Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.4003
Peer-review started: March 22, 2023
First decision: April 11, 2023
Revised: April 15, 2023
Accepted: May 12, 2023
Article in press: May 12, 2023
Published online: June 16, 2023
Processing time: 82 Days and 6.2 Hours
Acute esophageal variceal hemorrhage (AEVH) is a common complication of cirrhosis and might precipitate multi-organ failure, causing acute-on-chronic liver failure (ACLF).
To analyze if the presence and grading of ACLF as defined by European Society for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) is able to predict mortality in cirrhotic patients presenting AEVH.
Retrospective cohort study executed in Hospital Geral de Caxias do Sul. Data from medical records from 2010 to 2016 were obtained by searching the hospital electronic database for patients who received terlipressin. Medical records were reviewed in order to determine the diagnosis of cirrhosis and AEVH, including 97 patients. Kaplan-Meier survival analysis was used for univariate analysis and a stepwise approach to the Cox regression for multivariate analysis.
All- cause mortality for AEVH patients was 36%, 40.2% and 49.4% for 30-, 90- and 365-day, respectively. The prevalence of ACLF was 41.3%. Of these, 35% grade 1, 50% grade 2 and 15% grade 3. In multivariate analysis, the non-use of non-selective beta-blockers, presence and higher grading of ACLF and higher Model for End-Stage Liver Disease scores were independently associated with higher mortality for 30-day with the addition of higher Child-Pugh scores for 90-day period.
Presence and grading of ACLF according to the EASL-CLIF criteria was independently associated with higher 30- and 90-day mortality in cirrhotic patients admitted due to AEVH.
Core Tip: Acute esophageal variceal hemorrhage (AEVH) is a common complication of cirrhosis and might precipitate multi-organ failure, causing acute-on-chronic liver failure (ACLF). The purpose of this study is to analyze if the presence and grading of ACLF as defined by European Society for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) is able to predict mortality in cirrhotic patients presenting AEVH. This is a retrospective cohort study executed in Hospital Geral de Caxias do Sul, which gathered data from medical records from 2010 to 2016 were obtained by searching the hospital electronic database for patients who received terlipressin. Medical records were reviewed in order to determine the diagnosis of cirrhosis and AEVH, including 97 patients. Kaplan-Meier survival analysis was used for univariate analysis and a stepwise approach to the Cox regression for multivariate analysis. All- cause mortality for AEVH patients was 36%, 40.2% and 49.4% for 30-, 90- and 365-day, respectively. The prevalence of ACLF was 41.3%. Of these, 35% grade 1, 50% grade 2 and 15% grade 3. In multivariate analysis, the non-use of non-selective beta-blockers, presence and higher grading of ACLF and higher Model for End-Stage Liver Disease scores were independently associated with higher mortality for 30-day with the addition of higher Child-Pugh scores for 90-day period. In conclusion, the presence and grading of ACLF according to the EASL-CLIF criteria was independently associated with higher 30- and 90-day mortality in cirrhotic patients admitted due to AEVH.
- Citation: Terres AZ, Balbinot RS, Muscope ALF, Longen ML, Schena B, Cini BT, Rost Jr GL, Balensiefer JIL, Eberhardt LZ, Balbinot RA, Balbinot SS, Soldera J. Acute-on-chronic liver failure is independently associated with higher mortality for cirrhotic patients with acute esophageal variceal hemorrhage: Retrospective cohort study. World J Clin Cases 2023; 11(17): 4003-4018
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/4003.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.4003
Portal hypertension occurs in 90% of cirrhotic patients[1], caused by structural abnormalities of the liver and intra-hepatic vasoconstriction due to sinusoidal endothelial dysfunction[2,3]. This will in turn cause the recanalization of collateral veins, leading to the development of esophageal varices[4]. These varices will increase in size and become more likely to rupture, causing acute esophageal variceal hemorrhage (AEVH). This is secondary to the growing portal hypertension, and not to the thrombocytopenia caused by hypersplenism[5,6]. Therefore, esophageal varices are expected to be present in 42.7% of Child-Pugh class A patients, 70.7% of Child-Pugh class B patients and 75.5% of Child-Pugh class C patients[4].
Around one third of cirrhotic patients will present AEVH, which is one of the most common life-threatening complications of cirrhosis and is responsible for 80% to 90% of gastrointestinal bleedings in these patients[7-9]. The first episode of AEVH is associated with a mortality of 15% to 20%[10] and a high rate of recurrence[9].
When AEVH occurs, it is defined as decompensated cirrhosis. Nevertheless, an additional step called acute-on-chronic liver failure (ACLF) has been postulated to take place in-between decompensated cirrhosis and death, characterized by multi-organ failure[11]. Although the discussion between Hepatology and Intensive Care has began over a decade ago[12-14] in a critical care journal[15-18], a definitive definition of ACLF has only been introduced by the multi-centric prospective study CANONIC, developed in Europe and published in 2013[19]. This was done when the European Society for the Study of the Liver – Chronic Liver Failure (EASL-CLIF) group adapted the Sequential Organ Failure Assessment (SOFA) score into the CLIF-SOFA score. This dichotomizes systems as sufficient or insufficient and is able to stratify patients as having an acute decompensation (AD) or ALCF, which was stratified into three grades[19]. In the general population of decompensated cirrhotic patients, the grade of ACLF was associated with higher mortality. CANONIC study was designed with a prospective approach to determine the levels of organ dysfunction that are associated with a 28-day mortality rate exceeding 15%.
In the presence of AEVH, liver-specific scores have been shown to be superior to predict mortality than those used for acute esophageal non-variceal hemorrhage, as they indirectly evaluate liver function and portal hypertension, such as Child-Turcotte-Pugh (CTP), Model for End-Stage Liver Disease (MELD) and CLIF-SOFA[20-22]. CLIF-SOFA, in turn, score has been shown to be superior to CTP and MELD for prognosticating AEVH[23,24].
Prior to the first episode of AEVH, an upper digestive endoscopy is performed to assess the presence of esophageal varices and undertake primary prophylaxis with non-selective beta-blockers (NSBB), such as propranolol, nadolol or carvedilol[25]. Once AEVH occurs, it is generally treated with the association of terlipressin with endoscopic variceal banding (EVB)[26,27]. This demands afterwards a lifelong secondary prophylaxis with NSBB and EVB[28,29].
The purpose of this study is to analyze if the presence and grading of ACLF as defined by EASL-CLIF is able to predict mortality in cirrhotic patients presenting AEVH.
This study was approved by the Research ethics committee of Universidade de Caxias do Sul on June 20, 2017, under protocol no. 66646617.3.0000.5341. This was done in conformity to the ethical guidelines of the 1975 Declaration of Helsinki. As this study analyzed solely medical records, the need for an informed consent was waived by this human research ethics committee.
A search for every patient which received terlipressin from 2010 to 2016 was conducted in the hospital electronic database, as the hospital protocol for suspected AEVH mandates that patients receive terlipressin for 48 h. Data from electronic and physical medical records were retrieved.
Patients were included if they were over 18 years old, had a definitive diagnosis of cirrhosis using laboratory and imaging data and had a diagnosis of AEVH confirmed by endoscopy, defined as a bleeding esophageal varices, signs of recent AEVH or the presence of blood in the stomach with no other cause of hemorrhage other than esophageal varices. Patients who did not have cirrhosis, had incomplete medical records that could not safely confirm the diagnosis of AEVH, had no varices in the index endoscopy, had hemorrhage caused by gastric varices or had used terlipressin solely for the treatment of hepatorenal syndrome (HRS) were excluded. Then, data was collected in specific forms for each patient, with extensive data on clinical and laboratory variables.
Electronic and physical medical records were gathered for each case and individually assessed. Standardized imaging criteria findings were considered as sufficient for the diagnosis of hepatocellular carcinoma[30]. For the diagnosis of HRS type 1, an evidence-based protocol based on clinical criteria published in 2007 was used[31,32]: Hepatic encephalopathy was graded and diagnosed as per West-Haven criteria[33]. Laboratory data was noted using the units from the hospital laboratory.
Commonly used liver-specific scores were calculated and assessed, using online calculators for standardization. CTP is a classic score, mostly used for prognosticating 1-year mortality for compen
MELD[36] and MELD-Na[37], initially developed to prognosticate 90-day survival for cirrhotic patients, are currently used to assess the need and urgency for liver transplantation.
CLIF-SOFA was developed by the EASL-CLIF group and adapted from the SOFA score, in order to define and classify ACLF[19]. This score defines failure of each organic system, stratifying ACLF into grade 1, 2 and 3:
AD (non-ACLF): No organ failure; or an isolated non-renal organ failure with creatinine < 1.5 mg/dL and absence of encephalopathy; or an isolated neurological failure with creatinine < 1.5 mg/dL.
ACLF grade 1: An isolated kidney failure; or an isolated non-renal and non-neurological organ failure with creatinine ranging between 1.5 and 1.9 mg/dL or mild to moderate hepatic encephalopathy; or an isolated neurological failure with creatinine ranging between 1.5 to 1.9 mg/dL.
ACLF grade 2: Two organ failures.
ACLF grade 3: Three organ failures.
CLIF Consortium (CLIF-C) AD score and CLIF-C ACLF, are a couple of other scores developed by the EASL-CLIF group, intended to predict mortality for 28-day, 90-day, 180-day and 365-day for AD and ACLF patients[38]. These scores were calculated using an online calculator developed by the CLIF Research Group (https://www.clifresearch.com/ToolsCalculators.aspx), which after defining the presence and grade ACLF and calculating CLIF-SOFA, automatically analyzes if CLIF-C AD or ACLF applies and calculates its value for each case.
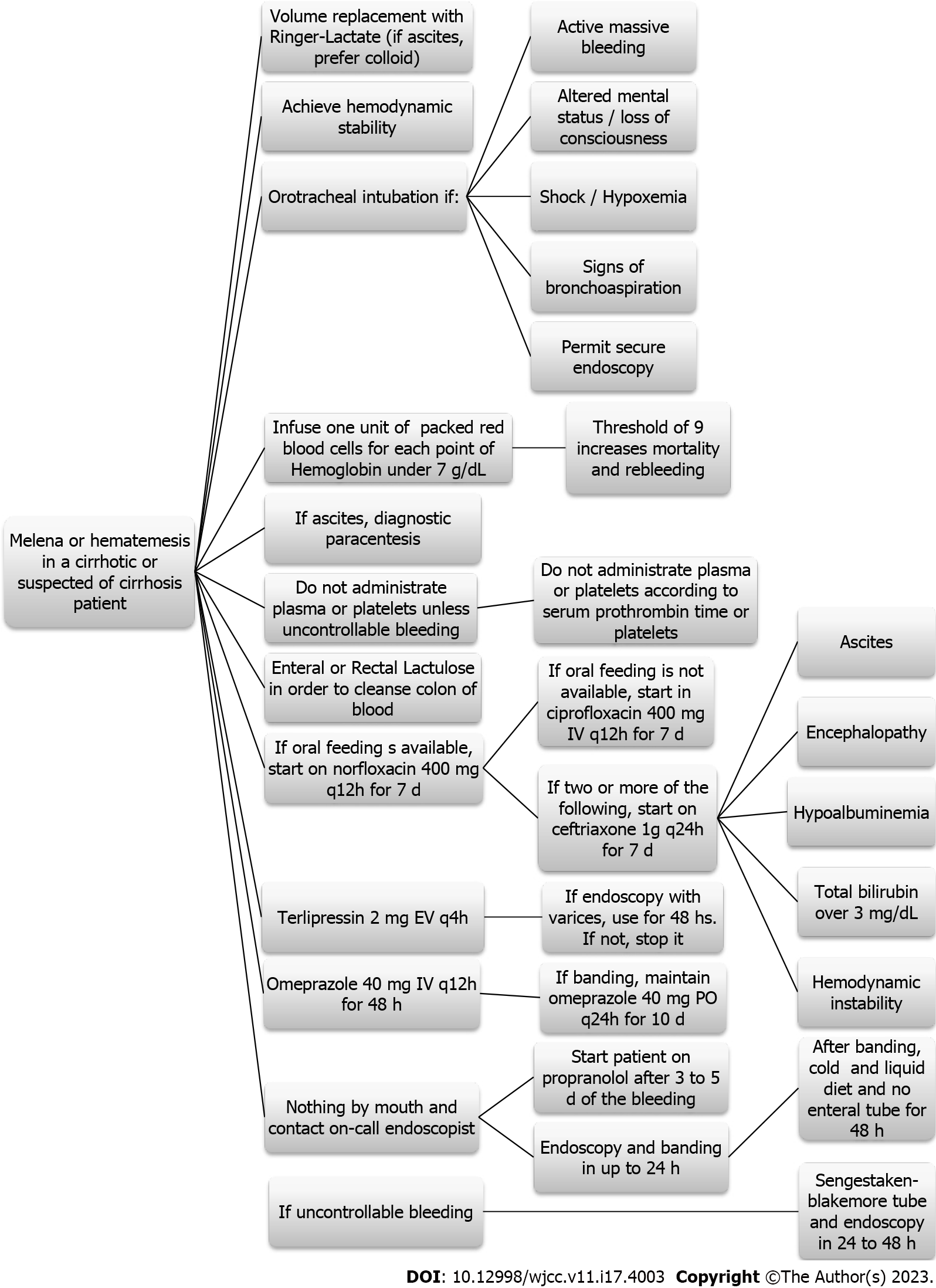
Our hospital follows an evidence-based institutional protocol to manage suspected cases of AEVH (Figure 1). When suspected, the patient starts to use terlipressin 2 mg q4h for 48 h and the on-call endoscopy team is called in action to perform an upper digestive endoscopy with EVB or sclerotherapy within 24 h of the hospital admission.
The choice whether to treat the varices and between EVB or sclerotherapy was done by the on-call endoscopist. Besides, every patient receives prophylactic antibiotics and lactulose. Prophylactic NSBB are started after three to five days of the AEVH resolution.
Death from all causes was primary outcome, gathered via medical records or the search on national death databases (https://www.falecidosnobrasil.org.br/). If the patient was admitted to the hospital with more than one episode of AEVH, data regarding the first episode was collected. Treatment failure was defined as either persistent bleeding or rebleeding in a time frame of 5 days.
Statistical analysis was performed using Statistical Package for the Social Sciences 15.0 (International Business Machines Corporation, Chicago, United States). Frequency was used to describe categorical variables and mean and standard deviation for continuous variables. Univariate analysis was performed by a Cox regression and a multivariate analysis by a stepwise progression to the Cox regression. Every statistical test performed for the analysis excluded missing data. The graphical description of survival was done via Kaplan-Meier curves.
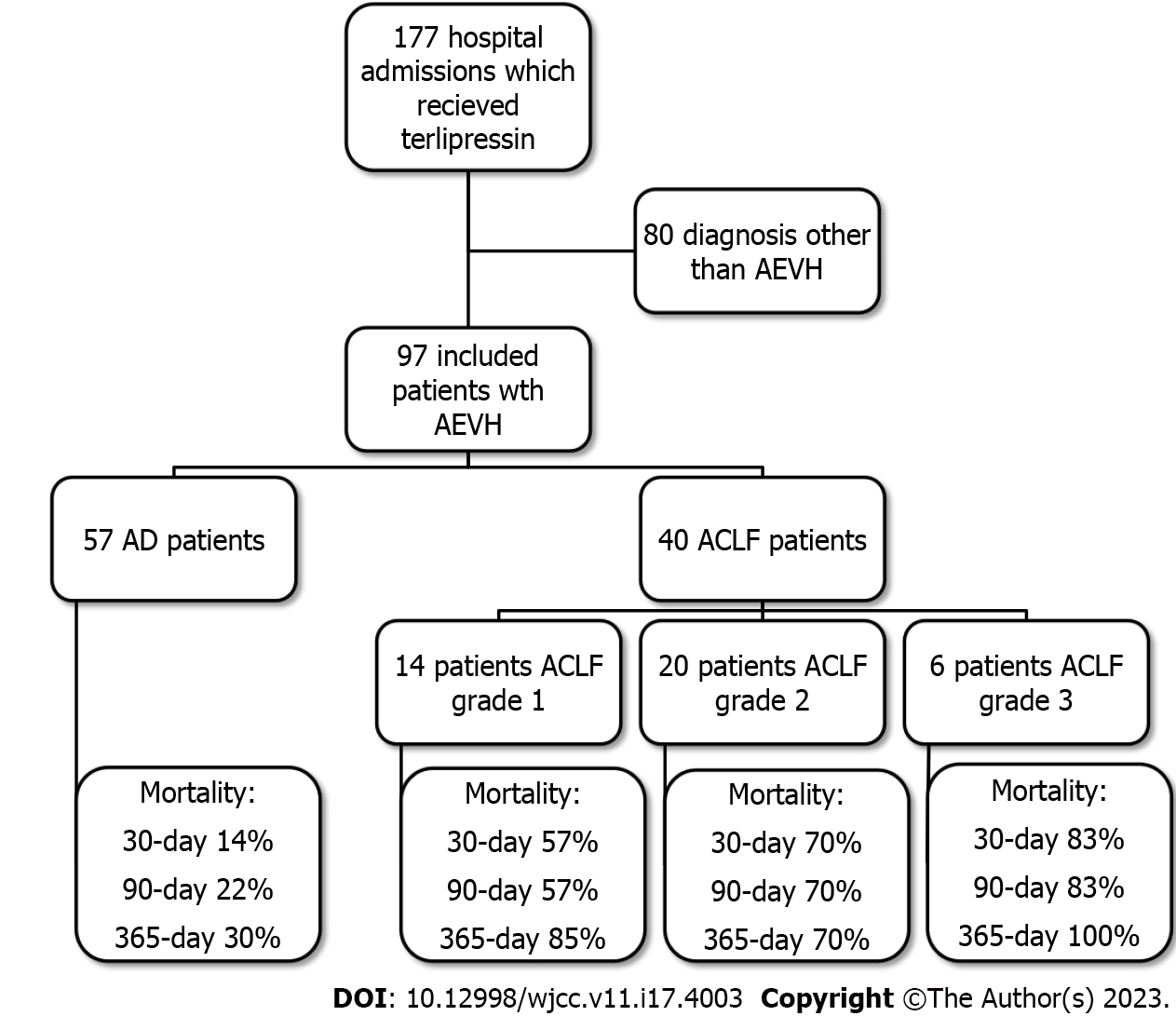
The search in the electronic database of the hospital retrieved 177 hospital admissions of patients who received terlipressin. Of these, 46 were diagnosed with HRS, whereas 16 were admitted because of a suspicion of AEVH not confirmed by index endoscopy, 4 had incomplete records and other 14 cases were re-admissions. Therefore, only 97 hospital admissions were diagnosed as AEVH and included in the study (Figure 2). Demographic, clinical, and laboratory data are described in Table 1 for the study population and stratified by AD or ACLF and its grade. Mean age was 56 years-old and 77.3% of the patients were male. The most common cause of cirrhosis was alcohol abuse (61.8%).
| Variable | Study population (n = 97) | AD (n = 57) | ACLF (n = 40) | ACLF grade 1 (n = 14) | ACLF grade 2 (n = 20) | ACLF grade 3 (n = 6) |
| Age (yr)1 | 56 (9) | 58 (10) | 54 (8.7) | 52 (9.8) | 55 (8.0) | 53 (92.0) |
| Male sex2 | 75 (77.3) | 40 (70.2) | 35 (87.5) | 14 (100) | 17 (85) | 4 (66.6) |
| Etiology of cirrhosis2 | ||||||
| Alcohol | 60 (61.8) | 32 (56.1) | 28 (70) | 10 (71.4) | 14 (70) | 4 (66.6) |
| Hepatitis C | 15 (15.4) | 10 (17.5) | 5 (12.5) | 2 (14.3) | 2 (10) | 1 (16.6) |
| Alcohol and hepatitis C | 13 (13.4) | 9 (15.8) | 4 (10) | 1 (7.1) | 2 (10) | 2 (33.3) |
| Other | 9 (9.2) | 6 (10.5) | 3 (7.5) | 1 (7.1) | 2 (10) | 0 |
| Active alcoholism2 | 45 (46.3) | 24 (42) | 21 (52.5) | 7 (50) | 11 (55) | 3 (50) |
| Previous use of medications2 | ||||||
| PPI | 28 (28.8) | 20 (35.1) | 8 (20) | 4 (28.6) | 3 (15) | 1 (16.6) |
| Spironolactone | 16 (16.4) | 5 (8.8) | 11 (27.5) | 3 (21.4) | 6 (30) | 2 (33.3) |
| Furosemide | 16 (16.4) | 4 (7) | 12 (30) | 4 (28.6) | 6 (30) | 2 (33.3) |
| NSBB | 23 (23.7) | 15 (26.3) | 8 (20) | 3 (21.4) | 5 (25) | 0 |
| Renal replacement therapy2 | 2 (2) | 1 (1.8) | 1 (2.5) | 1 (7.1) | 0 | 0 |
| Portal vein thrombosis2 | 3 (3.1) | 3 (5.3) | 0 | 0 | 0 | 0 |
| Hepatocellular carcinoma2 | 9 (9.2) | 6 (10.5) | 3 (7.5) | 1 (7.1) | 1 (5) | 1 (16.6) |
| Hepatorenal syndrome2 | 19 (19.5) | 3 (5.3) | 16 (40) | 6 (14.9) | 7 (35) | 3 (50) |
| Ascites2 | 47 (48.4) | 31 (54.4) | 26 (65) | 10 (71.4) | 12 (60) | 4 (66.6) |
| Hepatic encephalopathy2 | ||||||
| Absent | 72 (74.3) | 46 (80.7) | 26 (65) | 9 (64.3) | 13 (35) | 4 (66.6) |
| Grade 1 | 6 (6.2) | 2 (3.5) | 4 (10) | 3 (21.4) | 1 (5) | 0 |
| Grade 2 | 7 (7.2) | 3 (5.3) | 4 (10) | 2 (14.3) | 2 (10) | 0 |
| Grade 3 | 8 (8.2) | 4 (7.0) | 4 (10) | 0 | 3 (15) | 1 (16.6) |
| Grade 4 | 4 (4.1) | 2 (3.5) | 2 (5) | 0 | 1 (5) | 1 (16.6) |
| Esophageal varices2 | ||||||
| Small caliber | 14 (14.5) | 12 (21) | 2 (5) | 2 (14.2) | 0 | 0 |
| Medium caliber | 28 (28.8) | 16 (28.1) | 12 (30) | 6 (42.9) | 6 (30) | 0 |
| Large caliber | 55 (56.7) | 29 (50.9) | 26 (65) | 6 (42.9) | 14 (70) | 6 (100) |
| Infection2 | ||||||
| Absent | 67 (69) | 43 (75.4) | 24 (60) | 8 (57.1) | 14 (70) | 2 (33.3) |
| SBP | 5 (5.1) | 3 (5.3) | 2 (5) | 2 (14.3) | 0 | 0 |
| RTI | 9 (9.2) | 6 (10.5) | 3 (7.5) | 1 (7.1) | 1 (5) | 1 (16.6) |
| UTI | 5 (5.1) | 3 (5.3) | 2 (5.0) | 0 | 2 (10) | 0 |
| Sepsis with undefined source of infection | 8 (8.2) | 0 | 8 (20) | 3 (21.4) | 2 (10) | 3 (50) |
| Other | 3 (3.1) | 2 (3.5) | 1 (2.5) | 0 | 1 (5) | 0 |
| Index endoscopy | ||||||
| Red marks2 | 36 (37.1) | 24 (42.1) | 12 (30) | 4 (28.6) | 5 (25) | 3 (50) |
| Rupture point2 | 28 (28.8) | 14 (24.6) | 14 (35) | 6 (42.9) | 7 (35) | 1 (16.6) |
| Active bleeding2 | 12 (12.3) | 5 (8.8) | 7 (17.5) | 3 (21.4) | 3 (15) | 1 (16.6) |
| Variceal banding2 | 40 (41.2) | 21 (36.8) | 19 (47.5) | 10 (71.4) | 7 (35) | 2 (33.3) |
| Esclerotherapy2 | 7 (7.2) | 4 (7) | 3 (7.5) | 0 | 2 (10) | 1 (16.6) |
| Door to endoscopy time (hours)1 | 31.2 (35.9) | 29.4 (35) | 35 (37) | 32 (36) | 39 (43) | 33 (12) |
| Laboratory – in admission1 | ||||||
| Hemoglobin (g/dL) | 8.4 (2.4) | 8.5 (2.6) | 8.4 (2.8) | 9.7 (2.5) | 7.4 (2.0) | 8.9 (4.3) |
| Hematocrit (%) | 26.4 (19.4) | 28.4 (24.9) | 24.4 (7.6) | 26.9 (8.0) | 22.2 (5.3) | 26 (11.7) |
| Leukocyte (/mm³) | 12038 (5565) | 7974 (5211) | 9850 (4026) | 9887 (6202) | 12870 (5695) | 9635 (5069) |
| Platelets (10³/mm³) | 102 (63) | 90 (55) | 115 (72) | 115 (76) | 120 (67) | 98 (88) |
| Total bilirubin (mg/dL) | 3.2 (3.9) | 2.2 (2.0) | 4.7 (5.3) | 2.8 (2.1) | 4.2 (5.0) | 10.0 (7.9) |
| INR | 1.6 (0.6) | 1.3 (0.3) | 1.9 (0.8) | 1.5 (0.3) | 2.1 (0.9) | 2.0 (0.9) |
| AST (U/L) | 174 (303) | 138 (257) | 211 (351) | 141 (136) | 281 (473) | 139 (160) |
| ALT (U/L) | 78 (128) | 70 (136) | 85 (118) | 84 (118) | 99 (136) | 42 (26) |
| GGT (U/L) | 238 (333) | 305 (366) | 161 (284) | 138 (101) | 198 (374) | 86 (68) |
| Creatinine (mg/dL) | 1.3 (1.1) | 0.8 (0.3) | 1.9 (1.4) | 1.3 (0.5) | 2.1 (1.4) | 2.8 (2.3) |
| Sodium (mg/dL) | 136 (14) | 135 (18) | 138 (5) | 137 (4) | 139 (5) | 135 (6) |
| Potassium (mg/dL) | 5.7 (1.7) | 6.7 (1.8) | 4.5 (1.0) | 4.3 (0.8) | 4.7 (1.1) | 4.2 (0.8) |
| Albumin (mg/dL) | 2.6 (0.5) | 2.7 (0.5) | 2.5 (0.5) | 2.4 (0.5) | 2.5 (0.5) | 2.7 (0.6) |
| CRP (mg/L) | 27 (29) | 23 (25) | 31 (32) | 3 (1) | 35 (26) | 47 (63) |
| Blood products1 | ||||||
| Packed red blood cells (units) | 5.4 (5.7) | 6.1 (6.4) | 4.4 (4.5) | 3.8 (3.1) | 4.7 (4.7) | 4.8 (6.8) |
| Packed red blood cells (mL) | 1427 (976) | 1607 (1746) | 1171 (1092) | 1026 (823) | 1212 (983) | 1372 (1936) |
| Fresh frozen plasma (units) | 4.1 (6.2) | 3.4 (5.8) | 5.2 (6.7) | 3.4 (5.3) | 6.2 (7.1) | 6.5 (8.4) |
| Fresh frozen plasma (mL) | 744 (1121) | 626 (1100) | 912 (1114) | 621 (928) | 1069 (1051) | 1066 (1287) |
| Platelets (units) | 2.7 (7.7) | 2 (4.7) | 4 (10.5) | 4.6 (12.8) | 1.6 (3.8) | 9.8 (18.0) |
| Platelets (mL) | 196 (535) | 142 (349) | 273 (721) | 314 (816) | 116 (267) | 703 (1,315) |
| Liver-specific scores1 | ||||||
| CTP | 9 (2) | 8 (2) | 10 (2) | 9 (2) | 9 (2) | 11 (2) |
| MELD | 16 (7) | 12 (5) | 21 (7) | 17 (4) | 23 (6) | 27 (10) |
| MELD-Na | 17 (7) | 13 (5) | 22 (7) | 18 (5) | 24 (6) | 27 (11) |
| CLIF-SOFA | 8.8 (1.5) | 8 (0.9) | 10 (1.5) | 8 (0.9) | 10 (0.8) | 12 (1.3) |
| CLIF-C AD or ACLF | 49 (9.9) | 17 (10.3) | 50 (9.2) | 46 (7) | 52 (10) | 56 (6) |
| Time to death (days)1 | 94 (203) | 144 (243) | 71 (183) | 99 (156) | 66 (234) | 25 (51) |
| Treatment failure1 | 7 (7.2) | 5 (8.8) | 2 (5) | 1 (7.1) | 0 | 1 (16.6) |
| All-cause mortality2 | ||||||
| 30-day | 35 (36) | 8 (14) | 27 (67.5) | 8 (57.1) | 14 (70) | 5 (83.3) |
| 90-day | 39 (40.2) | 12 (21.1) | 27 (67.5) | 8 (57.1) | 14 (70) | 5 (83.3) |
| 365-day | 48 (49.4) | 17 (29.8) | 31 (77.5) | 12 (85) | 14 (70) | 6 (100) |
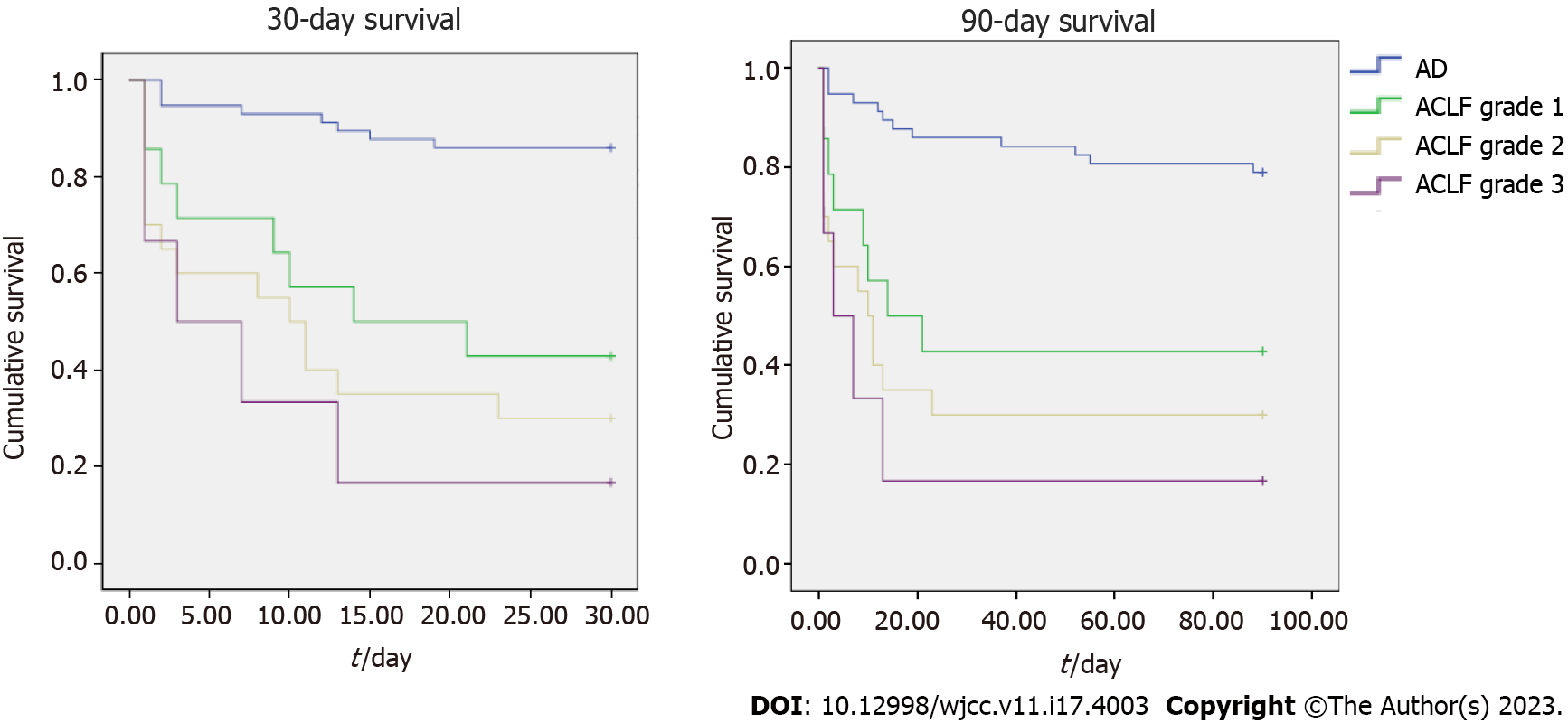
All-cause mortality for AEVH was 36%, 40.2% and 49.4% for 30-, 90- and 365-day, respectively. The prevalence of ACLF was 41.3%. Of these, 35% grade 1, 50% grade 2 and 15% grade 3. All cause-mortality was, respectively, 14%, 21.1% and 29.8% for 30-, 90- and 365-day for AD, 57.1%, 57.1% and 85% for 30-, 90- and 365-day for ACLF grade 1, 70% for 30-, 90- and 365-day for ACLF grade 2 and 83%, 83% and 100% for 30-, 90- and 365-day for ACLF grade 3.
Cox regression univariate analysis was performed. Etiology of cirrhosis, non-use of PPI (proton-pump inhibitor) and NSBB, hepatic encephalopathy, red marks or active bleeding in the index endoscopy, presence of infection, leukocytes > 10000/mm³, total bilirubin > 2 mg/dL, International normalized ratio > 1.3, creatinine > 2 mg/dL, albumin < 3.5 mg/dL, higher use of fresh frozen plasma, CTP, MELD, MELD-Na, CLIF-SOFA, CLIF-C and ACLF scores and ACLF presence and grade were associated with higher 30-day and 90-day mortality (Table 2), using as statistically significant a P value below 0.2 for inclusion in the multivariate analysis. Figure 3 presents a Kaplan-Meier curve for 30- and 90-day survival for AEVH patients according to either AD or ACLF grade.
| Variable | Hazard ratio (95%CI) | |
| 30-day mortality | 90-day mortality | |
| Age (yr)1 | 0.98 (0.95-1.02), P = 0.42 | 0.98 (0.95-1.01), P = 0.36 |
| Male sex | 1.04 (0.47-2.29), P = 0.90 | 1.05 (0.50-2.20), P = 0.88 |
| Etiology of cirrhosis | 0.48 (0.22-1.01), P =0.055 | 0.51 (0.25-1.02), P = 0.06 |
| Active alcoholism | 1.39 (0.73-2.66), P = 0.31 | 1.19 (0.65-2.19), P = 0.55 |
| Previous use of medications | ||
| PPI | 0.44 (0.18-1.07), P = 0.072 | 0.53 (0.24-1.15), P = 0.10 |
| Spironolactone | 1.18 (0.51-2.69), P = 0.69 | 1.22 (0.56-2.64), P = 0.60 |
| Furosemide | 1.21 (0.53-2.76), P = 0.64 | 1.05 (0.46-2.37), P = 0.89 |
| NSBB | 0.33 (0.12-0.95), P = 0.04 | 0.45 (0.19-1.07), P = 0.07 |
| Renal replacement therapy | 1.21 (0.16-8.87), P = 0.84 | 1.10 (0.15-8.03), P = 0.92 |
| Portal vein thrombosis | 0.04 (0.001-39.9), P = 0.37 | 0.99 (0.24-4.11), P = 0.99 |
| Hepatocellular carcinoma | 1.15 (0.41-3.27), P = 0.78 | 1.29 (0.50-3.29), P = 0.59 |
| Hepatic encephalopathy | 2.03 (1.04-3.96), P = 0.03 | 1.73 (0.91-3.30), P = 0.09 |
| Treatment failure | 1.05 (0.24-4.51), P = 0.94 | 0.84 (0.19-3.55), P = 0.81 |
| Index endoscopy | ||
| Red marks | 0.44 (0.17-1.15), P = 0.09 | 0.39 (0.16-0.94), P = 0.03 |
| Rupture point | 1.75 (0.74-4.12), P = 0.20 | 1.68 (0.78-3.65), P = 0.18 |
| Active bleeding | 2.65 (1.02-6.84), P = 0.043 | 2.08 (0.83-5.19), P = 0.11 |
| Variceal banding or sclerotherapy | 0.28 (0.09-0.84), P = 0.02 | 0.43 (0.18-1.03), P = 0.06 |
| Esophageal varices caliber | 1.14 (0.48-2.70), P = 0.75 | 0.93 (0.43-2.04), P = 0.87 |
| Infection | ||
| SBP | 0.97 (0.13-7.26), P = 0.98 | 1.19 (0.168.78), P = 0.86 |
| RTI | 0.01 (0.001-1.70), P = 0.97 | 0.01 (0.001-1.30), P = 0.97 |
| UTI | 1.64 (0.19-14.04), P = 0.65 | 1.72 (0.20-14.80), P = 0.61 |
| Sepsis with undefined source of infection | 0.55 (0.03-8.89), P = 0.67 | 0.54 (0.03-8.74), P = 0.67 |
| Other | 6.49 (0.79-85.86), P = 0.08 | 7.62 (0.93-62.41), P = 0.05 |
| Laboratory | ||
| Hemoglobin < 9 g/dL | 1.25 (0.65-2.39), P = 0.49 | 1.23 (0.67-2.26), P = 0.50 |
| Leukocyte > 10000/mm3 | 0.50 (0.26-0.97), P = 0.04 | 0.50 (0.27-0.93), P = 0.03 |
| Total bilirubin > 2 mg/dL | 0.37 (0.18-0.75), P = 0.005 | 0.40 (0.21-0.78), P = 0.008 |
| INR > 1.31 | 0.31 (0.13-0.72), P = 0.006 | 0.35 (0.21-0.78), P = 0.006 |
| AST > 40 U/L | 0.66 (0.27-1.60), P = 0.35 | 0.59 (0.24-1.42), P = 0.24 |
| ALT > 40 U/L | 67 (0.34-1.34), P = 0.26 | 0.72 (0.37-1.39), P = 0.33 |
| GGT > 60 U/L | 1.04 (0.43-2.49), P = 0.91 | 0.99 (0.44-2.23), P = 0.99 |
| Creatinine > 2 mg/dL | 0.50 (0.25-0.98), P = 0.046 | 0.52 (0.27-1.00), P = 0.05 |
| Sodium > 135 mg/dL | 0.64 (0.30-1.33), P = 0.23 | 0.51 (0.26-1.00), P = 0.052 |
| Potassium > 3.5 mg/dL | 1.61 (0.38-6.7), P = 0.50 | 1.88 (0.45-7.82), P = 0.38 |
| Albumin > 3.5 mg/dL | 0.40 (0.16-0.99), P = 0.048 | 0.39 (0.17-0.89), P = 0.02 |
| Terlipressin – total dose (mg) | 1.04 (0.94-1.15), P = 0.43 | 1.05 (0.95-1.15), P = 0.30 |
| Blood products (used versus not used) | ||
| Packed red blood cells | 0.61 (0.28-1.3), P = 0.22 | 0.61 (0.29-1.29), P = 0.20 |
| Fresh frozen plasma | 2.9 (1.33-6.39), P = 0.007 | 3.07 (1.46-6.44), P = 0.003 |
| Platelets | 0.94 (0.43-2.06), P = 0.88 | 0.93 (0.44-1.96), P = 0.86 |
| Liver-specific scores1 | ||
| CTP | 1.35 (1.17-1.56), P < 0.001 | 1.35 (1.18-1.55), P < 0.001 |
| MELD | 1.10 (1.06-1.15), P < 0.001 | 1.11 (1.07-1.15), P < 0.001 |
| MELD-Na | 1.10 (1.06-1.15), P < 0.001 | 1.11 (1.07-1.15), P < 0.001 |
| CLIF-SOFA | 1.51 (1.29-1.76), P < 0.001 | 1.49 (1.27-1.73), P < 0.001 |
| CLIF-C AD or ACLF | 1.04 (1.01-1.07), P = 0.007 | 1.03 (1.004-1.06), P = 0.02 |
| ACLF2 | 1.13 (1.60-3.0), P < 0.001 | 1.19 (1.09-1.39), P < 0.001 |
Each one of these variables was used for the multivariate analysis using Cox regression. Using the stepwise approach, the model was reduced until every variable had a level of independent significance of P ≤ 0.1. Previous use of NSBB was protective for 30- and 90-day mortality. MELD score and ACLF grade were associated with higher 30- and 90- day mortality. CTP score was only predictive of 90-day mortality (Table 3).
AEVH is one of the most common causes for decompensation of cirrhotic patients. Treatment has advanced greatly in the past few decades, but AEVH still carries a mortality of 10% to 20% in six weeks[39]. This study has sought to determine whether the presence of ACLF according to EASL-CLIF criteria might be useful for prognostication of AEVH.
The concept that there is an additional step between AD and death has been in the making for a long time. The initial concept was that ACLF would take place after a triggering event, precipitating multi-organ failure and eventually death. Possible events were infection, AEVH, drug or herb-induced liver injury, alcoholic hepatitis or acute viral hepatitis[12-16]. The introduction of well-researched clinical criteria would come only in 2013 by the CANONIC study. In this study, the EASL-CLIF group developed and validated the CLIF-SOFA score and definitions of ACLF, analyzing their role in prognosticating end-stage liver disease (ESLD)[19].
For patients with ACLF and AEVH, 30-day mortality was 67.5%, considerably higher than the 33.9% described in the CANONIC study[19] and the 39% described in other studies[40-42]. For patients with ACLF diagnosed with Spontaneous Bacterial peritonitis, a 28-day-mortality of 65% has been described[43,44], while a 30-day mortality of 67.3% has been described for HRS patients[32,45].
Prognosticating AEVH is challenging. There are many scores developed to stratify patients with non-variceal acute upper digestive hemorrhage such as the Rockall score, Glasgow Blatchford Score, AIMS65 score, Almela score, score, Baylor Bleeding score and Cedars-Sinai Medical Center predictive index[46-50]. Nevertheless, it is paramount to remember that these scores were not developed to prognosticate AEVH. Therefore, it has been demonstrated that such scores are more accurate to prognosticate non-variceal acute upper digestive hemorrhage than cirrhotic patients with AEVH[20,21,51,52].
It comes to no surprise, therefore, that scores that predict mortality using liver function tests in cirrhotic patients are superior to prognosticate AEVH. This has been demonstrated in a study that found that CLIF-SOFA and MELD scores have better predict hospital mortality and post-EVB 6-week mortality for AEVH patients than other generally used scores[22,23,24,53]. In such a complex scenario, artificial intelligence might just outperform every commonly used score[54,55].
Once used to allocate organs for liver transplantation (LT), CTP is a score known by heart by every hepatologist, and helps allocate resources and adjust follow-up for both inpatients and outpatients. It is by large the most researched score, and is extremely important to prognosticate outpatients[34,35], intensive care patients[56] and hepatorenal syndrome patients[45,57]. In the present study, higher CTP has been independently associated with higher 90-day mortality.
Currently used for LT organ allocation[58], MELD and MELD-Na scores are useful for predicting 90-day mortality for ESLD patients[36,37]. This has been true even for AEVH patients[22], where they have been shown to be similar to CTP score for prognosticating AEVH[59,60]. In the present study, higher MELD has been independently associated with higher 30- and 90-day mortality.
EASL-CLIF consortium developed the CLIF-SOFA for the CANONIC study[19], a recent score superior to other liver-specific scores in prognosticating ACLF patients[61-65], for both acute decompensations and chronic liver injuries[19,65-69]. Although, in the present study, CLIF-SOFA score was not independently associated with higher mortality.
The use of NSBB (either carvedilol or propranolol) is a mainstay of AEVH primary and secondary prophylaxis[28,29]. In this study, the previous use of NSBB was independently associated with lower 30- and 90-day mortality for all patients. This corroborates the findings of a previous randomized controlled trial, where the use of carvedilol reduced mortality for ACLF patients[70]. Since a cohort has shown propranolol and carvedilol to be equivalent in clinical outcomes, it is expected that both NSBB are effective in reducing mortality[71].
Transjugular intrahepatic portosystemic shunt (TIPS) has been more recently recommended for refractory and severe AEVH[72,73]. In our setting, this is unavailable and might have impacted our results regarding mortality. Although in the studied hospital, protocol mandates the use of prophylactic antibiotics, there was still a high rate of infection. Nevertheless, in a recent study, almost one-fifth of patients with AEVH developed bacterial infections despite antibiotic prophylaxis[74]. This rate was not so different from the presented population (24.6% for AD and 40% for ACLF patients). Another factor that might contribute for decompensation and AEVH is portal vein thrombosis[75]. This condition did not impact our results, as it was rather uncommon in the presented population.
ACLF has been associated with systemic inflammation, which might cause impairment of the functions of the major organ systems and might act synergistically with the traditional mechanisms involved in the development of AD and ACLF, impairing organ system functions[76]. In the present study, the presence and grade of ACLF in AEVH patients were associated with higher 30- and 90-day mortality. This has been demonstrated in a few previous retrospective cohorts[73,77-81]. A recent systematic review investigated the ability of the CLIF-SOFA, CLIF-C ACLF, and CLIF-C AD scores for prognosticating acute-on-chronic liver failure and acute decompensation of cirrhosis and it was found that these scores are accurate as short-term and long-term mortality prognosticating scores, with CLIF-SOFA being the most effective in predicting mortality in ACLF patients, especially in the short-term[82].
The major limitation of our study is the small sample size. Nevertheless, most of the studies that analyzed ACLF were multi-centric, gathering large data banks. Our institution does not have TIPS or liver transplantation facilities, and patients with AEVH require transfer to another city, which is challenging. Moreover, this study is a historical cohort, without sufficient numbers of patients to stratify them accurately, and hence adequate results may not be obtained. The present study provides a thorough analysis of the data, shedding light on the role of ACLF definitions in predicting AEVH prognosis. What sets our paper apart is its novel finding of a significant association between ACLF grade and mortality in AEVH patients.
In conclusion, the use of NSBB was protective for mortality associated with AEVH, while MELD and CTP scores and the presence and grading of ACLF according to the EASL-CLIF criteria was independently associated with higher 30- and 90-day mortality in cirrhotic patients admitted due to AEVH. A large prospective cohort study on AEVH and ACLF would be beneficial to better understand the association between the two. It is becoming paramount in this era to develop more accurate tools for predicting outcomes and optimizing medical therapy in these patients.
Acute-on-chronic liver failure (ACLF) is a severe syndrome affecting patients with liver disease, characterized by decompensation, organ failure, and high mortality rates. ACLF diagnosis is based on clinical criteria, while treatment options remain limited, underscoring the need for predictive tools and targeted therapies to improve outcomes. This study aimed to identify prognostic factors and therapeutic targets associated with ACLF and acute variceal hemorrhage (AEVH) to improve patient management. The mechanisms of ACLF remain unclear, highlighting the importance of further research in this area.
The study on ACLF and AEVH aims to identify predictors of poor outcomes in AEVH and the development of ACLF, which has gained significant attention due to poor prognosis and limited treatment options. Understanding the pathophysiology and clinical implications of ACLF remains crucial, and identifying risk factors for its development could enhance our knowledge of this condition and inform future therapies. The study thus has important clinical implications for managing patients with liver disease.
The main objective of the study on ACLF and AEVH was to identify risk factors for mortality and to evaluate the role of non-selective beta-blockers (NSBB) in improving patient outcomes.
The study analyzed data of cirrhosis patients who received terlipressin for AEVH from 2010 to 2016. Patients with incomplete medical records or without cirrhosis were excluded. Extensive clinical and laboratory data was collected for each patient, and liver-specific scores were calculated. AEVH therapy involved terlipressin, prophylactic antibiotics, and lactulose. Outcomes were recorded as all-cause deaths, collected from medical records or national death databases.
This study provides insights into the prognosis of AEVH and ACLF. The study found that the all-cause mortality rate for AEVH ranged from 36% to 49.4% depending on the time point, while the prevalence of ACLF was 41.3%, with a higher mortality rate ranging from 57.1% to 100%. Various factors were associated with higher mortality rates, including etiology of cirrhosis, laboratory abnormalities, and scoring systems. The study emphasizes the importance of early identification and treatment of AEVH and ACLF, with previous use of NSBB being protective and MELD score and ACLF grade associated with higher mortality rates.
The study aimed to assess the utility of ACLF criteria for predicting AEVH prognosis. Patients with ACLF and AEVH had a high 30-day mortality rate of 67.5%. CLIF-SOFA and MELD scores were better predictors of hospital mortality and 6-week post-EVB mortality than other scores. Higher CTP and MELD scores were associated with higher 90-day and 30- and 90-day mortality rates, respectively. The previous use of NSBB was associated with lower 30- and 90-day mortality.
Future research on AEVH and ACLF should investigate long-term outcomes, including factors associated with better outcomes and novel therapeutic approaches. Developing new biomarkers for early detection and diagnosis is necessary, as current methods are limited in accuracy and specificity. Further research is needed to understand the underlying mechanisms of these conditions, particularly inflammation and immune dysregulation. Randomized controlled trials evaluating the efficacy and safety of various treatments, including pharmacological and non-pharmacological approaches, are necessary to manage AEVH and ACLF and improve patient outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Sociedade Brasileira de Hepatologia; Federação Brasileira De Gastroenterologia.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Garbuzenko DV, Russia; Paparoupa M, Germany S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7:141-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 2. | Gracia-Sancho J, Maeso-Díaz R, Bosch J. Pathophysiology and a Rational Basis of Therapy. Dig Dis. 2015;33:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121-S131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 405] [Article Influence: 21.3] [Reference Citation Analysis (14)] |
| 4. | Boregowda U, Umapathy C, Halim N, Desai M, Nanjappa A, Arekapudi S, Theethira T, Wong H, Roytman M, Saligram S. Update on the management of gastrointestinal varices. World J Gastrointest Pharmacol Ther. 2019;10:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (12)] |
| 5. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 6. | Bosch J, Pizcueta P, Feu F, Fernández M, García-Pagán JC. Pathophysiology of portal hypertension. Gastroenterol Clin North Am. 1992;21:1-14. [PubMed] |
| 7. | Sharara AI, Rockey DC. Gastroesophageal variceal hemorrhage. N Engl J Med. 2001;345:669-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 229] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 640] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 9. | Habib A, Sanyal AJ. Acute variceal hemorrhage. Gastrointestinal endoscopy clinics of North America. Gastrointest Endosc Clin N Am. 2007;17:223-252. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Garcia-Tsao G, Bosch J. Varices and Variceal Hemorrhage in Cirrhosis: A New View of an Old Problem. Clin Gastroenterol Hepatol. 2015;13:2109-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 35.2] [Reference Citation Analysis (1)] |
| 12. | Jalan R. Acute-on-chronic liver failure: from concept to a new syndrome. Curr Opin Crit Care. 2011;17:152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Olson JC, Kamath PS. Acute-on-chronic liver failure: concept, natural history, and prognosis. Curr Opin Crit Care. 2011;17:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Mookerjee RP. Acute-on-chronic liver failure: the liver and portal haemodynamics. Curr Opin Crit Care. 2011;17:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | García-Martínez R, Córdoba J. Acute-on-chronic liver failure: the brain. Curr Opin Crit Care. 2011;17:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Cárdenas A, Ginès P. Acute-on-chronic liver failure: the kidneys. Curr Opin Crit Care. 2011;17:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Liu H, Lee SS. Acute-on-chronic liver failure: the heart and systemic hemodynamics. Curr Opin Crit Care. 2011;17:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Hassanein TI, Schade RR, Hepburn IS. Acute-on-chronic liver failure: extracorporeal liver assist devices. Curr Opin Crit Care. 2011;17:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2171] [Article Influence: 180.9] [Reference Citation Analysis (5)] |
| 20. | Budimir I, Gradišer M, Nikolić M, Baršić N, Ljubičić N, Kralj D, Budimir I Jr. Glasgow Blatchford, pre-endoscopic Rockall and AIMS65 scores show no difference in predicting rebleeding rate and mortality in variceal bleeding. Scand J Gastroenterol. 2016;51:1375-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Thanapirom K, Ridtitid W, Rerknimitr R, Thungsuk R, Noophun P, Wongjitrat C, Luangjaru S, Vedkijkul P, Lertkupinit C, Poonsab S, Ratanachu-ek T, Hansomburana P, Pornthisarn B, Thongbai T, Mahachai V, Treeprasertsuk S. Prospective comparison of three risk scoring systems in non-variceal and variceal upper gastrointestinal bleeding. J Gastroenterol Hepatol. 2016;31:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Al-Freah MA, Gera A, Martini S, McPhail MJ, Devlin J, Harrison PM, Shawcross D, Abeles RD, Taylor NJ, Auzinger G, Bernal W, Heneghan MA, Wendon JA. Comparison of scoring systems and outcome of patients admitted to a liver intensive care unit of a tertiary referral centre with severe variceal bleeding. Aliment Pharmacol Ther. 2014;39:1286-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Wong MW, Chen MJ, Chen HL, Kuo YC, Lin IT, Wu CH, Lee YK, Cheng CH, Bair MJ. Application of chronic liver failure-sequential organ failure assessment score for the predication of mortality after esophageal variceal hemorrhage post endoscopic ligation. PLoS One. 2017;12:e0182529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Terres AZ, Balbinot RS, Muscope ALF, Eberhardt LZ, Balensiefer JIL, Cini BT, Luis Rost G Jr, Longen ML, Schena B, Balbinot RA, Balbinot SS, Soldera J. Predicting mortality for cirrhotic patients with acute oesophageal variceal haemorrhage using liver-specific scores. GastroHep. 2021;3:236-246. [DOI] [Full Text] |
| 25. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2294] [Article Influence: 229.4] [Reference Citation Analysis (3)] |
| 26. | Dai C, Liu WX, Jiang M, Sun MJ. Endoscopic variceal ligation compared with endoscopic injection sclerotherapy for treatment of esophageal variceal hemorrhage: a meta-analysis. World J Gastroenterol. 2015;21:2534-2541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 27. | Sridharan K, Sivaramakrishnan G. Vasoactive Agents for the Management of Variceal Bleeding: A Mixed Treatment Comparison Network Meta-analysis and Trial Sequential Analysis of Randomized Clinical Trials. Drug Res (Stuttg). 2019;69:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1441] [Article Influence: 180.1] [Reference Citation Analysis (3)] |
| 29. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1499] [Article Influence: 499.7] [Reference Citation Analysis (2)] |
| 30. | Soldera J, Balbinot SS, Balbinot RA, Cavalcanti AG. Diagnostic and Therapeutic Approaches to Hepatocellular Carcinoma: Understanding the Barcelona Clínic Liver Cancer Protocol. Clin Med Insights Gastroenterol. 2016;9:67-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 356] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 32. | Terres AZ, Balbinot RS, Muscope ALF, Longen ML, Schena B, Cini BT, Luis Rost G Jr, Balensiefer JIL, Eberhardt LZ, Balbinot RA, Balbinot SS, Soldera J. Evidence-based protocol for diagnosis and treatment of hepatorenal syndrome is independently associated with lower mortality. Gastroenterol Hepatol. 2022;45:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1408] [Article Influence: 128.0] [Reference Citation Analysis (1)] |
| 34. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 35. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5736] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 36. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3678] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 37. | Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1057] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 38. | Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 441] [Article Influence: 55.1] [Reference Citation Analysis (1)] |
| 39. | Bittencourt PL, Strauss E, Farias AQ, Mattos AA, Lopes EP. Variceal bleeding: update of recommendations from the Brazilian association of hepatology. Arq Gastroenterol. 2017;54:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Silva PE, Fayad L, Lazzarotto C, Ronsoni MF, Bazzo ML, Colombo BS, Dantas-Correa EB, Narciso-Schiavon JL, Schiavon LL. Single-centre validation of the EASL-CLIF consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int. 2015;35:1516-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Grochot RM, Luz LB, Garcia R, Balbinot RA, Balbinot SS, Soldera J. CLIF-SOFA is superior to other liver-specific scores for predicting mortality in acute-on-chronic liver failure and decompensated cirrhosis. Austin J Gastroenterol. 2019;6:1105. |
| 42. | Grochot RM, Luz LB, Garcia R, Balbinot RÂ, Balbinot SS, Soldera J. Acute–on–chronic liver failure data from a teaching hospital in Brazil. A Historical Cohort. Inter J Sci Res. 2020;9:1-6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 43. | Jacques ROC, Massignan LDS, Winkler MS, Balbinot RS, Balbinot SS, Soldera J. Acute-on-chronic liver failure is independently associated with lower survival in patients with spontaneous bacterial peritonitis. Arq Gastroenterol. 2021;58:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 44. | Jacques ROC, Massignan LS, Winkler MS, Balbinot RS, Balbinot SS, Soldera J. Liver-specific scores as predictors of mortality in spontaneous bacterial peritonitis. GastroHep. 2020;2:224-231. [DOI] [Full Text] |
| 45. | Terres AZ, Balbinot RS, Muscope ALF, Longen ML, Schena B, Cini BT, Rost Jr GL, Leichtweis Balensiefer JI, Eberhardt LZ, Balbinot RA, Balbinot SS, Soldera J. Predicting mortality for hepatorenal syndrome with liver-specific scores. GastroHep. 2020;2:336-343. [DOI] [Full Text] |
| 46. | Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 684] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 47. | Hyett BH, Abougergi MS, Charpentier JP, Kumar NL, Brozovic S, Claggett BL, Travis AC, Saltzman JR. The AIMS65 score compared with the Glasgow-Blatchford score in predicting outcomes in upper GI bleeding. Gastrointest Endosc. 2013;77:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 48. | Kuipers EJ. Endoscopy: Risk assessment in upper gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2010;7:480-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 49. | Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 896] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 50. | Almela P, Benages A, Peiró S, Añón R, Pérez MM, Peña A, Pascual I, Mora F. A risk score system for identification of patients with upper-GI bleeding suitable for outpatient management. Gastrointest Endosc. 2004;59:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Chandnani S, Rathi P, Udgirkar SS, Sonthalia N, Contractor Q, Jain S. Clinical utility of risk scores in variceal bleeding. Arq Gastroenterol. 2019;56:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Aluizio CLS, Montes CG, Reis GFSR, Nagasako CK. Risk stratification in acute variceal bleeding: Far from an ideal score. Clinics (Sao Paulo). 2021;76:e2921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Karvellas CJ, Bagshaw SM. Advances in management and prognostication in critically ill cirrhotic patients. Curr Opin Crit Care. 2014;20:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Simsek C, Sahin H, Emir Tekin I, Koray Sahin T, Yasemin Balaban H, Sivri B. Artificial intelligence to predict overall survivals of patients with cirrhosis and outcomes of variceal bleeding. Hepatol Forum. 2021;2:55-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 55. | Soldera J, Tomé F, Corso LL, Rech MM, Ferrazza AD, Terres AZ, Cini BT, Eberhardt LZ, Longen JL, Schena B, Rost Jr GL, Furlan RG, Balbinot RA, Balbinot SS. Use of a machine learning algorithm to predict rebleeding and mortality for oesophageal variceal bleeding in cirrhotic patients. EMJ Gastroenterol. 2020;9:46-48. |
| 56. | Weil D, Levesque E, McPhail M, Cavallazzi R, Theocharidou E, Cholongitas E, Galbois A, Pan HC, Karvellas CJ, Sauneuf B, Robert R, Fichet J, Piton G, Thevenot T, Capellier G, Di Martino V; METAREACIR Group. Prognosis of cirrhotic patients admitted to intensive care unit: a meta-analysis. Ann Intensive Care. 2017;7:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 57. | Licata A, Maida M, Bonaccorso A, Macaluso FS, Cappello M, Craxì A, Almasio PL. Clinical course and prognostic factors of hepatorenal syndrome: A retrospective single-center cohort study. World J Hepatol. 2013;5:685-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Thapar M, Bonkovsky HL. Indications for liver transplant and AASLD guidelines. Hepatology. 2015;61:408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Peng Y, Qi X, Dai J, Li H, Guo X. Child-Pugh versus MELD score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis. Int J Clin Exp Med. 2015;8:751-757. [PubMed] |
| 60. | Krige J, Spence RT, Jonas E, Hoogerboord M, Ellsmere J. A New Recalibrated Four-Category Child-Pugh Score Performs Better than the Original Child-Pugh and MELD Scores in Predicting In-Hospital Mortality in Decompensated Alcoholic Cirrhotic Patients with Acute Variceal Bleeding: a Real-World Cohort Analysis. World J Surg. 2020;44:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | Kim HY, Kim CW, Kim TY, Song DS, Sinn DH, Yoon EL, Jung YK, Suk KT, Lee SS, Lee CH, Kim TH, Kim JH, Yim HJ, Kim SE, Baik SK, Lee BS, Jang JY, Kim YS, Kim SG, Yang JM, Sohn JH, Lee HJ, Park SH, Choi EH, Kim DJ; Korean Acute-on-Chronic Liver Failure Study Group. Assessment of scoring systems for acute-on-chronic liver failure at predicting short-term mortality in patients with alcoholic hepatitis. World J Gastroenterol. 2016;22:9205-9213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Maipang K, Potranun P, Chainuvati S, Nimanong S, Chotiyaputta W, Tanwandee T, Charatcharoenwitthaya P. Validation of the prognostic models in acute-on-chronic liver failure precipitated by hepatic and extrahepatic insults. PLoS One. 2019;14:e0219516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Lee M, Lee JH, Oh S, Jang Y, Lee W, Lee HJ, Yoo JJ, Choi WM, Cho YY, Cho Y, Lee DH, Lee YB, Yu SJ, Yi NJ, Lee KW, Kim YJ, Yoon JH, Suh KS, Lee HS. CLIF-SOFA scoring system accurately predicts short-term mortality in acutely decompensated patients with alcoholic cirrhosis: a retrospective analysis. Liver Int. 2015;35:46-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 64. | Kulkarni S, Sharma M, Rao PN, Gupta R, Reddy DN. Acute on Chronic Liver Failure-In-Hospital Predictors of Mortality in ICU. J Clin Exp Hepatol. 2018;8:144-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | Sy E, Ronco JJ, Searle R, Karvellas CJ. Prognostication of critically ill patients with acute-on-chronic liver failure using the Chronic Liver Failure-Sequential Organ Failure Assessment: A Canadian retrospective study. J Crit Care. 2016;36:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 66. | Zou D, Qi X, Zhu C, Ning Z, Hou F, Zhao J, Peng Y, Li J, Deng H, Guo X. Albumin-bilirubin score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis: A retrospective study. Turk J Gastroenterol. 2016;27:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 67. | Peng Y, Qi X, Tang S, Deng H, Li J, Ning Z, Dai J, Hou F, Zhao J, Wang R, Guo X. Child-Pugh, MELD, and ALBI scores for predicting the in-hospital mortality in cirrhotic patients with acute-on-chronic liver failure. Expert Rev Gastroenterol Hepatol. 2016;10:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 68. | Shi Y, Yang Y, Hu Y, Wu W, Yang Q, Zheng M, Zhang S, Xu Z, Wu Y, Yan H, Chen Z. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. 2015;62:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 243] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 69. | Zhang Q, Li Y, Han T, Nie C, Cai J, Liu H, Liu Y. Comparison of current diagnostic criteria for acute-on-chronic liver failure. PLoS One. 2015;10:e0122158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Kumar M, Kainth S, Choudhury A, Maiwall R, Mitra LG, Saluja V, Agarwal PM, Shasthry SM, Jindal A, Bhardwaj A, Kumar G, Sarin SK. Treatment with carvedilol improves survival of patients with acute-on-chronic liver failure: a randomized controlled trial. Hepatol Int. 2019;13:800-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Sharma S, Agarwal S, Gunjan D, Kaushal K, Anand A, Mohta S, Shalimar, Saraya A. Long-term Outcomes with Carvedilol versus Propranolol in Patients with Index Variceal Bleed: 6-year Follow-up Study. J Clin Exp Hepatol. 2021;11:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Walter A, Rudler M, Olivas P, Moga L, Trépo E, Robic MA, Ollivier-Hourmand I, Baiges A, Sutter O, Bouzbib C, Peron JM, Le Pennec V, Ganne-Carrié N, Garcia-Pagán JC, Mallet M, Larrue H, Dao T, Thabut D, Hernández-Gea V, Nault JC, Bureau C, Allaire M; Salvage TIPS Group. Combination of Model for End-Stage Liver Disease and Lactate Predicts Death in Patients Treated With Salvage Transjugular Intrahepatic Portosystemic Shunt for Refractory Variceal Bleeding. Hepatology. 2021;74:2085-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 73. | Trebicka J, Gu W, Ibáñez-Samaniego L, Hernández-Gea V, Pitarch C, Garcia E, Procopet B, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Silva-Junior G, Martinez J, Genescà J, Bureau C, Llop E, Laleman W, Palazon JM, Castellote J, Rodrigues S, Gluud L, Ferreira CN, Barcelo R, Cañete N, Rodríguez M, Ferlitsch A, Mundi JL, Gronbaek H, Hernández-Guerra M, Sassatelli R, Dell'Era A, Senzolo M, Abraldes JG, Romero-Gómez M, Zipprich A, Casas M, Masnou H, Primignani M, Weiss E, Catalina MV, Erasmus HP, Uschner FE, Schulz M, Brol MJ, Praktiknjo M, Chang J, Krag A, Nevens F, Calleja JL, Robic MA, Conejo I, Albillos A, Rudler M, Alvarado E, Guardascione MA, Tantau M, Bosch J, Torres F, Pavesi M, Garcia-Pagán JC, Jansen C, Bañares R; International Variceal Bleeding Observational Study Group and Baveno Cooperation. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol. 2020;73:1082-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 74. | Martínez J, Hernández-Gea V, Rodríguez-de-Santiago E, Téllez L, Procopet B, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Ibañez-Samaniego L, Silva-Junior G, Genescà J, Bureau C, Trebicka J, Bañares R, Krag A, Llop E, Laleman W, Palazon JM, Castellote J, Rodrigues S, Gluud LL, Noronha-Ferreira C, Cañete N, Rodríguez M, Ferlitsch A, Schwarzer R, Mundi JL, Gronbaek H, Hernández-Guerra M, Sassatelli R, Dell'Era A, Senzolo M, Abraldes JG, Romero-Gomez M, Zipprich A, Casas M, Masnou H, Primignani M, Nevens F, Calleja JL, Jansen C, Robic MA, Conejo I, Catalina MV, Rudler M, Alvarado E, Perez-Campuzano V, Guardascione MA, Fischer P, Bosch J, García-Pagán JC, Albillos A; International Variceal Bleeding Observational Study Group and Baveno Cooperation. Bacterial infections in patients with acute variceal bleeding in the era of antibiotic prophylaxis. J Hepatol. 2021;75:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 75. | Zhang Y, Xu BY, Wang XB, Zheng X, Huang Y, Chen J, Meng ZJ, Gao YH, Qian ZP, Liu F, Lu XB, Shi Y, Shang J, Li H, Wang SY, Yin S, Sun SN, Hou YX, Xiong Y, Li BL, Lei Q, Gao N, Ji LJ, Li J, Jie FR, Zhao RH, Liu JP, Lin TF, Chen LY, Tan WT, Zhang Q, Zou CC, Huang ZB, Jiang XH, Luo S, Liu CY, Zhang YY, Li T, Ren HT, Wang SJ, Deng GH, Xiong SE, Liu XX, Wang C, Yuan W, Gu WY, Qiao L, Wang TY, Wu DD, Dong FC, Hua J. Prevalence and Clinical Significance of Portal Vein Thrombosis in Patients With Cirrhosis and Acute Decompensation. Clin Gastroenterol Hepatol. 2020;18:2564-2572.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 76. | Arroyo V, Angeli P, Moreau R, Jalan R, Clària J, Trebicka J, Fernández J, Gustot T, Caraceni P, Bernardi M; investigators from the EASL-CLIF Consortium, Grifols Chair and European Foundation for the Study of Chronic Liver Failure (EF-Clif). The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2021;74:670-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 287] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 77. | Heo NY. Acute-on-chronic liver failure: A predictor of poor prognosis in patients with variceal bleeding or a risk factor for variceal bleeding? Clin Mol Hepatol. 2020;26:487-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 78. | Shin J, Yu JH, Jin YJ, Yim HJ, Jung YK, Yang JM, Song DS, Kim YS, Kim SG, Kim DJ, Suk KT, Yoon EL, Lee SS, Kim CW, Kim HY, Jang JY, Jeong SW. Acute-on-chronic liver failure as a major predictive factor for mortality in patients with variceal bleeding. Clin Mol Hepatol. 2020;26:540-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 79. | Lv Y, Wang Z, Li K, Wang Q, Bai W, Yuan X, Yu T, Niu J, Yang Z, Zhu X, Zhao J, Xue H, Jiang Z, Zhuge Y, Zhang C, Sun J, Ding P, Ren W, Li Y, Zhang K, Zhang W, Guo W, Luo B, Li X, Yuan J, Han N, Zhu Y, He C, Yin Z, Fan D, Han G. Risk Stratification Based on Chronic Liver Failure Consortium Acute Decompensation Score in Patients With Child-Pugh B Cirrhosis and Acute Variceal Bleeding. Hepatology. 2021;73:1478-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 80. | Kumar R, Kerbert AJC, Sheikh MF, Roth N, Calvao JAF, Mesquita MD, Barreira AI, Gurm HS, Ramsahye K, Mookerjee RP, Yu D, Davies NH, Mehta G, Agarwal B, Patch D, Jalan R. Determinants of mortality in patients with cirrhosis and uncontrolled variceal bleeding. J Hepatol. 2021;74:66-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 81. | Al-Mahtab M, Akbar SM, Garg H. Influence of variceal bleeding on natural history of ACLF and management options. Hepatol Int. 2016;10:436-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Rashed E, Soldera J. CLIF-SOFA and CLIF-C scores for the prognostication of acute-on-chronic liver failure and acute decompensation of cirrhosis: A systematic review. World J Hepatol. 2022;14:2025-2043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (2)] |