Published online Jun 6, 2023. doi: 10.12998/wjcc.v11.i16.3706
Peer-review started: December 27, 2022
First decision: January 20, 2023
Revised: February 18, 2023
Accepted: May 4, 2023
Article in press: May 4, 2023
Published online: June 6, 2023
Processing time: 157 Days and 5.5 Hours
Idiopathic normal pressure hydrocephalus (iNPH) is caused by impaired cerebrospinal fluid absorption in the elderly; it is a surgically treatable form of dementia. Gait disturbance, dementia, and urinary incontinence are the triad of signs for iNPH. In addition to these clinical findings, imaging studies show characteristic ventricular enlargement. High Evans Index and ‘disproportionately enlarged subarachnoid hydrocephalus’ are other well-known imaging findings of iNPH. If the tap test shows improved symptoms, shunt surgery is performed. The disease was first described by Hakim and Adams in 1965, followed by the publication of the first, second, and third editions of the guidelines in 2004, 2012, and 2020, respectively. Recent studies signal the glymphatic system and classical cerebrospinal fluid (CSF) absorption from the dural lymphatics as aetiological mechanisms of CSF retention. Research is also underway on imaging test and biomarker developments for more precise diagnosis, shunting technique options with fewer sequelae and complications, and the influence of genetics. Particularly, the newly introduced ‘suspected iNPH’ in the third edition of the guidelines may be useful for earlier diagnosis. However, less well-studied areas remain, such as pharmacotherapy in non-operative indications and neurological findings other than the triadic signs. This review briefly presents previous research on these and future issues.
Core Tip: Idiopathic normal pressure hydrocephalus (iNPH) presents with gait disturbance, dementia, and urinary incontinence. Improvement in these symptoms by tap testing, and imaging studies showing characteristic ventricular enlargement, are important for the diagnosis. iNPH is a dementia that is treatable by shunt surgery. This review describes recent pathophysiology, diagnosis, and treatment in iNPH.
- Citation: Ishida T, Murayama T, Kobayashi S. Current research of idiopathic normal pressure hydrocephalus: Pathogenesis, diagnosis and treatment. World J Clin Cases 2023; 11(16): 3706-3713
- URL: https://www.wjgnet.com/2307-8960/full/v11/i16/3706.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i16.3706
The initial documentation of idiopathic normal pressure hydrocephalus (iNPH) was made by Hakim and Adams[1] in 1965. In 2004, the first global guidelines for iNPH were published[2]. The guidelines placed the tap test at the centre of the diagnostic algorithm. A second edition of the guidelines was published in 2008[3]. These guidelines emphasised the importance of clinical features such as gait disturbance, urinary incontinence, and cognitive impairment, as well as ‘disproportionately enlarged subarachnoid hydrocephalus’ (DESH).
Classically, the third circulation theory (bulk flow theory) has been considered the established theory for classical cerebrospinal fluid (CSF) production and absorption. In this theory, CSF is produced from blood in the choroid plexus within the ventricles, and the CSF has a steady flow. CSF flows from the uppermost lateral ventricles and drains through the foramen of Monroe, the third ventricle, the middle cerebral aqueduct, and the fourth ventricle, into the subarachnoid space. In the subarachnoid space, CSF further flows from the posterior cranial fossa through the basilar, sylvian, and cerebral hemispheric fissures to the higher arcuate region in the vicinity of the superior sagittal arteriovenous system. However, more recently, involvement of the glymphatic system and a mechanism of CSF absorption from the dural lymphatics has been considered more appropriate[4,5]. According to these theories, CSF is produced from the interstitial fluid produced by brain cells via the glymphatic system. Some CSF drains into the ventricles and has a steady flow, as in the classical theory. However, the majority of CSF is absorbed from the intradural lymphatics by anomalous flow. Future research is needed on the relationship between these new theories and clinical ventricular enlargement mechanisms.
Future research is needed to identify the relationship between these new theories and clinical ventricular enlargement mechanisms. iNPH has been classified as secondary hydrocephalus, with it being secondary to subarachnoid haemorrhage and other conditions, and the actual cause of iNPH may be unknown. Although there have been reports of sibling cases, there are no reports of familial or hereditary hydrocephalus. However, familial normal pressure hydrocephalus (fNPH) has recently been reported, with affected families being reported from Japan in 2011[6], Canada in 2012[7], and Greece in 2014[8]. There is also a family history of shunting in 5% of patients with iNPH in Finland[9]. Thus, fNPH may exist in clinically important numbers. In addition, the gene encoding the protein ‘Scm-like with four MBT domains protein1 (SFMBT1)’ has been reported as a risk gene for iNPH, and the cilia-and flagella-associated protein 43 (CFAP43) as a causative gene for fNPH in the field of genetic medicine[10,11]. SFMBT1 localises to cells constituting the subarachnoid space, cerebral arteries, veins, and ventricular walls. CFAP43 affects proteins related to villus structure and function. Therefore, research on fNPH may also help in understanding the pathogenesis of iNPH and developing treatment.
Traditionally, the diagnosis of iNPH was classified into three levels: possible, probable, and definite. However, “suspected iNPH” was added in the third edition of the guidelines. Suspected iNPH is defined by two conditions: (1) An age of 60 years or older; and (2) enlarged ventricles (Evans Index > 0.3) on head imaging. Clinical symptoms such as dementia, gait disturbance, and dysuria are not required. This classification is expected to lead to earlier diagnosis of iNPH and earlier treatment[12]. In the study of asymptomatic iNPH, there is also the concept of asymptomatic ventriculomegaly with features of iNPH on MRI (AVIM)[13]. AVIM characterizes the supplementary observation of con
In the diagnosis of iNPH, head imaging findings are as important as abnormal neurological findings such as dementia, gait disturbance, and dysuria. It is important to recognize that solely relying on imaging results should not exclude iNPH. Techniques such as diffusion tensor imaging (DTI) and single photon emission computed tomography should also be considered, and fluorodeoxyglucose positron emission tomography imaging are also useful in the diagnosis of iNPH. However, they are omitted from this discussion owing to word count limitations.
In the diagnostic process, it's essential to distinguish between Alzheimer's dementia and iNPH, though this can be difficult in real-world situations. Interestingly, iNPH is viewed as a distinct disease in Europe and Japan, while in the United States, it's classified as an Alzheimer's disease subtype[14].
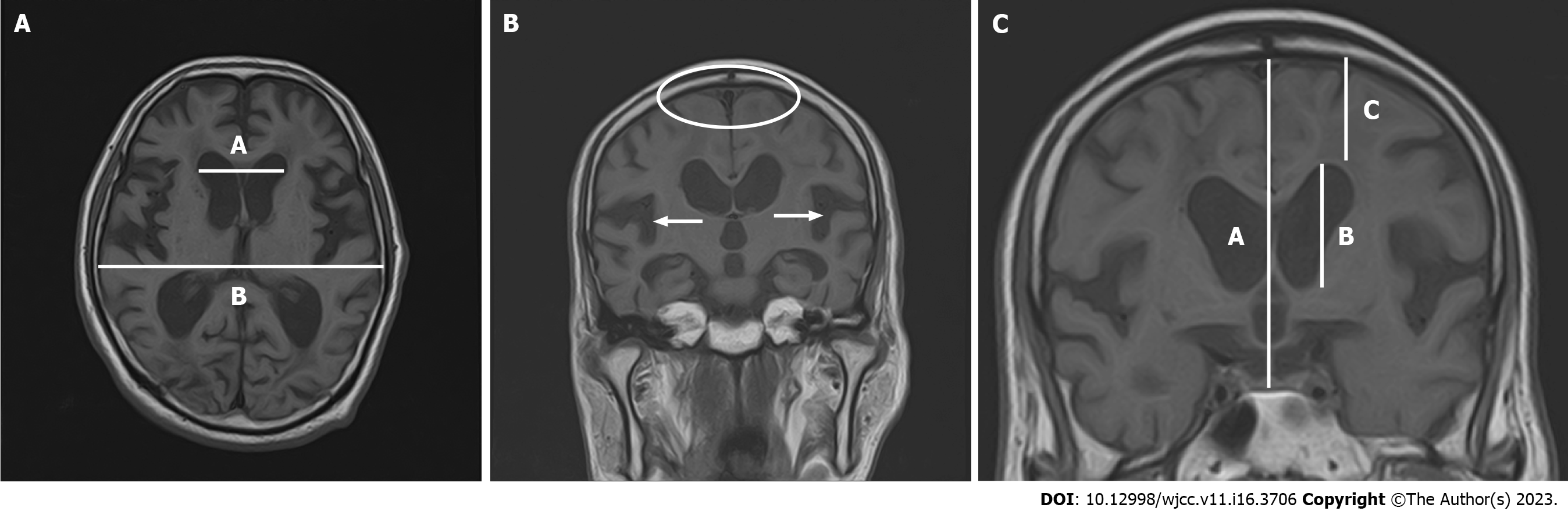
In cases of iNPH, the Evans Index typically exceeds 0.3 (Figure 1A). Frequently, the subarachnoid space expands within the sylvian fissure and in a downward direction, accompanied by narrowing in the elevated curvature area[15-18]. DESH is also an important imaging finding, as discussed in ‘Changes in iNPH guidelines’ (Figure 1B). DESH and cerebral atrophy due to Alzheimer's disease can be differentiated with high sensitivity and specificity[16-18]. DESH can be assessed visually, but previous studies have examined atrophic changes in more detail using voxel-based morphometry[18]. In addition to DESH, other evaluation criteria of interest include the Z-Evans Index (zEI) and brain/ventricle ratio (BVR) (Figure 1C). The (classic) Evans Index is a useful indicator of lateral ventricular enlargement in the horizontal section direction. However, in practice, iNPH patients often have lateral ventricles that show high-convexity tightness visible in the z-axis rather than in the horizontal section. The zEI is defined as the maximum z-axis length of the cranium from the maximum z-axis length of the frontal horn. This index is useful for detecting ventricular enlargement in the z-axis direction rather than in the horizontal sectional direction[19]. Yamada suggested that zEI may be associated with tap-test positivity. BVR is defined as the width of the anterior horn of the lateral ventricle/intracranial width immediately above the lateral ventricle[20]. A BVR < 1.0 in coronal sections on anterior commissure points, or a BVR < 1.5 in coronal sections on posterior commissure points, is considered an indicator of DESH findings[20].
The radiological scale (Rad scale) has a total score of 12 points and is used to assess iNPH over the following seven items: (1) Widening of the ventricular/intracranial cavity width ratio (Evans Index > 3.0); (2) widening of the sylvian fissure; (3) narrowing of the high circumflex and median subarachnoid space, (4) steepening of the cerebral corpuscle angle; (5) focal widening of the cerebral sulci (a pooling phenomenon of cerebrospinal fluid); (6) widening of the lateral subventricular angle; and (7) pe
Proteins and neuropeptides in cerebrospinal fluid have been studied as biomarkers for iNPH. Previous studies reported comparisons of total tau, phosphorylated tau, and amyloid-β42 between iNPH and Alzheimer's disease[24-31]. In addition, leucine-rich α2-glycoprotein (LRG), protein tyrosine phosphatase receptor type Q (PTPRQ), and neurofilament light chain (NfL) are biomarkers that have recently received attention[32-34]. These are useful markers not only for diagnosis, but also for predicting the effect of shunting. In iNPH, total tau and phosphorylated tau are generally lower than in Alzheimer's disease, with higher levels predicting a poor response to shunting.
Amyloid-β42 is also lower in patients with iNPH than in healthy subjects, and low levels are an indicator of a poor response to shunting procedures. NfL-PTPRQ-LRG is higher in patients with iNPH than in healthy subjects. High NfL-LRG values are indicative of poor effectiveness of shunting procedures, but the relevance of high PTPRQ is unknown. Future work in this area is likely to focus on whether these biomarkers can be combined to make them more sensitive for diagnosis and prediction of treatment response.
Shunting procedures for iNPH include ventriculo-peritoneal (VP), lumbo-peritoneal (LP), and ventriculo-atrial (VA) shunts. Of these, VP and LP shunts are the most common, while VA shunts are less common[35]. There is no significant difference in efficacy and complications between LP and VP shunts, and the technique considered most appropriate for each case is selected[36]. LP shunts do not puncture the brain and are considered safe in elderly patients, but are not suitable for patients with scoliosis, peritonitis, severe constipation, or obesity[37].
VP shunts are often chosen when LP shunts cannot be performed for the above reasons. Although VA shunts are used in fewer cases than LP and VP shunts, as described above, it is reported that adverse events and dysfunctions are less common than with VP shunts[38,39]. Endoscopic transtentorial ventriculostomy (ETV), which differs from the above shunting techniques, should also be described. ETV is a procedure to create a short-circuit pathway to avoid an obstruction in the ventricle. Traditionally, ETV has had narrower indications than shunting. However, recent studies have shown that ETV may improve not only CSF outflow, but also the compliance of the periventricular wall parenchyma[40-42]. Thus, iNPH surgeries continue to be investigated according to their theoretical bases and actual results to determine the best method. Although the safety of the above-mentioned shunt surgeries has improved over the years, adverse events are still common. A previous study showed that re-operations due to problems such as infection are more frequently associated with adverse events than first-time shunting procedures[43].
The two main types of shunt valves currently in use are pressure-fixed and pressure-variable valves. Pressure-fixed valves have a simpler mechanism and are cheaper. However, variable-pressure valves allow pressure to be set non-invasively from outside the body after shunt placement. The 2020 Cochrane Systematic Review did not show any superiority or inferiority between these two shunt valves[44]. However, the Japanese Guidelines for Idiopathic Normal Pressure Hydrocephalus, 3rd edition, recommend the use of variable valves for safety reasons[45]. In addition, antimicrobial-impregnated catheters have recently been used in many cases to prevent shunt infection. Previous studies have shown that antimicrobial-impregnated catheters significantly reduce shunt reconstruction associated with infection[46]. Therefore, the use of antimicrobial-impregnated catheters may be beneficial in shunt surgery in infection-prone children and immunocompromised patients.
As mentioned in the surgery section, surgical intervention carries risks and potential complications. In cases where a tap test doesn't demonstrate a significant enhancement in cognitive abilities, surgery is not required. If surgery is not performed, the treatment of patients with iNPH is limited to symptomatic treatment. This section describes symptomatic pharmacotherapy.
First, there are anti-dementia drugs. These anti-dementia drugs inhibit the progression of cognitive decline but do not improve cognitive function. Four anti-dementia drugs are used in Japan: donepezil, galantamine, rivastigmine, and memantine. All of these are indicated for Alzheimer's disease, while only donepezil is also indicated for dementia with Lewy bodies. Clinically, these anti-dementia drugs are often prescribed for patients with other types of dementia, including iNPH. However, there is no clear evidence on their efficacy. Therefore, below we introduce the previous case reports. Moriuchi et al[47] reported that donepezil was effective in four patients with iNPH with residual cognitive decline after shunting surgery. Takaya[48] reported a case report in which memantine was effective for psychiatric symptoms of iNPH. Basic experiments also suggest that memantine may reduce hydro
Lately, attention has been drawn to patients experiencing late-onset epilepsy, who frequently appear in outpatient dementia clinics[52]. Past research indicates that while uncommon, postoperative complications of iNPH can involve seizures, with a mere 0.16% incidence rate in cases[53]. Two case reports have been published on nonsurgical iNPH presenting with seizures. In the first case, hyponatraemia associated with the administration of laxatives following lower gastrointestinal endoscopy triggered an epileptic seizure[54]. In the other case, a change in diagnosis and treatment from donepezil treatment for Alzheimer's disease to levetiracetam treatment for symptomatic epileptic seizures associated with iNPH resulted in improved cognitive function[55].
Promptness and individualisation are needed in the treatment of iNPH. In particular, the fact that suspected iNPH is defined solely by age and imaging findings leads to a more rapid diagnosis. Other novel indicators such as z-EI, BVR, and Rad scale have increased the sensitivity and specificity of the diagnosis.In terms of individualisation, research on biomarkers in spinal fluid has also developed. This has enabled the effect of shunting to be predicted preoperatively. In addition, further research is needed on drug therapy in cases where surgery is not expected to improve symptoms. The development of biomarkers for less invasive samples such as urine and blood is also expected in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gokce E, Turkey; Wijaya JH, Indonesia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci. 1965;2:307-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 778] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Ishikawa M; Guideline Committe for Idiopathic Normal Pressure Hydrocephalus, Japanese Society of Normal Pressure Hydrocephalus. Clinical guidelines for idiopathic normal pressure hydrocephalus. Neurol Med Chir (Tokyo). 2004;44:222-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Mori E, Ishikawa M, Kato T, Kazui H, Miyake H, Miyajima M, Nakajima M, Hashimoto M, Kuriyama N, Tokuda T, Ishii K, Kaijima M, Hirata Y, Saito M, Arai H; Japanese Society of Normal Pressure Hydrocephalus. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir (Tokyo). 2012;52:775-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 424] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 4. | Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2426] [Cited by in RCA: 3684] [Article Influence: 307.0] [Reference Citation Analysis (0)] |
| 5. | Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2359] [Cited by in RCA: 3050] [Article Influence: 305.0] [Reference Citation Analysis (0)] |
| 6. | Takahashi Y, Kawanami T, Nagasawa H, Iseki C, Hanyu H, Kato T. Familial normal pressure hydrocephalus (NPH) with an autosomal-dominant inheritance: a novel subgroup of NPH. J Neurol Sci. 2011;308:149-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | McGirr A, Cusimano MD. Familial aggregation of idiopathic normal pressure hydrocephalus: novel familial case and a family study of the NPH triad in an iNPH patient cohort. J Neurol Sci. 2012;321:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Liouta E, Liakos F, Koutsarnakis C, Katsaros V, Stranjalis G. Novel case of familial normal pressure hydrocephalus. Psychiatry Clin Neurosci. 2014;68:583-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Huovinen J, Kastinen S, Komulainen S, Oinas M, Avellan C, Frantzen J, Rinne J, Ronkainen A, Kauppinen M, Lönnrot K, Perola M, Pyykkö OT, Koivisto AM, Remes AM, Soininen H, Hiltunen M, Helisalmi S, Kurki M, Jääskeläinen JE, Leinonen V. Familial idiopathic normal pressure hydrocephalus. J Neurol Sci. 2016;368:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Sato H, Takahashi Y, Kimihira L, Iseki C, Kato H, Suzuki Y, Igari R, Sato H, Koyama S, Arawaka S, Kawanami T, Miyajima M, Samejima N, Sato S, Kameda M, Yamada S, Kita D, Kaijima M, Date I, Sonoda Y, Kayama T, Kuwana N, Arai H, Kato T. A Segmental Copy Number Loss of the SFMBT1 Gene Is a Genetic Risk for Shunt-Responsive, Idiopathic Normal Pressure Hydrocephalus (iNPH): A Case-Control Study. PLoS One. 2016;11:e0166615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Morimoto Y, Yoshida S, Kinoshita A, Satoh C, Mishima H, Yamaguchi N, Matsuda K, Sakaguchi M, Tanaka T, Komohara Y, Imamura A, Ozawa H, Nakashima M, Kurotaki N, Kishino T, Yoshiura KI, Ono S. Nonsense mutation in CFAP43 causes normal-pressure hydrocephalus with ciliary abnormalities. Neurology. 2019;92:e2364-e2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Yamada S, Kimura T, Jingami N, Atsuchi M, Hirai O, Tokuda T, Miyajima M, Kazui H, Mori E, Ishikawa M; SINPHONI-2 Investigators. Disability risk or unimproved symptoms following shunt surgery in patients with idiopathic normal-pressure hydrocephalus: post hoc analysis of SINPHONI-2. J Neurosurg. 2017;126:2002-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Iseki C, Kawanami T, Nagasawa H, Wada M, Koyama S, Kikuchi K, Arawaka S, Kurita K, Daimon M, Mori E, Kato T. Asymptomatic ventriculomegaly with features of idiopathic normal pressure hydrocephalus on MRI (AVIM) in the elderly: a prospective study in a Japanese population. J Neurol Sci. 2009;277:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Tan C, Wang X, Wang Y, Wang C, Tang Z, Zhang Z, Liu J, Xiao G. The Pathogenesis Based on the Glymphatic System, Diagnosis, and Treatment of Idiopathic Normal Pressure Hydrocephalus. Clin Interv Aging. 2021;16:139-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Sasaki M, Honda S, Yuasa T, Iwamura A, Shibata E, Ohba H. Narrow CSF space at high convexity and high midline areas in idiopathic normal pressure hydrocephalus detected by axial and coronal MRI. Neuroradiology. 2008;50:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Takagi K, Watahiki R, Machida T, Onouchi K, Kato K, Oshima M. Reliability and Interobserver Variability of Evans' Index and Disproportionately Enlarged Subarachnoid Space Hydrocephalus as Diagnostic Criteria for Idiopathic Normal Pressure Hydrocephalus. Asian J Neurosurg. 2020;15:107-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Kitagaki H, Mori E, Ishii K, Yamaji S, Hirono N, Imamura T. CSF spaces in idiopathic normal pressure hydrocephalus: morphology and volumetry. AJNR Am J Neuroradiol. 1998;19:1277-1284. [PubMed] |
| 18. | Ishii K, Kawaguchi T, Shimada K, Ohkawa S, Miyamoto N, Kanda T, Uemura T, Yoshikawa T, Mori E. Voxel-based analysis of gray matter and CSF space in idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 2008;25:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Yamada S, Ishikawa M, Yamamoto K. Optimal Diagnostic Indices for Idiopathic Normal Pressure Hydrocephalus Based on the 3D Quantitative Volumetric Analysis for the Cerebral Ventricle and Subarachnoid Space. AJNR Am J Neuroradiol. 2015;36:2262-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Yamada S, Ishikawa M, Yamamoto K. Fluid Distribution Pattern in Adult-Onset Congenital, Idiopathic, and Secondary Normal-Pressure Hydrocephalus: Implications for Clinical Care. Front Neurol. 2017;8:583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Kockum K, Lilja-Lund O, Larsson EM, Rosell M, Söderström L, Virhammar J, Laurell K. The idiopathic normal-pressure hydrocephalus Radscale: a radiological scale for structured evaluation. Eur J Neurol. 2018;25:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Kockum K, Virhammar J, Riklund K, Söderström L, Larsson EM, Laurell K. Diagnostic accuracy of the iNPH Radscale in idiopathic normal pressure hydrocephalus. PLoS One. 2020;15:e0232275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Fällmar D, Andersson O, Kilander L, Löwenmark M, Nyholm D, Virhammar J. Imaging features associated with idiopathic normal pressure hydrocephalus have high specificity even when comparing with vascular dementia and atypical parkinsonism. Fluids Barriers CNS. 2021;18:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Miyajima M, Nakajima M, Ogino I, Miyata H, Motoi Y, Arai H. Soluble amyloid precursor protein α in the cerebrospinal fluid as a diagnostic and prognostic biomarker for idiopathic normal pressure hydrocephalus. Eur J Neurol. 2013;20:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Moriya M, Miyajima M, Nakajima M, Ogino I, Arai H. Impact of cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus on the amyloid cascade. PLoS One. 2015;10:e0119973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Nakajima M, Miyajima M, Ogino I, Akiba C, Sugano H, Hara T, Fusegi K, Karagiozov K, Arai H. Cerebrospinal fluid biomarkers for prognosis of long-term cognitive treatment outcomes in patients with idiopathic normal pressure hydrocephalus. J Neurol Sci. 2015;357:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Akiba C, Nakajima M, Miyajima M, Ogino I, Motoi Y, Kawamura K, Adachi S, Kondo A, Sugano H, Tokuda T, Irie K, Arai H. Change of Amyloid-β 1-42 Toxic Conformer Ratio After Cerebrospinal Fluid Diversion Predicts Long-Term Cognitive Outcome in Patients with Idiopathic Normal Pressure Hydrocephalus. J Alzheimers Dis. 2018;63:989-1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Jeppsson A, Zetterberg H, Blennow K, Wikkelsø C. Idiopathic normal-pressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology. 2013;80:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Jeppsson A, Wikkelsö C, Blennow K, Zetterberg H, Constantinescu R, Remes AM, Herukka SK, Rauramaa T, Nagga K, Leinonen V, Tullberg M. CSF biomarkers distinguish idiopathic normal pressure hydrocephalus from its mimics. J Neurol Neurosurg Psychiatry. 2019;90:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Kawamura K, Miyajima M, Nakajima M, Kanai M, Motoi Y, Nojiri S, Akiba C, Ogino I, Xu H, Kamohara C, Yamada S, Karagiozov K, Ikeuchi T, Kondo A, Arai H. Cerebrospinal Fluid Amyloid-β Oligomer Levels in Patients with Idiopathic Normal Pressure Hydrocephalus. J Alzheimers Dis. 2021;83:179-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Kokkinou M, Beishon LC, Smailagic N, Noel-Storr AH, Hyde C, Ukoumunne O, Worrall RE, Hayen A, Desai M, Ashok AH, Paul EJ, Georgopoulou A, Casoli T, Quinn TJ, Ritchie CW. Plasma and cerebrospinal fluid ABeta42 for the differential diagnosis of Alzheimer's disease dementia in participants diagnosed with any dementia subtype in a specialist care setting. Cochrane Database Syst Rev. 2021;2:CD010945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Johnson ECB, Dammer EB, Duong DM, Ping L, Zhou M, Yin L, Higginbotham LA, Guajardo A, White B, Troncoso JC, Thambisetty M, Montine TJ, Lee EB, Trojanowski JQ, Beach TG, Reiman EM, Haroutunian V, Wang M, Schadt E, Zhang B, Dickson DW, Ertekin-Taner N, Golde TE, Petyuk VA, De Jager PL, Bennett DA, Wingo TS, Rangaraju S, Hajjar I, Shulman JM, Lah JJ, Levey AI, Seyfried NT. Large-scale proteomic analysis of Alzheimer's disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat Med. 2020;26:769-780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 751] [Cited by in RCA: 652] [Article Influence: 130.4] [Reference Citation Analysis (0)] |
| 33. | Pfanner T, Henri-Bhargava A, Borchert S. Cerebrospinal Fluid Biomarkers as Predictors of Shunt Response in Idiopathic Normal Pressure Hydrocephalus: A Systematic Review. Can J Neurol Sci. 2018;45:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Agren-Wilsson A, Lekman A, Sjöberg W, Rosengren L, Blennow K, Bergenheim AT, Malm J. CSF biomarkers in the evaluation of idiopathic normal pressure hydrocephalus. Acta Neurol Scand. 2007;116:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Kuriyama N, Miyajima M, Nakajima M, Kurosawa M, Fukushima W, Watanabe Y, Ozaki E, Hirota Y, Tamakoshi A, Mori E, Kato T, Tokuda T, Urae A, Arai H. Nationwide hospital-based survey of idiopathic normal pressure hydrocephalus in Japan: Epidemiological and clinical characteristics. Brain Behav. 2017;7:e00635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Kazui H, Miyajima M, Mori E, Ishikawa M; SINPHONI-2 Investigators. Lumboperitoneal shunt surgery for idiopathic normal pressure hydrocephalus (SINPHONI-2): an open-label randomised trial. Lancet Neurol. 2015;14:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 37. | Miyajima M, Kazui H, Mori E, Ishikawa M; , on behalf of the SINPHONI-2 Investigators. One-year outcome in patients with idiopathic normal-pressure hydrocephalus: comparison of lumboperitoneal shunt to ventriculoperitoneal shunt. J Neurosurg. 2016;125:1483-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 38. | Hung AL, Vivas-Buitrago T, Adam A, Lu J, Robison J, Elder BD, Goodwin CR, Jusué-Torres I, Rigamonti D. Ventriculoatrial versus ventriculoperitoneal shunt complications in idiopathic normal pressure hydrocephalus. Clin Neurol Neurosurg. 2017;157:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | McGovern RA, Kelly KM, Chan AK, Morrissey NJ, McKhann GM 2nd. Should ventriculoatrial shunting be the procedure of choice for normal-pressure hydrocephalus? J Neurosurg. 2014;120:1458-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Greitz D. Paradigm shift in hydrocephalus research in legacy of Dandy's pioneering work: rationale for third ventriculostomy in communicating hydrocephalus. Childs Nerv Syst. 2007;23:487-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Tomycz LD, Hale AT, George TM. Emerging Insights and New Perspectives on the Nature of Hydrocephalus. Pediatr Neurosurg. 2017;52:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Cinalli G, Maixner WJ, Sainte-Rose C. Pediatric hydrocephalus. 2nd ed. Cham: Springer, 2019: 784-785. |
| 43. | Patwardhan RV, Nanda A. Implanted ventricular shunts in the United States: the billion-dollar-a-year cost of hydrocephalus treatment. Neurosurgery. 2005;56:139-44; discussion 144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 237] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Garegnani L, Franco JV, Ciapponi A, Garrote V, Vietto V, Portillo Medina SA. Ventriculo-peritoneal shunting devices for hydrocephalus. Cochrane Database Syst Rev. 2020;6:CD012726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Nakajima M, Yamada S, Miyajima M, Ishii K, Kuriyama N, Kazui H, Kanemoto H, Suehiro T, Yoshiyama K, Kameda M, Kajimoto Y, Mase M, Murai H, Kita D, Kimura T, Samejima N, Tokuda T, Kaijima M, Akiba C, Kawamura K, Atsuchi M, Hirata Y, Matsumae M, Sasaki M, Yamashita F, Aoki S, Irie R, Miyake H, Kato T, Mori E, Ishikawa M, Date I, Arai H; research committee of idiopathic normal pressure hydrocephalus. Guidelines for Management of Idiopathic Normal Pressure Hydrocephalus (Third Edition): Endorsed by the Japanese Society of Normal Pressure Hydrocephalus. Neurol Med Chir (Tokyo). 2021;61:63-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 300] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 46. | Mallucci CL, Jenkinson MD, Conroy EJ, Hartley JC, Brown M, Dalton J, Kearns T, Moitt T, Griffiths MJ, Culeddu G, Solomon T, Hughes D, Gamble C; BASICS Study collaborators. Antibiotic or silver versus standard ventriculoperitoneal shunts (BASICS): a multicentre, single-blinded, randomised trial and economic evaluation. Lancet. 2019;394:1530-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 47. | Moriuchi S, Makoto D, Takeshi S, Shogo F, Takanori F, Yasushi H, Takao S. Lumbar-peritoneal shunt followed by donepezil administration for residual cognitive impairment in idiopathic normal pressure hydrocephalus: a case report. J Neurol Neurophysiol. 2015;6:2. [DOI] [Full Text] |
| 48. | Takaya M. Memantine treatment for neuropsychiatric symptoms in a patient with probable idiopathic normal pressure hydrocephalus: a case report. J Med Case Rep. 2013;7:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 49. | Köktürk S, Ceylan S, Etus V, Yasa N. Morinda citrifolia L. (noni) and memantine attenuate periventricular tissue injury of the fourth ventricle in hydrocephalic rabbits. Neural Regen Res. 2013;8:773-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 50. | Tanaka T, Momozaki N, Liu X. Case report: a study of seven cases of chronic subdural haematoma in patients with normal pressure hydrocephalus shunt implantation treated with goreisan (in Japanese). Science of Kampo Medicine. 2014;38:131-134. |
| 51. | Alperin N, Oliu CJ, Bagci AM, Lee SH, Kovanlikaya I, Adams D, Katzen H, Ivkovic M, Heier L, Relkin N. Low-dose acetazolamide reverses periventricular white matter hyperintensities in iNPH. Neurology. 2014;82:1347-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Giordan E, Palandri G, Lanzino G, Murad MH, Elder BD. Outcomes and complications of different surgical treatments for idiopathic normal pressure hydrocephalus: a systematic review and meta-analysis. J Neurosurg. 2018;1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 54. | Eleftheriou A, Amezcua S, Nilsson M. Idiopathic normal pressure hydrocephalus presenting with epileptic seizure as a cardinal symptom: a case presentation. Interdiscip Neurosurg. 2020;19:100618. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Ishida T, Murayama T, Kobayashi S. A case report of nonsurgical idiopathic normal pressure hydrocephalus differentiated from Alzheimer's dementia: levetiracetam was effective in symptomatic epilepsy. PCN Rep. 2022;1:43-48. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |