Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.3967
Peer-review started: February 5, 2023
First decision: March 24, 2023
Revised: April 15, 2023
Accepted: May 6, 2023
Article in press: May 6, 2023
Published online: June 16, 2023
Processing time: 126 Days and 21.7 Hours
Regional pressure differences between sites within the left ventricular cavity have long been identified, and the potential clinical value of diastolic and systolic intraventricular pressure differences (IVPDs) is of increasing interest. This study concluded that the IVPD plays an important role in ventricular filling and emptying and is a reliable indicator of ventricular relaxation, elastic recoil, diastolic pumping, and effective left ventricular filling. Relative pressure imaging, as a novel and potentially clinically applicable measure of left IVPDs, enables early and more comprehensive identification of the temporal and spatial characteristics of IVPD. In the future, as research related to relative pressure imaging continues, this measurement method has the possibility to become more refined and serve as an additional clinical aid that can replace the gold standard cardiac catheterization technique for the diagnosis of diastolic dysfunction.
Core Tip: Cardiac catheterization is currently the gold standard for assessing ventricular diastolic function, but it is invasive. There has been a need for a non-invasive alternative to cardiac catheterization. In the course of cardiac research, the phenomenon of local intraventricular pressure differences has been explored, and researchers have continued to develop imaging techniques. Particular advancements have been made in magnetic resonance imaging and ultrasound techniques. Relative pressure imaging has been developed based on ultrasound blood flow vector imaging, is able to measure intraventricular pressure differences visually and non-invasively, and has significant advantages over magnetic resonance imaging and color M-mode Doppler ultrasound.
- Citation: Zheng AS, Yu HX. Value of clinical applications of differential pressure and relative pressure imaging in the left ventricle. World J Clin Cases 2023; 11(17): 3967-3975
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/3967.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.3967
The assessment of cardiac function is a fundamental problem in all cardiac imaging techniques and is usually determined by studying the movement of the myocardial wall, deformation of the valve, or the velocity of blood flow through the valve[1]. Another way to study cardiac function is to analyze the blood flow within the heart. Non-invasive assessment of intraventricular pressure differences (IVPDs) is an emerging topic in cardiology. The study of IVPDs is expected to provide information that is relevant to cardiac function and that may be useful in clinical applications. Non-invasive IVPD measurement has been the subject of several recent clinical and academic studies, which have been performed with different techniques ranging from M-mode color Doppler to 2D color Doppler. Thus, a review on this subject dedicated to the vector flow mapping (VFM) method to evaluate the IVPD can be a useful contribution. This study reviewed the use of 2D color Doppler technology to evaluate IVPDs.
Important pathophysiological information on ventricular ejection and filling kinetics has been derived from invasive studies[2]. In the left ventricle (LV), high-fidelity pressure tracing at different sites within the LV showed regional pressure differences in both diastole and systole[3,4]. IVPDs throughout the cardiac cycle have provided insight into their complex interplay with cardiac function[2]. The intraventricular pressure gradients (IVPGs) in the LV exist over the entire cycle of the heart and have been shown to correlate with cardiac function[5,6]. During normal left ventricular systole and diastole, longitudinal hemodynamic forces (along the basal-apical axis) predominate. This coincides with the main direction of LV inflow and outflow through the mitral valve[1]. The IVPD is the difference in pressure between the different parts of the left ventricular cavity that drives the filling of the LV from the left atrium during diastole and the ejection of blood from the LV into the aorta during systole, maintaining circulation[7]. The IVPD is a sensitive reflection of heart function during the circulatory period[8].
There are several phases to the circulatory period: (1) The isovolumic systole phase. The basal pressure is lower than the apical pressure, peaking just before the aortic valve opens; (2) The rapid ejection phase. As the aortic valve opens, the differential pressure continues to drive the left ventricular blood rapidly into the aorta; (3) The slow ejection phase. The aortic pressure rises gradually, the basal pressure is higher than the apical pressure, and the left ventricular blood flows slowly towards the aorta due to inertia until the aortic valve closes[7]; (4) The isovolumic diastolic phase. The left ventricular decompression releases the potential energy stored inside and outside the cells during cardiac contraction, the pressure in the LV decreases at the apex, a small pressure gradient develops within the ventricle from basal to apical, and blood flows slowly towards the apex[9]. This contributes to the smooth transition of subsequent LV pumping, which peaks before the mitral valve opens; (5) The rapid filling phase. As the pressure differential within the LV gradually increases, the mitral valve opens, and blood flows rapidly into the LV, where the creation of eddy currents also helps to prevent energy loss and the formation of adverse pressure gradients[1]. The vortex is a fluid structure that rotates around a virtual central axis and is capable of storing kinetic energy as it rotates. The momentum thrust generated by the ventricular muscle on the blood in the heart chambers is the combined result of an IVPG that is highly matched to the left ventricular geometry in order to create a normal vortex flow, ensure a seamless conversion from diastole to systole, and achieve apical flow filling and strict left ventricular apical-basal longitudinal pressure gradients[10,11]; (6) The slow filling phase. As LV intraventricular pressure increases, blood continues to flow towards the apex, and blood in the left atrium continues to enter the LV due to inertia, with a small negative left ventricular IVPG. This bidirectional pressure difference may play an important role in intraventricular blood flow and vortex formation[12]; and (7) The left atrial systole phase. In the late diastolic, the left atrial contraction develops a positive pressure gradient again from the base of the LV to the apex until the mitral valve closes and causes the residual blood of the left atrium to flow into the LV[13].
During isovolumic diastole, apical directional blood flow has been detected in the LV by color M-mode ultrasound and pulsed wave Doppler echocardiography, despite the closure of both the aortic and mitral valves[14,15]. This demonstrates that there is a pressure difference between the apical and basal regions of the LV during isovolumic diastole, which is thought to redistribute blood in the LV and promote left ventricular filling in early diastole[14]. The IVPD during isovolumic diastole reflects suction force in the LV and provides a valuable evaluation of systolic and diastolic function[16]. The mechanism explaining the relationship between the IVPD and contractile force in early diastole was found to exist in cardiac myocytes and the extracellular matrix. Myosin (activated by titin, a bidirectional molecular spring), which stretches in reverse with the shortening of micronodular length during myocardial contraction, forms a restoring force in early diastole with the elastic retraction of myocardial relaxation and extension, helping to create an active suction force in early diastole[17]. The shorter the systolic segment length, the more pronounced the titin-dependent restoring force is. Correspondingly, the smaller the end-systolic volume, the more prominent the early diastolic suction is[18].
Acute or chronic ventricular dysfunction reduces the amount of myocardium available in a region for systole and subsequent elastic retraction, losing synchrony of myocardial contraction or diastole[19]. The reduction in systolic stored elastic potential energy, together with an increase in end-systolic volume and a reduction in elastic retraction of the myocardium, leads to a decline in the left ventricular pressure difference in the early diastolic phase. Further studies to clarify the relationship between the early diastolic left ventricular pressure difference and myocardial function would help to understand the coupling of the systolic and diastolic phases as well as the key role of ventricular elastic retraction[11].Elastic recoil plays an important role in generating left ventricular suction, and left ventricular suction capacity is closely related to systolic function[20]. When the LV contracts below its equilibrium volume, active left ventricular diastolic relaxation is facilitated by the release of potential energy stored in early diastole[21].
The normal LV exhibits a dynamic balance between systolic performance, active suction and elastic properties, and spatial distribution of the IVPG that can vary over time[21]. Indeed, the left ventricular IVPD between systole and diastole is physiologically regulated by asynchrony between the basal and apical myocardial segments. The outflow tract segments restretch throughout diastole; the apical segments first shorten and then lengthen in late diastole[22]. The physiological asynchrony observed during ventricular filling affects the physiological IVPG and contributes to the filling of the left ventricular outflow tract, thereby facilitating ventricular emptying[4]. This suggests that the IVPD is essential to ensure effective left ventricular filling and emptying and is closely related to left ventricular systolic and diastolic function[22].
In this study, during systole, we recorded the gradient from the apex to the outflow tract during the rapid ejection phase, which reversed during the slow ejection phase[19]. This pressure gradient pattern parallels the ventricular-aortic pressure gradient and facilitates ventricular emptying. When ventricular emptying is opposite to afterload elevation, we demonstrated that the positive IVPG is reversed, indicating impaired ventricular ejection[19].
Regional pressure differences in the LV are used in both pathophysiological studies and in the clinical assessment of cardiac function[23]. In the cardiovascular system, invasive pressure transducers can be used to obtain absolute pressures and assess the IVPD[7]. In 1980, Falsetti et al[24] first demonstrated the existence of IVPDs in animal studies, but the invasiveness of the necessary techniques limited its use in clinical practice[2]. This analysis was made possible by the development of non-invasive cardiovascular imaging techniques, which allowed the visualization and quantification of intracardiac hemodynamics. As an alternative, Greenberg et al[2] were the first to use color Doppler M-mode echocardiography for non-invasive estimation of transient pressure differences across the mitral valve, applying the one-dimensional Euler equation to color Doppler velocities to calculate intracardiac pressure gradients.
The echocardiographic particle image velocimetry technique is based on the intravenous injection of low doses of ultrasound contrast (microbubbles that have the same rheological behavior as red blood cells and act as blood flow tracers) to demonstrate intracardiac gyratory motion[25]. This technique uses contrast to assess vorticity. Blood speckle tracking, which provides an estimate of the blood velocity vector by tracking the speckles produced by moving blood cells frame by frame[26], requires a very high frame rate acquisition and is currently only available for pediatric and transesophageal probes[27].
Correlation of the IVPD to cardiac function (sometimes called hemodynamic force) has become more prevalent in recent years, mainly due to the technology of phase-contrast magnetic resonance imaging (4D flow MRI) and, more recently, with regular echocardiography. Since the 1990s, methods have been applied to visualize multidimensional pressure distributions using 4D flow MRI to determine a physiological relationship between flow and relative pressure[28]. Finally, VFM combines color Doppler imaging with speckle tracking analysis and has been applied to children and adults[29,30]. The idea of combining the Navier–Stokes equation of motion to the velocity field to obtain the IVPG was introduced earlier in 4D flow MRI and echocardiographic particle image velocimetry and later applied in VFM[8].
VFM is a technique that reflects changes in the flow field within the cardiac chambers and detects cardiac pathophysiological and functional changes from a hemodynamic perspective of intraventricular blood flow field changes[31-33]. Color Doppler converts the acquired two-dimensional velocity flow field distribution into two flow components: vortex and basal flow[34]. Based on the principle of continuity equations and combined with speckle tracking analysis[35], axial velocity can be analyzed by color Doppler assessment while horizontal velocity speckle tracking can be estimated with an analytic calculation. Together, these produce a vector representing the direction and velocity of blood flow.
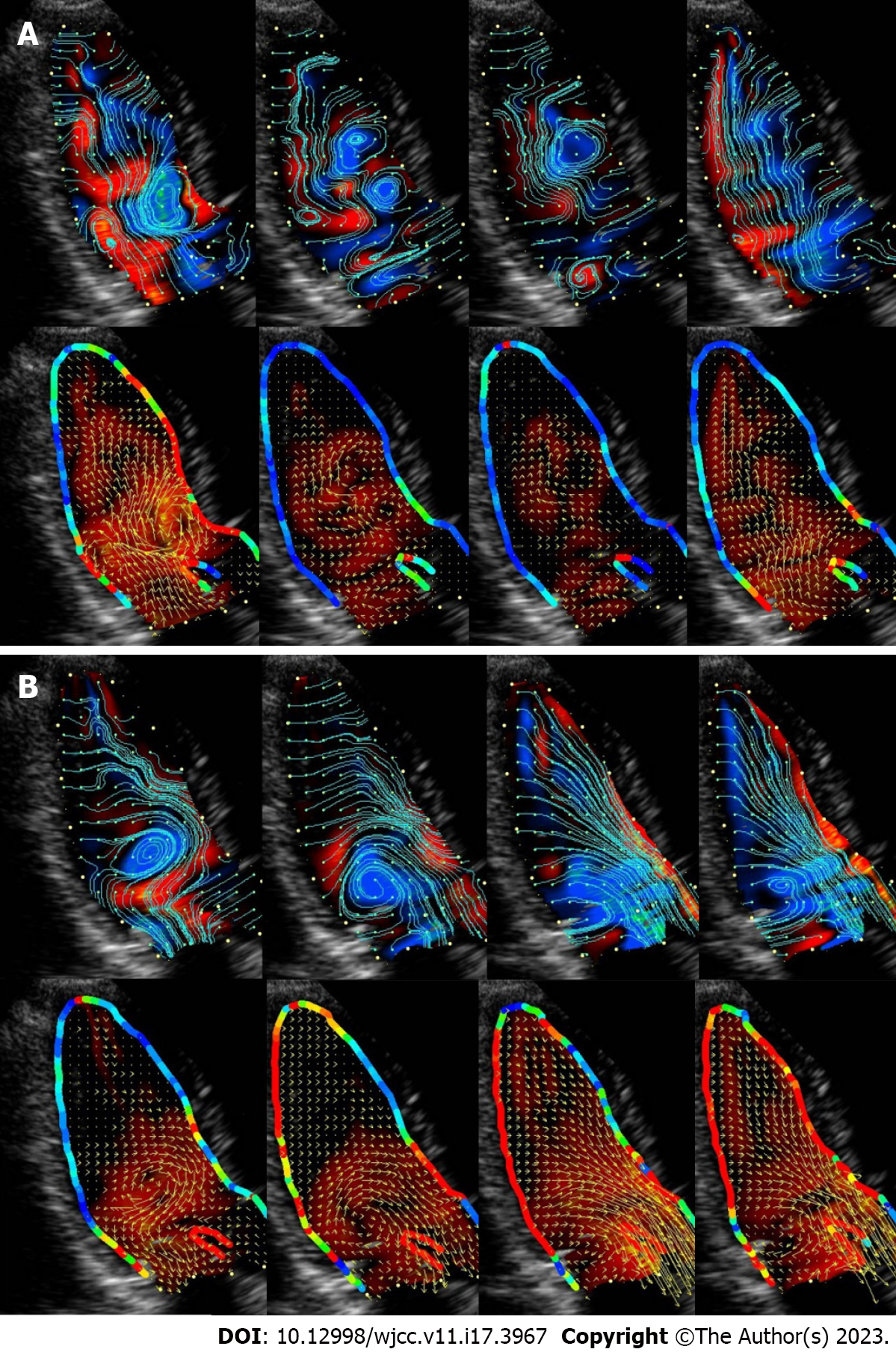
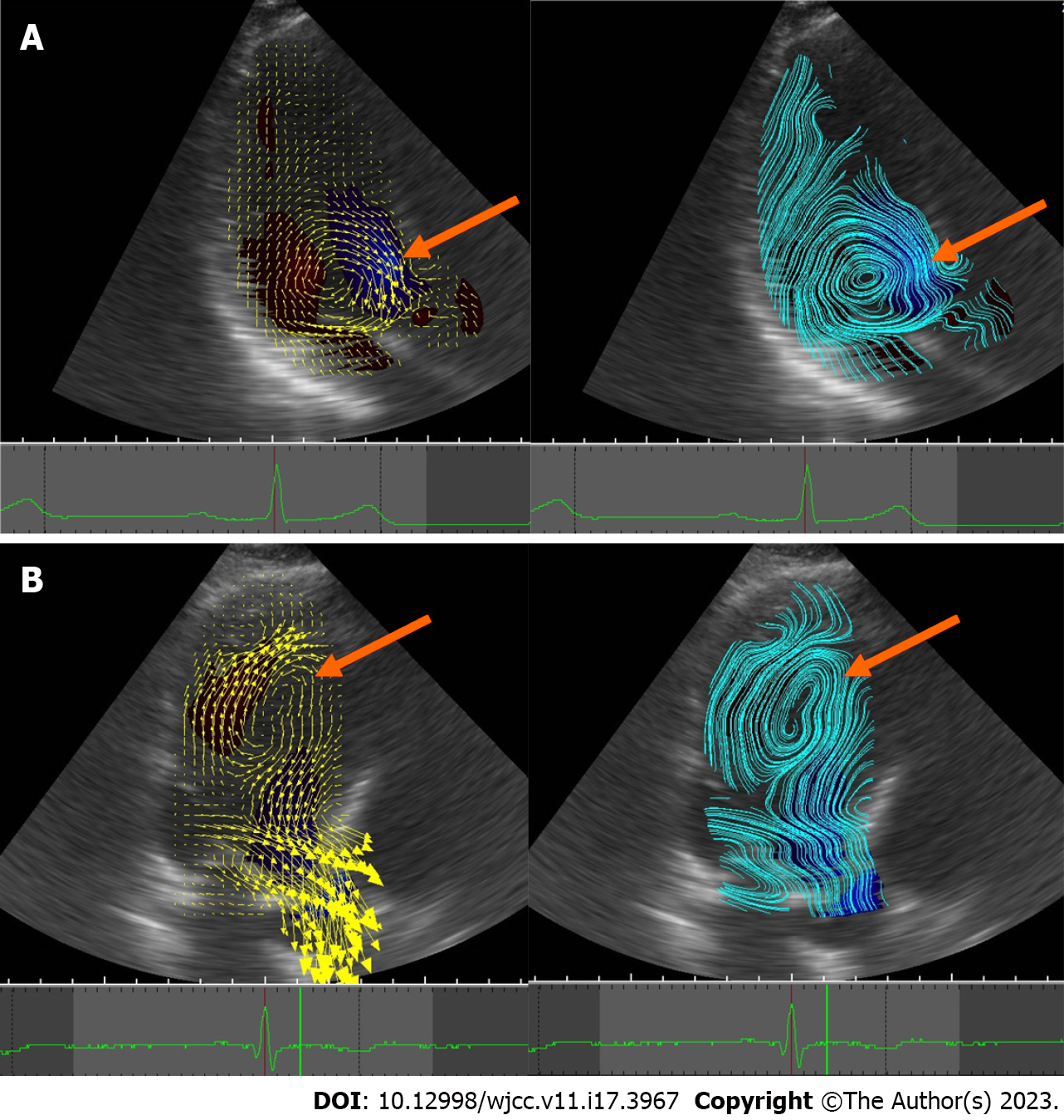
The current VFM technique was developed by further modifying Garcia’s method and improving the weighting functions of the two numerically oriented velocity components derived from the anterior and posterior wall boundaries[36]. This technology provides a rich and accurate visualization and quantification of the flow field in the cardiovascular cavity, as seen in Video 1 and Figure 1. As seen in Figure 2, there are two types of vortices: effective and ineffective.
In the normal LV, vortices conserve energy and avoid energy consumption due to deceleration and re-acceleration of blood flow. Diastolic vorticity temporarily accumulates kinetic energy to support the change of the direction of blood flow to the outflow tract in the pre-ejection phase while generating the thrust to close the mitral valve in time. Conversely, in pathological states, vortex flow sometimes causes energy expenditure. Vortex flow may therefore be a good tool to assess hemodynamic status before and after treatment, to clarify the severity of the disease, and to help determine treatment options.
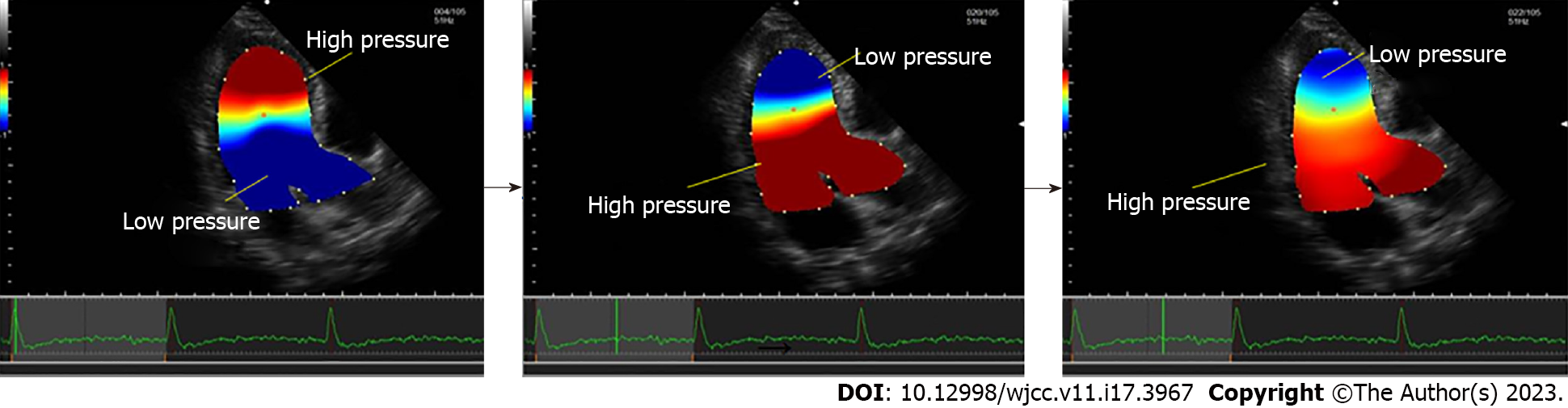
With the development of new cardiovascular technologies, accurate evaluation of cardiac function has become possible. Relative pressure imaging (RPI) is used for the visual quantification and non-invasive assessment of the IVPD and the IVPG, which can sensitively reflect cardiac function during the cardiac cycle[8]. After the initial implementation of RPI in 2014[37], Tanaka et al[8] combined the Navier–Stokes equation of motion with the momentum conservation of viscous incompressible fluids and validated the accuracy and feasibility of non-invasive measurement of the IVPD based on VFM by visualizing the distribution of the two-dimensional-field pressure in the left heart cavity. The RPI technique can be used to quantify the functional degradation caused by fluid convection in the 0.1 mmHg range, which correlates well with transcatheter measurements[8]. RPI is a novel method that may reveal how various cardiac conditions regulate pressure distribution[16] and overcome the disadvantages of two assessment methods, color Doppler M-mode echocardiography[38], and cardiac MRI[39], as shown in Figure 3.
Dynamic images of more than four cardiac cycles are usually acquired in the apical three-chamber cardiac view using a 5.0 or 8.0 MHz phased-array cardiac ultrasound probe. The Nyquist limit is usually set at 60–80 cm/s, but it should be adjusted to minimize aliasing. The color Doppler frame rate is usually kept at around 60 fps. Data can be stored as DICOM files on the ultrasound machine or a dedicated VFM workstation. The opening and closing of the mitral and aortic valves are marked on the electrocardiogram traces, and the detected areas of blending can be corrected manually by phase shifting. At end-systole, the endocardium is manually traced for speckle-tracking analysis starting from the posterior of the left ventricular mitral annulus. It should be noted that part of the left atrium and LV outflow tract are included along with the LV myocardium. The software is then started. After a few seconds, a flow-through analysis can begin, in which velocity information can be converted to pressure information, allowing the intraventricular relative pressures to be assessed through the cardiac cycle. The pressure distribution is displayed as a colored map with automatic reference points in the center of the region of interest, where warm and cool colors indicate higher and lower pressures relative to each other[40].
The longitudinal pressure gradient in the LV during isovolumic systole and rapid ejection is directed from the apex to the base of the heart. During slow ejection, the direction of the longitudinal pressure gradient is reversed from the base of the heart to the apex, and the systolic left ventricular IVPD curve shows a positive rise and a negative fall. During isovolumic diastole and rapid filling, the longitudinal pressure gradient in the ventricle is directed from the base of the heart to the apex; during slow filling the longitudinal pressure gradient in the ventricle is reversed from the apex to the base of the heart. After atrial systole, the gradient is again directed from the base of the heart to the apex, making the IVPD curve in the LV during diastole positive, then negative, and then positive[7,41].
The VFM technique is able to reflect changes in vascular and cardiac function in certain disease states through parameters such as energy loss, circulation, and ventricular wall shear stress. However, few studies have been conducted on the two more recently recognized parameters, the left ventricular IVPG (which shows the difference per unit length, a measure at a point, or averaged in a region), and IVPD (which shows the difference between two points). Compared with traditional diastolic function parameters, relative pressure parameters incorporate the effect of ventricular wall motion on blood flow into the calculation of blood flow vectors and solve the problem of Doppler angle dependence by utilizing digital computation and displaying the intracavity hemodynamic situation more accurately, intuitively, and specifically[40].
Zhong et al[42] used the RPI technique for studying and analyzing the early diastolic IVPG in patients with advanced chronic kidney disease (CKD) with preserved left ventricular ejection fraction (HFpEF). A total of 51 patients with advanced CKD and 39 healthy controls were included in the study, and the patients were divided into an HFpEF group (32 patients) and a non-HFpEF group (19 patients) according to the definition of HFpEF. An apical IVPG < 0.02 mmHg/cm (risk ratio: 9.82, 95% confidence interval: 2.01–48.01, P = 0.005) was an independent risk factor for death and cardiovascular hospitalization outcomes during a median follow-up period of 24 mo. This led to the conclusion that patients with advanced CKD with HFpEF exhibited reduced LV apical and mid-apical IVPGs and that the severity of the apical IVPG reduction was associated with poor patient outcomes.
Nakajima et al[43] used the RPI technique to non-invasively assess early diastolic LV apical and intracardiac IVPGs (ED-IVPGs). Peak LV untwisting and torsional velocities, an indicator of LV relaxation, were measured by speckle-tracking strain analysis for clinical assessment. Patients with impaired peak LV untwisting velocities had significantly lower peak ED-IVPGs compared with patients without impaired peak LV untwisting velocities. The optimal peak ED-IVPG cutoff value for an impaired peak LV untwisting velocity was 0.40 mmHg (sensitivity: 81%, specificity: 74%, area under the curve: 0.81). There was a good correlation between the peak ED-IVPG and the peak LV untwisting velocity (r = 0.64, P < 0.0001). A peak ED-IVPG as measured after aortic valve closure can be used as a non-invasive indicator for estimating an impaired LV untwisting velocity in the clinical setting.
The early diastolic IVPG is considered a useful tool for describing not only LV diastolic filling but also for describing LV systolic elastic recoil[44]. The LV has been shown to be of great value in evaluating intracavitary hemodynamics in different pathophysiological states. LV desynchronization leads to a reduction in the absolute value of the early diastolic IVPG[16]. In addition, in patients with severe aortic stenosis, these gradients are affected throughout the cardiac cycle but reappear immediately after valve replacement[22]. Very few clinically relevant studies of the RPI technique currently exist. Those that do exist focus on the clinical value of the early diastolic IVPG and specifically on the left ventricular segmental IVPG.
The RPI technique is based on using a two-dimensional flow field to assess three-dimensional relative pressures. This physical constraint is valid for the 3D velocity vector field but does not apply to the 2D slice. Therefore, the horizontal velocity is approximate, and the degree of approximation is not spatially uniform. Studies have shown that 3D VFM could provide full-volume echocardiographic information on left intraventricular hemodynamics from the clinical modality of triplane color Doppler[45] but would depend on RPI technology being further developed.
Ventricular IVPD can reflect intrinsic ventricular characteristics, and regional changes of IVPGs may be an important factor in the characteristic changes associated with impaired intraventricular blood flow that occur in specific cardiac diseases. They may also be an early sign of ventricular dysfunction. Identifying small changes in these gradients may help in the early diagnosis and intervention of diseases that lead to ventricular dysfunction. The RPI technique offers the potential to apply the IVPG indicators in the clinical setting to accurately assess LV diastolic dysfunction. Further research is needed to validate these techniques.
I am grateful to Xue Sun and Zhi-Ping Qin for their valuable suggestions, patience, and good counsel. For their encouragement, support, and research assistance, I would like to thank the following individuals who have contributed substantially to the completion of this work. In addition, I would like to thank the anonymous reviewers who have helped to improve the paper and have contributed considerably to its publication.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Vice Chairman of the Gynecology and Obstetrics Ultrasound Special Committee of Henan Ultrasonic Medical Engineering Society.
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta P, United States; Tudoran C, Romania S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Mele D, Smarrazzo V, Pedrizzetti G, Capasso F, Pepe M, Severino S, Luisi GA, Maglione M, Ferrari R. Intracardiac Flow Analysis: Techniques and Potential Clinical Applications. J Am Soc Echocardiogr. 2019;32:319-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Greenberg NL, Vandervoort PM, Thomas JD. Instantaneous diastolic transmitral pressure differences from color Doppler M mode echocardiography. Am J Physiol. 1996;271:H1267-H1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Bird JJ, Murgo JP, Pasipoularides A. Fluid dynamics of aortic stenosis: subvalvular gradients without subvalvular obstruction. Circulation. 1982;66:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Courtois M, Kovács SJ Jr, Ludbrook PA. Transmitral pressure-flow velocity relation. Importance of regional pressure gradients in the left ventricle during diastole. Circulation. 1988;78:661-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 248] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Yotti R, Bermejo J, Antoranz JC, Rojo-Alvarez JL, Allue C, Silva J, Desco MM, Moreno M, García-Fernández MA. Noninvasive assessment of ejection intraventricular pressure gradients. J Am Coll Cardiol. 2004;43:1654-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Yotti R, Bermejo J, Benito Y, Antoranz JC, Desco MM, Rodríguez-Pérez D, Cortina C, Mombiela T, Barrio A, Elízaga J, Fernández-Avilés F. Noninvasive estimation of the rate of relaxation by the analysis of intraventricular pressure gradients. Circ Cardiovasc Imaging. 2011;4:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Liu M, Cai Y, Huang H, Zhong Y, Wang F. [The preliminary value of vector flow mapping on assessment of left intraventricular pressure difference in patients with paroxysmal atrial fibrillation]. Shengwu Yixue Gongchengxue Zazhi. 2021;38:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Tanaka T, Okada T, Nishiyama T, Seki Y. Relative pressure imaging in left ventricle using ultrasonic vector flow mapping. Jpn J Appl Phys. 2017;56:07JF26. [DOI] [Full Text] |
| 9. | Guerra M, Sampaio F, Brás-Silva C, Leite-Moreira AF. Left intraventricular diastolic and systolic pressure gradients. Exp Biol Med (Maywood). 2011;236:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Suga H, Goto Y, Igarashi Y, Yamada O, Nozawa T, Yasumura Y. Ventricular suction under zero source pressure for filling. Am J Physiol. 1986;251:H47-H55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Jain S, Londono FJ, Segers P, Gillebert TC, De Buyzere M, Chirinos JA. MRI Assessment of Diastolic and Systolic Intraventricular Pressure Gradients in Heart Failure. Curr Heart Fail Rep. 2016;13:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Steine K, Stugaard M, Smiseth O. Mechanisms of diastolic intraventricular regional pressure differences and flow in the inflow and outflow tracts. J Am Coll Cardiol. 2002;40:983-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Ruskin J, McHale PA, Harley A, Greenfield JC Jr. Pressure-flow studies in man: effect of atrial systole on left ventricular function. J Clin Invest. 1970;49:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Yanada A, Ohte N, Narita H, Akita S, Miyabe H, Takada N, Goto T, Mukai S, Hayano J, Kimura G. The role of apically directed intraventricular isovolumic relaxation flow in speeding early diastolic left ventricular filling. J Am Soc Echocardiogr. 2003;16:1226-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Lin MS, Lin JL, Liu YB, Wu CC, Lin LC, Chen MF. Immediate impairment of left ventricular mechanical performance and force-frequency relation by rate-responsive dual-chamber, but not atrial pacing: Implications from intraventricular isovolumic relaxation flow. Int J Cardiol. 2006;109:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Minami S, Masuda K, Stugaard M, Kamimukai T, Asanuma T, Nakatani S. Noninvasive assessment of intraventricular pressure difference in left ventricular dyssynchrony using vector flow mapping. Heart Vessels. 2021;36:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 17. | LeWinter MM, Granzier H. Cardiac titin: a multifunctional giant. Circulation. 2010;121:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 194] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 18. | LeWinter MM, Zile MR. Could Modification of Titin Contribute to an Answer for Heart Failure With Preserved Ejection Fraction? Circulation. 2016;134:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Guerra M, Amorim MJ, Brás-Silva C, Leite-Moreira AF. Intraventricular pressure gradients throughout the cardiac cycle: effects of ischaemia and modulation by afterload. Exp Physiol. 2013;98:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Nakatani S, Beppu S, Nagata S, Ishikura F, Tamai J, Yamagishi M, Ohmori F, Kimura K, Takamiya M, Miyatake K. Diastolic suction in the human ventricle: observation during balloon mitral valvuloplasty with a single balloon. Am Heart J. 1994;127:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Udelson JE, Bacharach SL, Cannon RO 3rd, Bonow RO. Minimum left ventricular pressure during beta-adrenergic stimulation in human subjects. Evidence for elastic recoil and diastolic "suction" in the normal heart. Circulation. 1990;82:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Guerra M, F Leite-Moreira A. Relevance of intraventricular pressure gradients in left ventricular diastolic and systolic function: clinical implications. Rev Port Cir Cardiotorac Vasc. 2018;25:19-26. [PubMed] |
| 23. | Thomas JD, Popovic ZB. Intraventricular pressure differences: a new window into cardiac function. Circulation. 2005;112:1684-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Falsetti HL, Verani MS, Chen CJ, Cramer JA. Regional pressure differences in the left ventricle. Cathet Cardiovasc Diagn. 1980;6:123-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Hong GR, Pedrizzetti G, Tonti G, Li P, Wei Z, Kim JK, Baweja A, Liu S, Chung N, Houle H, Narula J, Vannan MA. Characterization and quantification of vortex flow in the human left ventricle by contrast echocardiography using vector particle image velocimetry. JACC Cardiovasc Imaging. 2008;1:705-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 215] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 26. | Fadnes S, Wigen MS, Nyrnes SA, Lovstakken L. In Vivo Intracardiac Vector Flow Imaging Using Phased Array Transducers for Pediatric Cardiology. IEEE Trans Ultrason Ferroelectr Freq Control. 2017;64:1318-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Nyrnes SA, Fadnes S, Wigen MS, Mertens L, Lovstakken L. Blood Speckle-Tracking Based on High-Frame Rate Ultrasound Imaging in Pediatric Cardiology. J Am Soc Echocardiogr. 2020;33:493-503.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 28. | Buyens F, Jolivet O, De Cesare A, Bittoun J, Herment A, Tasu JP, Mousseaux E. Calculation of left ventricle relative pressure distribution in MRI using acceleration data. Magn Reson Med. 2005;53:877-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Rodríguez Muñoz D, Moya Mur JL, Fernández-Golfín C, Becker Filho DC, González Gómez A, Fernández Santos S, Lázaro Rivera C, Rincón Díaz LM, Casas Rojo E, Zamorano Gómez JL. Left ventricular vortices as observed by vector flow mapping: main determinants and their relation to left ventricular filling. Echocardiography. 2015;32:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Hayashi T, Itatani K, Inuzuka R, Shimizu N, Shindo T, Hirata Y, Miyaji K. Dissipative energy loss within the left ventricle detected by vector flow mapping in children: Normal values and effects of age and heart rate. J Cardiol. 2015;66:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Wang Y, Hong J, Yu R, Xu D. Evaluation of left ventricular function by vector flow mapping in females with systemic lupus erythematosus. Clin Rheumatol. 2021;40:4049-4060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Rodriguez Muñoz D, Markl M, Moya Mur JL, Barker A, Fernández-Golfín C, Lancellotti P, Zamorano Gómez JL. Intracardiac flow visualization: current status and future directions. Eur Heart J Cardiovasc Imaging. 2013;14:1029-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Asami R, Tanaka T, Kawabata KI, Hashiba K, Okada T, Nishiyama T. Correction to: Accuracy and limitations of vector flow mapping: left ventricular phantom validation using stereo particle image velocimetory. J Echocardiogr. 2018;16:103. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Tanaka M, Sakamoto T, Sugawara S, Nakajima H, Katahira Y, Ohtsuki S, Kanai H. Blood flow structure and dynamics, and ejection mechanism in the left ventricle: analysis using echo-dynamography. J Cardiol. 2008;52:86-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Garcia D, Del Alamo JC, Tanne D, Yotti R, Cortina C, Bertrand E, Antoranz JC, Perez-David E, Rieu R, Fernandez-Aviles F, Bermejo J. Two-dimensional intraventricular flow mapping by digital processing conventional color-Doppler echocardiography images. IEEE Trans Med Imaging. 2010;29:1701-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 36. | Itatani K, Okada T, Uejima T, Tanaka T, Ono M, Miyaji K, Takenaka K. Intraventricular flow velocity vector visualization based on the continuity equation and measurements of vorticity and wall shear stress. Jpn J Appl Phys. 2013;52:07HF16. [DOI] [Full Text] |
| 37. | Pedrizzetti G, La Canna G, Alfieri O, Tonti G. The vortex--an early predictor of cardiovascular outcome? Nat Rev Cardiol. 2014;11:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 38. | Yotti R, Bermejo J, Benito Y, Sanz-Ruiz R, Ripoll C, Martínez-Legazpi P, del Villar CP, Elízaga J, González-Mansilla A, Barrio A, Bañares R, Fernández-Avilés F. Validation of noninvasive indices of global systolic function in patients with normal and abnormal loading conditions: a simultaneous echocardiography pressure-volume catheterization study. Circ Cardiovasc Imaging. 2014;7:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Ha H, Kim GB, Kweon J, Lee SJ, Kim YH, Lee DH, Yang DH, Kim N. Hemodynamic Measurement Using Four-Dimensional Phase-Contrast MRI: Quantification of Hemodynamic Parameters and Clinical Applications. Korean J Radiol. 2016;17:445-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Avesani M, Degrelle B, Di Salvo G, Thambo JB, Iriart X. Vector flow mapping: A review from theory to practice. Echocardiography. 2021;38:1405-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Thompson RB, McVeigh ER. Fast measurement of intracardiac pressure differences with 2D breath-hold phase-contrast MRI. Magn Reson Med. 2003;49:1056-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Zhong Y, Cai Y, Liu M, Bai W, Wang F, Tang H, Rao L. Left ventricular diastolic pressure gradient and outcome in advanced chronic kidney disease patients with preserved ejection fraction. Int J Cardiovasc Imaging. 2021;37:2663-2673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Nakajima Y, Hozumi T, Takemoto K, Fujita S, Wada T, Kashiwagi M, Shimamura K, Shiono Y, Kuroi A, Tanimoto T, Kubo T, Tanaka A, Akasaka T. Noninvasive estimation of impaired left ventricular untwisting velocity by peak early diastolic intra-ventricular pressure gradients using vector flow mapping. J Echocardiogr. 2021;19:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 44. | Firstenberg MS, Greenberg NL, Garcia MJ, Thomas JD. Relationship between ventricular contractility and early diastolic intraventricular pressure gradients: a diastolic link to systolic function. J Am Soc Echocardiogr. 2008;21:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Vixège F, Berod A, Courand PY, Mendez S, Nicoud F, Blanc-Benon P, Vray D, Garcia D. Full-volume three-component intraventricular vector flow mapping by triplane color Doppler. Phys Med Biol. 2022;67. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |