2193. [DOI] [Full Text] |
| 118. | Mada SB, Garba A, Mohammed HA, Muhammad A, Olagunju A. Antimicrobial activity and phytochemical screening of aqueous and ethanol extracts of Momordica charantia L. leaves. J Med Plant Res. 2013;. [DOI] [Full Text] |
| 119. | Prasad V, Jain V, Girish D, Dorle AK. Wound-healing property of Momordica charantia L. fruit powder. J Herb Pharmacother. 2006;6:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 120. | Hussan F, Teoh SL, Muhamad N, Mazlan M, Latiff AA. Momordica charantia ointment accelerates diabetic wound healing and enhances transforming growth factor-β expression. J Wound Care. 2014;23:400, 402, 404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 121. | Rosyid FN, Prasetyo TA, Prabawati CY, Heri SK, Hs N, Supratman S. The Effect of Bitter Melon (Momordica charantia L.) Leaves Extractonon Glycated Albumin in Diabetic Foot Ulcers: Randomized Controlled Trial. Bangladesh J Med Sci. 2021;. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 122. | Rosyid FN, Dharmana E, Suwondo A, HS KH, Sugiarto S. The effect of bitter melon (Momordica charantia L.) leaves extract on TNF-α serum levels and diabetic foot ulcers improvement: randomized controlled trial. Biomed Pharmacol J. 2018;11:1413-1421. [DOI] [Full Text] |
| 123. | El SN, Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr Rev. 2009;67:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 281] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 124. | Khan Y, Panchal S, Vyas N, Butani A, Kumar V. Olea europaea: a phyto-pharmacological review. Phcog Rev. 2007;1. |
| 125. | Ghanadian M, Soltani R, Homayouni A, Khorvash F, Jouabadi SM, Abdollahzadeh M. The Effect of Plantago major Hydroalcoholic Extract on the Healing of Diabetic Foot and Pressure Ulcers: A Randomized Open-Label Controlled Clinical Trial. Int J Low Extrem Wounds. 2022;15347346211070723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 126. | Sudjana AN, D’Orazio C, Ryan V, Rasool N, Ng J, Islam N, Riley TV, Hammer KA. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int J Antimicrob Agents. 2009;33:461-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 127. | Elnahas RA, Elwakil BH, Elshewemi SS, Olama ZA. Egyptian Olea europaea leaves bioactive extract: Antibacterial and wound healing activity in normal and diabetic rats. J Tradit Complement Med. 2021;11:427-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 128. | Casado-Díaz A, La Torre M, Priego-Capote F, Verdú-Soriano J, Lázaro-Martínez JL, Rodríguez-Mañas L, Berenguer Pérez M, Tunez I. EHO-85: A Multifunctional Amorphous Hydrogel for Wound Healing Containing Olea europaea Leaf Extract: Effects on Wound Microenvironment and Preclinical Evaluation. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 129. | Khadem HH, Koushan Y, Asgharzadeh A. Treatment of diabetic foot ulcer with propolis and olive oil: a case report. Knowledge Health J. 2012;6:35. |
| 130. | Abdoli A, Shahbazi R, Zoghi G, Davoodian P, Kheirandish S, Azad M, Kheirandish M. The effect of topical olive oil dressing on the healing of grade 1 and 2 diabetic foot ulcers: An assessor-blind randomized controlled trial in type 2 diabetes patients. Diabetes Metab Syndr. 2022;16:102678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 131. | Nasiri M, Fayazi S, Jahani S, Yazdanpanah L, Haghighizadeh MH. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 132. | Najafian Y, Hamedi SS, Farshchi MK, Feyzabadi Z. Plantago major in Traditional Persian Medicine and modern phytotherapy: a narrative review. Electron Physician. 2018;10:6390-6399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 133. | Farid A, Sheibani M, Shojaii A, Noori M, Motevalian M. Evaluation of anti-inflammatory effects of leaf and seed extracts of Plantago major on acetic acid-induced ulcerative colitis in rats. J Ethnopharmacol. 2022;298:115595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 134. | Tafazoli V, Taherifard E, Nimrouzi M, Pasalar M. Therapeutic effect of Plantago major on active severe pancolitis: A case report. Adv Integr Med. 2022;9:90-93. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 135. | Jazayeri SF, Ghods R, Hashem Dabaghian F, Shojaii A, Moravej SAA, Khadem E, Seyedian SS. The Efficacy of Plantago major Seed on Liver Enzymes in Nonalcoholic Fatty Liver Disease: A Randomized Double-Blind Clinical Trial. Evid Based Complement Alternat Med. 2021;2021:6693887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 136. | Rad NM, Shafie F, Chaghervand MM, Kashfi S, Rashidipour M, Chehelcheraghi F, Mozaffarpur SA, Rasoulian B. The Wound Healing Effect of Plantago Major Leaf Extract in a Rat Model: Pilot Experimental Confirmation of a Traditional Belief in Persian Medicine. J Herb Med. 2018;. |
| 137. | Ghiasian M, Niroomandi Z, Dastan D, Poorolajal J, Zare F, Ataei S. Clinical and phytochemical studies of Plantago major in pressure ulcer treatment: A randomized controlled trial. Complement Ther Clin Pract. 2021;43:101325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 138. | Keshavarzi A, Montaseri H, Akrami R, Moradi Sarvestani H, Khosravi F, Foolad S, Zardosht M, Zareie S, Saharkhiz MJ, Shahriarirad R. Therapeutic Efficacy of Great Plantain (Plantago major L.) in the Treatment of Second-Degree Burn Wounds: A Case-Control Study. Int J Clin Pract. 2022;2022:4923277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 139. | Punet K, Sangma S, Kumar N. Plectranthus amboinicus: a review on its pharmacological and pharmacognostical studies. Am J Physiol Bioche Pharmacol. 2020;10:55. [DOI] [Full Text] |
| 140. | Gurgel AP, da Silva JG, Grangeiro AR, Oliveira DC, Lima CM, da Silva AC, Oliveira RA, Souza IA. In vivo study of the anti-inflammatory and antitumor activities of leaves from Plectranthus amboinicus (Lour.) Spreng (Lamiaceae). J Ethnopharmacol. 2009;125:361-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 141. | Swamy MK, Arumugam G, Kaur R, Ghasemzadeh A, Yusoff MM, Sinniah UR. GC-MS Based Metabolite Profiling, Antioxidant and Antimicrobial Properties of Different Solvent Extracts of Malaysian Plectranthus amboinicus Leaves. Evid Based Complement Alternat Med. 2017;2017:1517683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 142. | de Oliveira FF, Torres AF, Gonçalves TB, Santiago GM, de Carvalho CB, Aguiar MB, Camara LM, Rabenhorst SH, Martins AM, Valença Junior JT, Nagao-Dias AT. Efficacy of Plectranthus amboinicus (Lour.) Spreng in a Murine Model of Methicillin-Resistant Staphylococcus aureus Skin Abscesses. Evid Based Complement Alternat Med. 2013;2013:291592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 143. | Sariozlu NY, Kivanc M. Gallnuts (Quercus infectoria Oliv. and Rhus chinensis Mill.) and their usage in health. Nuts Seeds Health Dis Prevention. 2011;505-511. [DOI] [Full Text] |
| 144. | Dar MS, Ikram M, Fakouhi T. Pharmacology of Quercus infectoria. J Pharm Sci. 1976;65:1791-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 145. | Ikram M, Nowshad F. Constituents of Quercus infectoria. Planta Med. 1977;31:286-287. [PubMed] [DOI] [Full Text] |
| 146. | Kaur G, Hamid H, Ali A, Alam MS, Athar M. Antiinflammatory evaluation of alcoholic extract of galls of Quercus infectoria. J Ethnopharmacol. 2004;90:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 147. | Basri DF, Fan SH. The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents. Indian J Pharmacol. 2005;37:26-29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 148. | Umachigi SP, Jayaveera KN, Kumar CA, Kumar GS, Kumar DK. Studies on wound healing properties of Quercus infectoria. Trop J Pharm Res. 2008;7:913-919. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 149. | Chokpaisarn J, Chusri S, Amnuaikit T, Udomuksorn W, Voravuthikunchai SP. Potential wound healing activity of Quercus infectoria formulation in diabetic rats. PeerJ. 2017;5:e3608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 150. | Sharba ZA, Hasoon BA, Maeah RK, Hussein NN. Cytotoxicity, antioxidant, and antimicrobial activities of crude extract of Quercus infectoria plant. Plant Arch. 2020;20:227-230. |
| 151. | Bonab FS, Farahpour MR. Topical co-administration of Pistacia atlantica hull and Quercus infectoria gall hydroethanolic extract improves wound-healing process. Comp Clin Path. 2017;26:1-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 152. | Chokpaisarn J, Chusri S, Voravuthikunchai SP. Clinical randomized trial of topical Quercus infectoria ethanolic extract for the treatment of chronic diabetic ulcers. J Herb Med. 2020;21:100301. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 153. | Sam URM. Nutritional analysis of Sesamum radiatum seeds. Final Year Project thesis, Universiti Malaysia Kelantan. 2019. [cited 2 January 2023]. Available from: http://discol.umk.edu.my/id/eprint/4855/. |
| 154. | Catarino L, Romeiras MM, Bancessi Q, Duarte D, Faria D, Monteiro F, Moldão M. Edible Leafy Vegetables from West Africa (Guinea-Bissau): Consumption, Trade and Food Potential. Foods. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 155. | Konan BA, Bouafou KM, Bléyéré NM, Zannou-Tchoko V, Amonkan KA, Oussou KR, Datté YJ. Acute toxicity study and effects of sesame (Sesamum radiatum) aqueous leaf extract on rabbit’s electrocardiogram. 2015. |
| 156. | Nep EI, Carnachan SM, Ngwuluka NC, Kontogiorgos V, Morris GA, Sims IM, Smith AM. Structural characterisation and rheological properties of a polysaccharide from sesame leaves (Sesamum radiatum Schumach. & Thonn.). Carbohydr Polym. 2016;152:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 157. | Ogunlesi M, Okiei W, Osibote EA. Analysis of the essential oil from the leaves of Sesamum radiatum, a potential medication for male infertility factor, by gas chromatography-mass spectrometry. Afr J Biotechnol. 2010;9:1060-1067. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 158. | Shittu LA. Antibacterial and antifungal activities of essential oils of crude extracts of Sesame radiatum against some common pathogenic micro-organisms. Iran J Pharmacol Ther. 2006;6:165-170. |
| 159. | Shittu L, Ahmed T, Akinsanya M, Bankole M, Shittu R, Ashiru OA. Differential antimicrobial activity of the various crude leaves extracts of Sesame radiatum against some common pathogenic micro-organisms. Sci Res Essays. 2006;1:108-111. |
| 160. | Tripathy R, Nair SV, Lakshman V, Raj S, Otta SP. Wound-healing potential of Nimbadi Kalka in diabetic foot ulcer: a clinical study. J Med. 2020;8:102. [DOI] [Full Text] |
| 161. | Rafieian-Kopaei M, Nasri H. Comment on: preventive effect of Teucrium polium on learning and memory deficits in diabetic rats. Med Sci Monit Basic Res. 2013;19:208-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 162. | Bahramikia S, Yazdanparast R. Phytochemistry and medicinal properties of Teucrium polium L. (Lamiaceae). Phytother Res. 2012;26:1581-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 163. | Tariq M, Ageel AM, al-Yahya MA, Mossa JS, al-Said MS. Anti-inflammatory activity of Teucrium polium. Int J Tissue React. 1989;11:185-188. [PubMed] |
| 164. | Sharififar F, Dehghn-Nudeh G, Mirtajaldini M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chemis. 2009;112:885-888. [RCA] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 165. | Darabpour E, Motamedi H, Nejad SM. Antimicrobial properties of Teucrium polium against some clinical pathogens. Asian Pac J Trop Med. 2010;3:124-127. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 166. | Alizadeh AM, Sohanaki H, Khaniki M, Mohaghgheghi MA, Ghmami G, Mosavi M. The effect of teucrium polium honey on the wound healing and tensile strength in rat. Iran J Basic Med Sci. 2011;14:499-505. [PubMed] |
| 167. | Fallah Huseini H, Abdolghaffari AH, Ahwazi M, Jasemi E, Yaghoobi M, Ziaee M. Topical Application of Teucrium polium Can Improve Wound Healing in Diabetic Rats. Int J Low Extrem Wounds. 2020;19:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 168. | Gharaboghaz MNZ, Farahpour MR, Saghaie S. Topical co-administration of Teucrium polium hydroethanolic extract and Aloe vera gel triggered wound healing by accelerating cell proliferation in diabetic mouse model. Biomed Pharmacother. 2020;127:110189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 169. | Fallah Huseini H, Yaghoobi M, Fallahi F, Boroumand F, Ezzati MH, Tabatabaei SM, Sotvan H, Ahvazi M, Badiee Aval S, Ziaee M. Topical Administration of Teucrium polium on Diabetic Foot Ulcers Accelerates Healing: A Placebo-Controlled Randomized Clinical Study. Int J Low Extrem Wounds. 2021;15347346211048371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 170. | Mehta PL, Kalra R, Prasad R. A Backdrop Case Study of AI-Drones in Indian Demographic Characteristics Emphasizing the Role of AI in Global Cities Digitalization. Wirel Pers Commun. 2021;118:301-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 171. | Thomas JV, Nair DVT, Noll S, Johnson TJ, Cardona C, Johny AK. Effect of Turkey-Derived Beneficial Bacteria Lactobacillus salivarius and Lactobacillus ingluviei on a Multidrug-Resistant Salmonella Heidelberg Strain in Turkey Poults. J Food Prot. 2019;82:435-440. [PubMed] [DOI] [Full Text] |
| 172. | Alugoju P, Chaitanya NS, Swamy VK, Kancharla PK. Phytotherapy for breast cancer. In: Malla RR, Nagaraju GP. A Theranostic and Precision Medicine Approach for Female-Specific Cancers. USA: Academic Press, 2021: 129-163. |
| 173. | Choudhary N, Siddiqui MB, Azmat S, Khatoon S. Tinospora cordifolia: ethnobotany, phytopharmacology and phytochemistry aspects. Int J Pharm Sci Res. 2013;4:891-899. |
| 174. | Patel MB, Mishra S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine. 2011;18:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 175. | Hashilkar NK, Patil PA, Bagi JG, Patil SY, Angadi NB. Influence of Tinospora cordifolia on wound healing in wistar rats. Int J Basic Clin Pharmacol. 2016;. [DOI] [Full Text] |
| 176. | Purandare H, Supe A. Immunomodulatory role of Tinospora cordifolia as an adjuvant in surgical treatment of diabetic foot ulcers: a prospective randomized controlled study. Indian J Med Sci. 2007;61:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 177. | Morales AL, Duque C. Aroma constituents of the fruit of the mountain papaya (Carica pubescens) from Colombia. J Agric Food Chem. 1987;35:538-540. [DOI] [Full Text] |
| 178. | Albuquerque RM, Pizzitola MP, Araújo E Silva AC, Dittz D, Freitas KM, Ferreira Ê, Salas CE, Lopes MTP. The Proteolytic Fraction From Vasconcellea cundinamarcensis Latex Displays Anti-Inflammatory Effect in A Mouse Model of Acute TNBS-Induced Colitis. Sci Rep. 2020;10:3074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 179. | Torres-Ossandón MJ, Vega-Gálvez A, Salas CE, Rubio J, Silva-Moreno E, Castillo L. Antifungal activity of proteolytic fraction (P1G10) from (Vasconcellea cundinamarcensis) latex inhibit cell growth and cell wall integrity in Botrytis cinerea. Int J Food Microbiol. 2019;289:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 180. | Freitas KM, Barcelos LS, Caliari MV, Salas CE, Lopes MTP. Healing activity of proteolytic fraction (P1G10) from Vasconcellea cundinamarcensis in a cutaneous wound excision model. Biomed Pharmacother. 2017;96:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 181. | Oliveira Silva R, da Costa BL, da Silva CN, da Mata Martins TM, Nunes Dourado LF, de Goes AM, Lopes MT, Salas CE, Silva-Cunha AD, da Silva FR. The proteolytic fraction from Vasconcellea cundinamarcensis accelerates wound healing after corneal chemical burn in rabbits. Burns. 2020;46:928-936. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 182. | Tonaco LAB, Gomes FL, Velasquez-Melendez G, Lopes MTP, Salas CE. The Proteolytic Fraction from Latex of Vasconcellea cundinamarcensis (P1G10) Enhances Wound Healing of Diabetic Foot Ulcers: A Double-Blind Randomized Pilot Study. Adv Ther. 2018;35:494-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 183. | Ifeoma II, Chukwunonso EE. Current perspectives on the medicinal potentials of Vernonia amygdalina Del. J Med Plants Res. 2011;5:1051-1061. |
| 184. | Igile GO, Oleszek W, Jurzysta M, Burda S, Fafunso M, Fasanmade AA. Flavonoids from Vernonia amygdalina and their antioxidant activities. J Agric Food Chem. 1993;42. [DOI] [Full Text] |
| 185. | Alara OR, Abdurahman NH, Mudalip SK, Olalere OA. Phytochemical and pharmacological properties of Vernonia amygdalina: a review. 2017. |
| 186. | Akinpelu DA. Antimicrobial activity of Vernonia amygdalina leaves. Fitoterapia. 1999;70:432-434. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 187. | Abosi AO, Raseroka BH. In vivo antimalarial activity of Vernonia amygdalina. Br J Biomed Sci. 2003;60:89-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 188. | Erasto P, Grierson DS, Afolayan AJ. Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina. J Ethnopharmacol. 2006;106:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 189. | Nafiu AB, Akinwale OC, Akinfe OA, Owoleye BV, Abioye AIA, Abdulazeez FI, Rahman MT. Histomorphological Evaluation of Wound Healing-Comparison between Use of Honey and Vernonia amygdalina Leaf Juice. 2016. |
| 190. | Soji-Omoniwa O, Adebayo SO, Ayinde RO, Oyeniyi AF, Adeleye DA, Ezechukwu TA, Chinedum C, Emeje EF, Alebiosu O, Ajibola OE, Adeniyi PO, Adeniyi A, Toluhi A, Adelaja A, Adebisi A, Olawoyin K, Asunloye O. Consumption of Cod Liver Oil-enriched Vernonia amygdalina Leaf-based Diet Promoted Wound Healing in Wound-inflicted Type 2 Diabetic Wistar Rats. 2022. |