Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.3993
Peer-review started: March 18, 2023
First decision: May 9, 2023
Revised: May 9, 2023
Accepted: May 15, 2023
Article in press: May 15, 2023
Published online: June 16, 2023
Processing time: 85 Days and 14.8 Hours
Preeclampsia (PE) is a multisystemic metabolic disease with an undetermined etiology. PE is a worldwide cause of maternal and perinatal morbidity, subdi
To test whether plasma Ela could serve as a reliable marker for predicting PE based on the time of onset (EoPE vs LoPE) compared to age and body mass matched healthy controls since no definitive treatment exists for PE but to terminate a pregnancy.
This case-control study recruited (n = 90) pregnant who fulfilled inclusion criteria; they were allocated into three groups: EoPE (30/90) (< 34 wk of gestation); LoPE (30/90) (≥ 34 wk of gestation); and healthy pregnant (30/90). Demographic criteria; biochemical, hematological, and maternal plasma Ela levels were recorded for comparison.
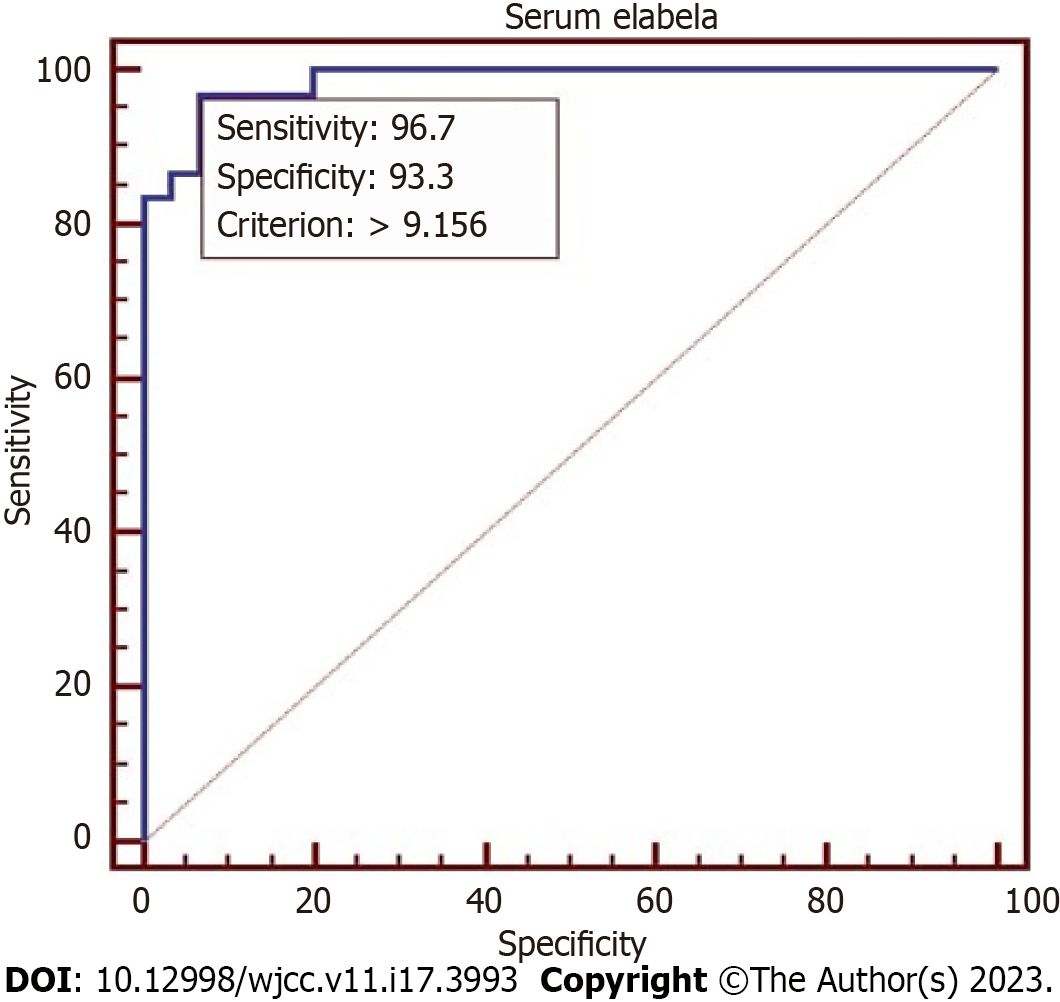
Serum Ela was significantly reduced in EoPE compared to LoPE and healthy controls (P = 0.0023). The correlation confirmed a strong inverse relationship with mean atrial blood pressure (r = -0.7, P < 0.001), while gestational age and platelets count showed a moderate correlation with (r = 0.4 with P < 0.0001). No correlation was confirmed between the body mass index (BMI) and urine albumin. The predictive ability of 25 centile serum Ela had an Odds ratio of 5.21, 95% confidence interval (1.28, 21.24), P = 0.02 for predicting EoPE. The receiver operator characteristic curve defined the Ela cutoff value at > 9.156 with 96.7% and 93.3% sensitivity and specificity, P < 0.0001 in predicting EoPE.
A strong correlation of serum Ela with PE parameters with excellent sensitivity and specificity in distinguishing EoPE independent of the BMI, age, and blood pressure which makes Ela a recommendable marker in screening. Further research is warranted to explore prognostic and therapeutic applications for Ela in PE.
Core Tip: Preeclampsia (PE) is a worldwide cause of increased maternal and perinatal morbidity; PE is divided into early-onset and late-onset subtypes. The precise pathophysiology of PE is obscured, and currently no treatment exists but to terminate pregnancy. Several researches seek a reliable biomarker to anticipate PE to mitigate its negative effects. Elabela (Ela), a recently discovered peptide hormone secreted by the fetus and human placenta; animal studies confirmed Ela’s critical role in maintaining blood pressure; its deficiency was linked to elevated blood pressure. The purpose of this study was to evaluate the accuracy of Ela in predicting PE based on the time of occurrence.
- Citation: Amer Ali E, Nori W, Salman AF, Al-Rawi TSS, Hameed BH, Al-Ani RM. Elabela is a reliable biomarker for predicting early onset preeclampsia: A comparative study. World J Clin Cases 2023; 11(17): 3993-4002
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/3993.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.3993
Preeclampsia (PE), a pregnancy-specific syndrome that increases feto-maternal morbidity and mortality, continues to fascinate scientists[1]. Despite the tremendous advance in the medical field, there is no definitive treatment for this enigmatic syndrome but to terminate the pregnancy. Scientists agree that PE is a 2-stage syndrome. Stage 1, in which defective trophoblast invasion and failure to model placental spiral vessels trigger placental hypoxia, eventually leading to diffuse inflammatory response and vascular endothelial dysfunction within the maternal circulation, is stage 2[2].
A growing body of research was directed to understand the pathophysiology of PE phenotypes; early onset (EoPE) and late-onset (LoPE) are differentiated by gestational age of < 34 wk and ≥ 34 wk, respectively. Furthermore, each type seems to have a different etiology, pathophysiology, and prognosis[3]. The primary issue in PE cases is a placental injury the more significant the placental insult, the graver its consequence. EoPE is a PE phenotype that is less common than LoPE (15% vs 80%); it presents higher materno-fetal morbidity in addition to an increased lifetime risk of cardiovascular diseases (CVD). Therefore, many researchers have addressed EoPE to halt its near and long complications and seek reliable prediction biomarkers or preventive interventions like Aspirin therapy[4,5].
Elabela (Ela), a recently discovered peptide hormone (also called Apela/Toddler) is a molecule composed of thirty-two amino acids. It’s an endogenous ligand for apelin (APJ), a G-protein-coupled-receptor[6,7].
There is a diverse expression of APJ receptors in many human tissues, and during pregnancy, it is secreted by the fetus and human placenta. Ela has a series of functions in embryonic life; it promotes skeletal development renewal of embryonic stem cells; inhibits renal remodeling, angiogenesis, and vascular morphogenesis in embryonic life[8]. In addition, the Ela-APJ system has an essential role in fetal cardiovascular development. It can lower the vascular tone by direct vasodilatory action on the aorta, lowering blood pressure[9].
Early animal studies confirm the vital role of Ela in controlling blood pressure rates, and its deficiency triggered PE development and various congenital disabilities in the offspring. However, human studies presented inconsistent and sometimes conflicting results about its role in PE[10]. This study aimed to examine the reliability of Ela in predicting PE based on time of occurrence (EoPE vs LoPE) to alleviate PE-related morbidity.
A case-control study was conducted in the University Hospital, a tertiary center in Baghdad/Iraq, from December 2020 to October 2021. The ethics committee of Mustansiriyah University issued the study approval IRB dated (IRB 126 on Nov 25, 2020). Our hospital receives hundreds of patients in many specialties; the maternity wards receive 150-300 cases per month. We sequentially recruited (90) pregnant who were aged and body mass index (BMI) matched. All pregnant gave written consent prior to enrollment; the declaration of Helsinki was followed. Cases that satisfied the study inclusion criteria were grouped into 3 main groups, early onset PE (EoPE) 30/90; Late-onset PE (LoPE) 30/90 and healthy controls 30/90. PE was defined as newly diagnosed hypertension after twenty weeks of pregnancy in a previously normotensive pregnant with /or without proteinuria. In the absence of proteinuria, PE is defined as hypertension that leads to end-organ damage. Early-onset PE was less than 34 wk; Late-onset PE was equal or more than 34 wk[11].
As follows: (1) Primigravida; age range 18-35 years pregnant with a singleton pregnancy with reliable dating; calculated by last menstrual period (LMP) and early pregnancy scanning; (2) pregnant should not start antihypertensive drugs; and (3) uneventful medical and surgical history.
As follows: (1) Multiparty, multiple pregnancies, abnormal fetuses, and those with fetal growth restriction; (2) pregnant with a BMI > 30 kg/m2, age < 18 years or > 35 years; and (3) past medical history of chronic hypertension, diabetes, thyroid, liver, and renal disease.
After a detailed history and clinical examination, which included measuring the blood pressure by sphygmomanometer in rest 4-h apart and measuring BMI. Overnight fast aspiration was made to 10 ccs of venous blood, which was sent for: (1) Complete blood counts, including hemoglobin and platelets; (2) biochemical parameters, including aspartate aminotransferase (AST), alanine transaminase (ALT), urea, and creatinine; (3) after centrifuging the blood at 3000 rpm/min for 15 min, the sera were refrigerated to -80 °C to analyze Ela later. The estimation was made by the Human Ela ELISA Kit (Cat. No. MBS 3803412) according to the manufacturer’s protocol; and (4) urinalysis for albuminuria.
It was achieved based on the following formulae[12]. r = stands for the ratio of control to cases, in our study, equal to 2. p* = denotes the average proportion exposed = proportion of exposed cases + proportion of control exposed /2. In our study the p*= 0.26 + 0.10/2 = 0.36/2 = 0.18; P1-P2 = different in proportion expected based on previous studies. P1 = proportion in cases, P2 = proportion in control. Zβ is the standard normal variant, and it is 0.84 for 80% power of the study. Zα/2 is the standard normal variant, and it is 1.96 at P = 0.05 value significance. Sample size = r + 1 (p*)(1-p*)(Zβ + Zα/2)2 /r (p1-p2)2 = 2 + 1 (0.18) (1-0.18) (0.84 + 1.96) 2/2 (0.19-0.03)2 = 3*0.18*0.82 *7.84/2*0.0256 = 70. So, the sample size is 70, and we collect 90 participants.
The Kolmogorov-Smirnov test checked the data normality. The expression for continuous data was as mean ± SD. ANOVA and Student’s t-test were used to compare different means when appropriate. Through the use of Pearson correlation analysis, the association between several PE indicators and Ela was evaluated. The correlation was interpreted as weak if r is equal to (0.2-0.4), moderate if r is equal to (0.4-0.6), strong if r is equal to (0.6-0.8), and very strong if r is equal to (0.8-1.0). Analysis of covariance (ANCOVA) was used to explore the impact of different PE parameters (taken as independent factors) on serum Ela levels taken as a dependent variable. The Odds ratio (OR), 95% confidence interval (95%CI), and respective P value for 25 centiles of serum Ela was tested as a predictive marker in early, late-onset PE and healthy controls. Finally, the receiver operator characteristic curve (ROC) calculated serum Ela cutoff value associated with the highest sensitivity, specificity, and area under the curve (AUC) in predicting early onset PE. Significance was set at a P value less than 0.05. All tests were conducted by the SPSS program (version 22.0)[13].
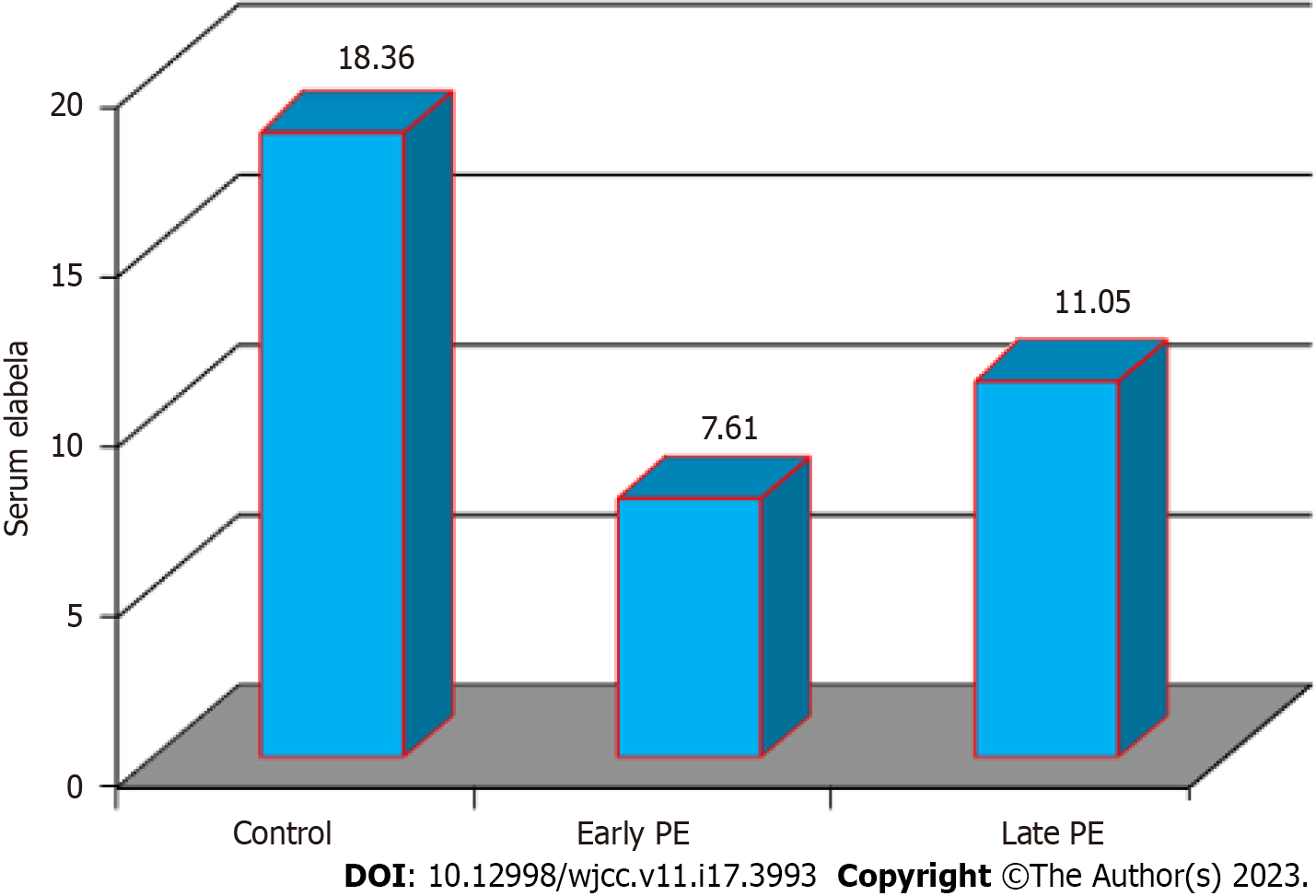
The analysis showed significant differences in all the study variables except for maternal age 26.53 years ± 1.18 years, 28.67 years ± 0.99 years, and 28.33 years ± 1.34 years for the healthy controls, EoPE, and LoPE respectively. BMI and blood urea showed insignificant differences across the three groups. Serum Ela scored the highest level among healthy controls, followed by late PE cases and early onset PE highlighted in Table 1 and Figure 1, respectively.
| Variable | Control (n = 30), mean ± SD | P value | Late-PE (n = 30), mean ± SD | Early-PE (n = 30), mean ± SD | P value |
| Age (yr) | 26.53 ± 1.18 | 0.39 NS | 28.33 ± 1.34 | 28.67 ± 0.99 | 0.39 NS |
| BMI (kg/m2) | 24.93 ± 0.34 | 0.77 NS | 26.19 ± 0.29 | 26.96 ± 3.42 | 0.77 NS |
| Gestational age (wk) | 34.93 ± 0.20b | 0.0001 | 36.30 ± 0.19a | 30.70 ± 0.34c | 0.0001 |
| Systolic BP (mmHg) | 106.00 ± 1.48b | 0.0001 | 152.33 ± 1.71a | 152.33 ± 1.64a | 0.0001 |
| Diastolic BP (mmHg) | 82.67 ± 1.85b | 0.0001 | 103.00 ± 1.80a | 102.66 ± 1.79a | 0.0001 |
| Mean arterial BP (MAP) | 90.44 ± 1.21b | 0.0001 | 119.44 ±1.69a | 119.22 ± 1.65a | 0.0001 |
| AST (IU/L) | 23.09 ± 1.07b | 0.05 | 34.05 ± 5.98a | 27.25 ± 1.41ab | 0.05 |
| ALT (IU/L) | 17.82 ± 0.88b | 0.0001 | 30.06 ± 2.75a | 25.17 ± 1.45a | 0.0001 |
| Urea (mg/dL) | 25.01 ± 1.32 | 0.84 NS | 25.93 ± 1.56 | 26.21 ± 1.64 | 0.84 NS |
| Creatinine (mg/dl) | 0.665 ± 0.02b | 0.0001 | 0.759 ± 0.02a | 0.759 ± 0.02a | 0.0001 |
| Albumin in urine | - | 0.19 NS | 460.50 ± 47.06 | 381.66 ± 35.43 | 0.19 NS |
| Hemoglobin (g/dL) | 12.68 ± 0.19a | 0.0001 | 11.69 ± 0.19b | 12.02 ± 0.21b | 0.0001 |
| Platelets counts × 109 L | 287.60 ± 11.23a | 0.0006 | 182.83 ± 10.48b | 204.90 ± 10.70b | 0.0006 |
| Elabela (pg/mL) | 18.36 ± 0.43a | 0.002 | 11.05 ± 0.22b | 7.61 ± 0.19c | 0.002 |
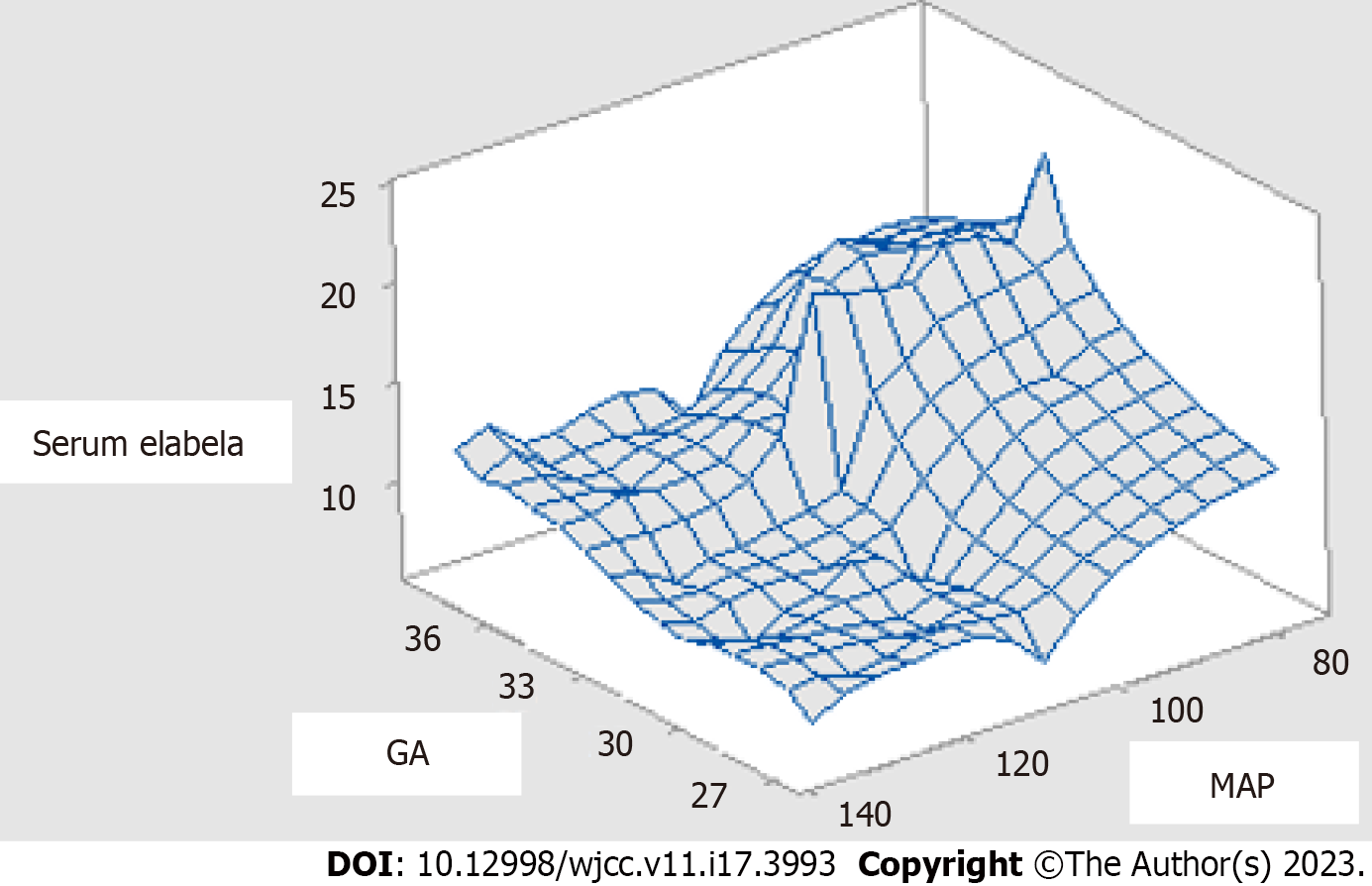
Ela correlation vs all study parameters was presented in Table 2; the strongest correlation was for SBP, MAP, and DBP with (r = -0.80, -0.70, and -0.64, P < 0.0001), respectively. Gestational age and platelets count showed a moderate correlation with (r = 0.44 and 0.48, P < 0.0001). None of the BMI, AST, Urea, and albumin in urine was significant. A three-dimensional figure highlighted the correlation of serum Ela concerning gestational age and degree of hypertension in Figure 2.
| Variable | Pearson correlation | P value |
| BMI (kg/m2) | -0.090 | 0.4200 |
| Gestational age (wk) | 0.440 | < 0.0001 |
| Systolic BP (mmHg) | -0.800 | < 0.0001 |
| Diastolic BP (mmHg) | -0.640 | < 0.0001 |
| Mean arterial BP | -0.700 | < 0.0001 |
| AST (IU/L) | -0.110 | 0.2000 |
| ALT (IU/L) | 0.200 | < 0.0030 |
| Urea (mg/dL) | 0.004 | 0.9700 |
| Creatinine (mg/dL) | -0.310 | < 0.0030 |
| Albumin in urine | 0.160 | 0.2000 |
| Hemoglobin (g/dL) | 0.290 | < 0.0040 |
| Platelets count (× 109/L) | 0.480 | < 0.0001 |
ANCOVA in Table 3 confirmed that none of the study variables had an impact on serum Ela as P value was > 0.05. In Table 4, the Odds ratio and 95%CI were tested to evaluate the predictive power of 25 centile serum Ela showed the highest Odds for predicting early onset PE with OR of 5.21, 95%CI: 1.28-21.24, P = 0.02. The ROC curve defined the Ela cutoff value at > 9.156 with 96.7%, 93.3%, sensitivity and specificity, and P < 0.0001 in predicting early onset PE (Figure 3).
| Variable | F-ratio | P value |
| Body mass index | 0.120 | 0.74 |
| Gestational age | 0.990 | 0.32 |
| Mean arterial pressure | 0.001 | 0.97 |
| Platelets count | 0.020 | 0.65 |
| Groups | Odds ratio | 95%CI | P value |
| Early-onset PE | 5.21 | 1.28-21.24 | 0.02 |
| Late-onset PE | 1.80 | 0.39-8.32 | 0.45 |
| Controls | Reference group |
Analysis showed a significant reduction of serum Ela in EoPE compared to LoPE and healthy controls. Ela correlated inversely and strongly with mean atrial blood pressure and moderately and positively with gestational age and platelet count. Ela did not correlate with BMI and urine albumin. None of the study variables impacted serum Ela levels. A 25-centile serum Ela had a significant Odds ratio for predicting EoPE.
In accordance with our result, Wang et al[14] confirmed a meaningful reduction of Ela concentration among EoPE vs healthy pregnant.
Conversely, Pritchard et al[15] examined Ela levels in healthy controls vs PE cases in addition to a subgroup analysis in PE cases below 34 wk of gestation vs matched age controls. Their result shows no statistical differences for both comparisons. Since earlier animal models support Ela’s role in PE pathogenesis, deficient rats in Ela had impaired placental and fetal development. The authors proposed that Ela levels are of no value once PE is clinically manifested[15]. Likewise, Huanga study did not recommend Ela levels in predicting gestational hypertension by screening pregnant in the first and second-trimester[16].
Panaitescu et al[17] declared higher Ela levels in PE (LoPE and EoPE) vs healthy pregnant (P = 0.32, < 0.001) at a gestational age of 37 and 30 wk, respectively. Different sampling times might explain the difference in our results. Still, serum Ela at EoPE was lower than LoPE; they attributed this to the different pathophysiology of the two subgroups of PE. Moreover, they discussed the long-term CVD risk among EoPE, which underlies insufficient protection by low Ela levels during pregnancy[18].
Deniz et al[19] investigated Ela serum levels among PE (36 wk), healthy pregnancy (38 wk), and their neonates’ blood. Ela was significantly low among PE cases, and that reduction was positively linked to PE severity in line with Ho et al[20] study. Furthermore, Deniz et al[19] correlated maternal Ela to low birth weight in the newborn. Reduced levels of Ela among PE cases caused insufficient placenta angiogenesis, which consequently reduced birth weight[21]. Georgiadou et al[22] tested Ela at 11-14 wk of pregnancy as a predictive marker of PE. Normal weight pregnant destined to have PE showed meaningfully reduced levels. However, Ela showed a wide variance in affected cases; moreover, it was BMI dependent, for it was not recommended for screening. However, a potential therapeutic avenue for Ela was proposed because it promotes extra villous trophoblastic differentiation.
Ela plays a crucial role in early developing fetuses and placenta by increasing trophoblast penetration into the uterine wall and angiogenic sprouting[8], reinforcing Ela’s role in preventing EoPE, a PE subtype caused by under-penetration of placental vessels. Scientists agree that PE is a 2-stage syndrome. First, defective trophoblast triggers placental hypoxia; this is (stage 1). Once PE is clinically evident around the 20th week of gestation (stage 2), the placenta will secret placental factors and cytokines to overcome reduced fetal blood supply and trigger a systemic maternal inflammatory cascade, a PE hallmark[23]. Apelin emerges later to promote fetal angiogenesis, placental vessel tone, and energy homeostasis[24].
Ela levels are high early in pregnancy and tend to decrease around 34 wk to be replaced by Apelin, which explains the difference in Ela’s reliability among PE cases[8]. That was highlighted in our result by the three-dimensional figure showing the inverse correlation between serum Ela vs mean arterial blood pressure and a positive correlation with gestational age; it can be noted that the lowest serum Ela is seen at 27-33 wk, i.e., early onset PE.
Inconsistency in reporting Ela links to early, late, and normal controls may be due to many factors[25]. First, inconsistency in gestational age at sampling time. Second, the use of different commercial kits. Third, the inclusion of heterogenous BMI participants[26-28]. Although earlier works have examined Ela’s role in PE onset, they did not address confounders that might impact Ela levels (proven by ANCOVA test) which forms the current study novelty. Interestingly BMI, gestational age, mean arterial pressure, and platelets count did not affect Ela levels, which added validity to our marker and implied its intimate link to PE pathogenies.
Earlier research discussed an Ela-BMI dependence among normal-weight women in LoPE but not for EoPE; the authors attribute this insignificant to a smaller size sample[21].
Our result highlighted Ela’s role in EoP by OR of 5.21, P = 0.02; conversely, LoPE had an OR of 1.80 and an insignificant P value. Additionally, the ROC estimated Ela cutoff value at > 9.156 with 96.7%, 93.3%, and P < 0.0001 sensitivity and specificity, respectively, in discriminating EoPE.
Many acknowledge PE as a 2-stage syndrome[23]. However, PE’s first stage fits with EoPE alongside the mother’s long-life risk of CVD; Panaitescu et al[17] discussed that the CVD risk among EoPE underlies insufficient protection by low Ela levels during pregnancy.
Ela plays a crucial role in early developing fetuses and placenta by increasing trophoblast penetration into the uterine wall and angiogenic, reinforcing Ela’s role in preventing EoPE, a PE subtype caused by under-penetration of placental vessels[29].
Qi et al[30] declared that Ela levels were markedly low among missed abortion cases vs healthy pregnant women, which suggests Ela’s critical role in supporting early pregnancy prosperity.
Ela is Apelin’s endogenous receptor against; Ela- and Apelin-APJ signaling orchestrates important aspects of placental growth by promoting extra-villous trophoblastic differentiation and invasiveness to the uterus, improving uterine blood supply, decreasing oxidative stress, and suppressing placenta apoptosis. Animal models support Ela’s role in PE pathogenesis. Ela-deficient rats had impaired placental and fetal development[20,31].
On the systemic level, Ela- and Apelin improve the cardiac output by producing nitric oxide that has vasodilatory action on the vasculature. Low Apelin concentrations were reported in cases with high blood pressure due to reduced vascular hemostasis protection[25,32,33].
Ho et al[20] discussed that Ela infusion in preeclamptic rates improved hypertension and albuminuria. Yang et al[32] reported that exogenous Ela infusion additionally ameliorates pulmonary hypertension by causing pulmonary vessel remodeling in rats.
Study limitation: Being a single center study and the small number of included studies due to scare number of published research. So, the current results need to be verified in large scale multi-centric studies. PE is a syndrome that is affected by race, a parameter we did not address. We hoped to recruit more patients; however COVID-19 pandemic affected many work aspects[34].
Study strengths: The study was well-powered with tight inclusion criteria; it estimated the risk ratio and Ela cutoff value in predicting EoPE with a reliable AUC and high sensitivity and specificity. Furthermore, Ela’s independency of gestational age, BMI, and mean arterial pressure, with high specificity and sensitivity, adds more credibility to Ela’s prediction power. Moreover, the therapeutic application suggested by earlier researchers may unveil prognostic application in the long run for reducing CVS risk for postpartum women.
Ela, a recently discovered peptide hormone, was found to be a reliable marker for screening EoPE independence of BMI, age, and blood pressure. It differentiated EoPE with high sensitivity, high specificity, and a significant area under the curve. Ela’s intimate link with predictors of PE opens a therapeutic and preventive avenue for Ela in PE.
Preeclampsia (PE) is a multisystemic disease that can cause problems for both the mother and the baby. It can start early or late, depending on how far along the pregnancy is. Elabela (Ela) is a recently found peptide hormone that was linked to the development of PE. Since the only safe way to treat PE is to end a pregnancy, the goal of this study was to see if plasma Ela could be used as a reliable way to predict PE based on the time of onset.
Endogenous ligand for apelin (APJ) receptors is found in many different parts of the human body, and Ela is produced by the fetus and placenta during pregnancy. Ela has a number of roles in embryonic life. During embryonic life, it stops renal remodeling, angiogenesis, and vascular morphogenesis. Also, the Ela-APJ system is very important for the development of the heart and blood vessels in a fetus. It can lower the tone of the blood vessels by directly relaxing the blood vessels in the aorta. This lowers blood pressure.
To determine the reliability of Ela in predicting PE based on time of incidence early (EoPE) vs late (LoPE) in order to reduce PE-related morbidity.
In a case-control study, pregnant women were divided into three groups: EoPE (30/90) (< 34 wk of gestation), LoPE (30/90) (≥ 34 wk of gestation), and healthy pregnant (30/90). Demographic criteria were examined, as well as biochemical, hematological, and maternal plasma Ela levels.
Serum Ela was significantly reduced in EoPE compared to LoPE and healthy controls (P = 0.0023); Ela had a strong inverse relationship to mean atrial blood pressure (r = -0.7, P < 0.001), and a moderate correlation with gestational age and platelets count (r = 0.4, P < 0.0001. The predictive ability of 25 centile serum Ela had an odds ratio of 5.21, 95% confidence interval (1.28, 21.24), P = 0.02 for predicting EoPE. Ela’s cutoff value (> 9.15) distinguished EoPE with 96.7% sensitivity, 93.3% specificity, and P < 0.0001.
Ela is a highly recommended marker in screening due to its high sensitivity and specificity in differentiating EoPE from other conditions such as body mass index, age, and blood pressure.
Ela’s specificity and sensitivity, independent of gestational age, body mass index, and mean arterial pressure, lend credence to Ela’s ability to predict. As an added bonus, the therapeutic use proposed by previous researchers may eventually reveal prognostic application for lowering CVS risk in postpartum women. Further study is required to verify its usefulness in clinical settings.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: Iraq
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rotondo JC, Italy; Shariati MBH, Iran; Wang T, China S-Editor: Chen YL L-Editor: A P-Editor: Zhao S
| 1. | Rahnemaei FA, Fashami MA, Abdi F, Abbasi M. Factors effective in the prevention of Preeclampsia:A systematic review. Taiwan J Obstet Gynecol. 2020;59:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Nori W, Hamed RM, Roomi AB, Akram W. Alpha-1antitrypsin in pre-eclampsia; from a clinical perspective. J Pak Med Assoc. 2021;71 Suppl 8:S53-S56. [PubMed] |
| 3. | Herzog EM, Eggink AJ, Willemsen SP, Slieker RC, Wijnands KPJ, Felix JF, Chen J, Stubbs A, van der Spek PJ, van Meurs JB, Steegers-Theunissen RPM. Early- and late-onset preeclampsia and the tissue-specific epigenome of the placenta and newborn. Placenta. 2017;58:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Aneman I, Pienaar D, Suvakov S, Simic TP, Garovic VD, McClements L. Mechanisms of Key Innate Immune Cells in Early- and Late-Onset Preeclampsia. Front Immunol. 2020;11:1864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 5. | Nori W, Shallal F, Zghair MAG. Aspirin effect on Mid luteal Phase Doppler Indices in Patients with Recurrent Pregnancy Loss. Int J Pharm Res. 2020;12:2929-2934. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Sharma M, Prabhavalkar KS, Bhatt LK. Elabela Peptide: An Emerging Target in Therapeutics. Curr Drug Targets. 2022;23:1304-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Liet B, Nys N, Siegfried G. Elabela/toddler: New peptide with a promising future in cancer diagnostic and therapy. Biochim Biophys Acta Mol Cell Res. 2021;1868:119065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Eberlé D, Marousez L, Hanssens S, Knauf C, Breton C, Deruelle P, Lesage J. Elabela and Apelin actions in healthy and pathological pregnancies. Cytokine Growth Factor Rev. 2019;46:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Zhou S, Wang J, Wang Q, Meng Z, Peng J, Song W, Zhou Y, Chen S, Chen F, Sun K. Essential Role of the ELABELA-APJ Signaling Pathway in Cardiovascular System Development and Diseases. J Cardiovasc Pharmacol. 2020;75:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Xu C. The Elabela in hypertension, cardiovascular disease, renal disease, and preeclampsia: an update. J Hypertens. 2021;39:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, McAuliffe F, da Silva Costa F, von Dadelszen P, McIntyre HD, Kihara AB, Di Renzo GC, Romero R, D'Alton M, Berghella V, Nicolaides KH, Hod M. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145 Suppl 1:1-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 706] [Article Influence: 117.7] [Reference Citation Analysis (0)] |
| 12. | Chow SC, Shao J, Wang H, Lokhnygina Y. Sample size calculations in clinical research. 3rd Ed. New York: Chapman and Hall/CRC, 2017. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Mazziotta C, Pellielo G, Tognon M, Martini F, Rotondo JC. Significantly Low Levels of IgG Antibodies Against Oncogenic Merkel Cell Polyomavirus in Sera From Females Affected by Spontaneous Abortion. Front Microbiol. 2021;12:789991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Wang L, Zhang Y, Qu H, Xu F, Hu H, Zhang Q, Ye Y. Reduced ELABELA expression attenuates trophoblast invasion through the PI3K/AKT/mTOR pathway in early onset preeclampsia. Placenta. 2019;87:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Pritchard N, Kaitu'u-Lino TJ, Gong S, Dopierala J, Smith GCS, Charnock-Jones DS, Tong S. ELABELA/APELA Levels Are Not Decreased in the Maternal Circulation or Placenta among Women with Preeclampsia. Am J Pathol. 2018;188:1749-1753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Huang R, Zhu J, Zhang L, Hua X, Ye W, Chen C, Sun K, Wang W, Feng L, Zhang J; Shanghai Birth Cohort study. Is ELABELA a reliable biomarker for hypertensive disorders of pregnancy? Pregnancy Hypertens. 2019;17:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Panaitescu B, Romero R, Gomez-Lopez N, Pacora P, Erez O, Vadillo-Ortega F, Yeo L, Hassan SS, Hsu CD. ELABELA plasma concentrations are increased in women with late-onset preeclampsia. J Matern Fetal Neonatal Med. 2020;33:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 651] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 19. | Deniz R, Baykus Y, Ustebay S, Ugur K, Yavuzkir Ş, Aydin S. Evaluation of elabela, apelin and nitric oxide findings in maternal blood of normal pregnant women, pregnant women with pre-eclampsia, severe pre-eclampsia and umbilical arteries and venules of newborns. J Obstet Gynaecol. 2019;39:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Ho L, van Dijk M, Chye STJ, Messerschmidt DM, Chng SC, Ong S, Yi LK, Boussata S, Goh GH, Afink GB, Lim CY, Dunn NR, Solter D, Knowles BB, Reversade B. ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice. Science. 2017;357:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 21. | Chng SC, Ho L, Tian J, Reversade B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev Cell. 2013;27:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 370] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 22. | Georgiadou D, Boussata S, Ranzijn WHM, Root LEA, Hillenius S, Bij de Weg JM, Abheiden CNH, de Boer MA, de Vries JIP, Vrijkotte TGM, Lambalk CB, Kuijper EAM, Afink GB, van Dijk M. Peptide hormone ELABELA enhances extravillous trophoblast differentiation, but placenta is not the major source of circulating ELABELA in pregnancy. Sci Rep. 2019;9:19077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Redman CWG, Staff AC, Roberts JM. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol. 2022;226:S907-S927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 196] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 24. | Liu Y, Wang L, Shi H. The biological function of ELABELA and APJ signaling in the cardiovascular system and pre-eclampsia. Hypertens Res. 2019;42:928-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Georgiadou D, Afink GB, van Dijk M. The apelinergic-axis in human preeclamptic pregnancies: A systematic review. Pregnancy Hypertens. 2019;17:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Daskalakis G, Bellos I, Nikolakea M, Pergialiotis V, Papapanagiotou A, Loutradis D. The role of serum adipokine levels in preeclampsia: A systematic review. Metabolism. 2020;106:154172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Georgiadou D, Boussata S, van Dijk M. ELABELA measurements by commercial ELISA kits require sample extraction. Am J Physiol Endocrinol Metab. 2019;317:E1218-E1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Zhou L, Sun H, Cheng R, Fan X, Lai S, Deng C. ELABELA, as a potential diagnostic biomarker of preeclampsia, regulates abnormally shallow placentation via APJ. Am J Physiol Endocrinol Metab. 2019;316:E773-E781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Womersley K, Ripullone K, Hirst JE. Tackling inequality in maternal health: Beyond the postpartum. Future Healthc J. 2021;8:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Qi Y, Hou Y, Ma M, Li X, Wu J. Circulating levels of Elabela in pregnant women with missed abortion. Gynecol Endocrinol. 2022;38:693-696. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Charo DN, Ho M, Fajardo G, Kawana M, Kundu RK, Sheikh AY, Finsterbach TP, Leeper NJ, Ernst KV, Chen MM, Ho YD, Chun HJ, Bernstein D, Ashley EA, Quertermous T. Endogenous regulation of cardiovascular function by apelin-APJ. Am J Physiol Heart Circ Physiol. 2009;297:H1904-H1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Yang P, Read C, Kuc RE, Buonincontri G, Southwood M, Torella R, Upton PD, Crosby A, Sawiak SJ, Carpenter TA, Glen RC, Morrell NW, Maguire JJ, Davenport AP. Elabela/Toddler Is an Endogenous Agonist of the Apelin APJ Receptor in the Adult Cardiovascular System, and Exogenous Administration of the Peptide Compensates for the Downregulation of Its Expression in Pulmonary Arterial Hypertension. Circulation. 2017;135:1160-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 33. | Yeganeh-Hajahmadi M, Najafipour H, Rostamzadeh F. The differential effects of low and high doses of apelin through opioid receptors on the blood pressure of rats with renovascular hypertension. Hypertens Res. 2017;40:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Al-Ani RM. Ear, nose, and throat manifestations of COVID-19 and its vaccines. World J Clin Cases. 2022;10:8808-8815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (1)] |