Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3542
Peer-review started: November 10, 2022
First decision: December 26, 2022
Revised: January 29, 2023
Accepted: April 17, 2023
Article in press: April 17, 2023
Published online: May 26, 2023
Processing time: 196 Days and 2.3 Hours
The pathophysiology of Fabry disease (FD)-induced progressive vital organ damage is irreversible. Disease progression can be delayed using enzyme replacement therapy (ERT). In patients with classic FD, sporadic accumulation of globotriaosylceramide (GL-3) in the heart and kidney begins in utero; however, until childhood, GL-3 accumulation is mild and reversible and can be restored by ERT. The current consensus is that ERT initiation during early childhood is paramount. Nonetheless, complete recovery of organs in patients with advanced FD is challenging.
Two related male patients, an uncle (patient 1) and nephew (patient 2), presented with classic FD. Both patients were treated by us. Patient 1 was in his 50s, and ERT was initiated following end-organ damage; this was subsequently ineffective. He developed cerebral infarction and died of sudden cardiac arrest. Patient 2 was in his mid-30s, and ERT was initiated when the patient was diagnosed with FD, during which the damage to vital organs was not overtly apparent. Although he had left ventricular hypertrophy at the beginning of this treatment, the degree of hypertrophy progression was limited to a minimal range after > 18 years of ERT.
We obtained discouraging ERT outcomes for older patients but encouraging outcomes for younger adults with classic FD.
Core Tip: Fabry disease (FD) is an inherited metabolic disorder, caused by a genetic mutation or decreased α-galactosidase activity in lysosomes. Enzyme replacement therapy (ERT) is a promising treatment for FD. Few reports have compared the effect of ERT in older adult populations vs. that in populations in their 30s. Here we report the effect of ERT in an older adult and a patient in his mid-30s from the same FD family. We demonstrated that ERT is sufficiently effective for patients in their mid-30s if the major organs such as the brain, heart and kidney are not severely damaged.
- Citation: Harigane Y, Morimoto I, Suzuki O, Temmoku J, Sakamoto T, Nakamura K, Machii K, Miyata M. Enzyme replacement therapy in two patients with classic Fabry disease from the same family tree: Two case reports. World J Clin Cases 2023; 11(15): 3542-3551
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3542.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3542
Fabry disease (FD) is an X-linked, inherited metabolic disorder[1] that is caused by a genetic mutation or decreased α-galactosidase activity in the lysosomes[2]. Globotriaosylceramide (GL-3), also known as ceramide trihexoside, is a substrate of α-galactosidase. When GL-3 is not selected for degradation, it accumulates in cells (specifically endothelial and smooth muscle cells), tissues, and organs (e.g., the heart, brain, kidneys, and cornea), leading to the onset of FD symptoms[3]. Limb pain, anhidrosis, and angiokeratoma are frequently observed as initial symptoms, and heart failure, renal failure, and cerebral infarction may also occur during adulthood.
Enzyme replacement therapy (ERT)[4,5] is a promising treatment for FD. However, most studies on ERT report outcomes after long durations of ERT in adults, usually after the onset of substantial organ damage[6,7]. There are few reports comparing the effect of ERT in older adult populations and those in their 30s[7]. Here we report the cases of two related male patients, one an older adult (patient 1) and the other in his 30s (patient 2), both with classic FD, from the same family. We compared the two cases, examined the degree of disease progression at initiation, and evaluated the treatment effects. Herein we discuss the differences in ERT efficacy between the cases after ERT initiation.
Case 1: Eruption (angiokeratoma), pain in extremities and anhidrosis.
Case 2: Pain in extremities, eruption (angiokeratoma), diarrhea and psychiatric symptoms.
Case 1: Diagnosed as FD because of the symptoms and his family history at age of 32 years.
Case 2: Diagnosed as FD because of the symptoms and his family history at age of 34 years.
Case 1: Schizophrenia and manic depression at age of 22 and 23 years, respectively.
Case 2: Psychiatric symptoms at age of 33 years.
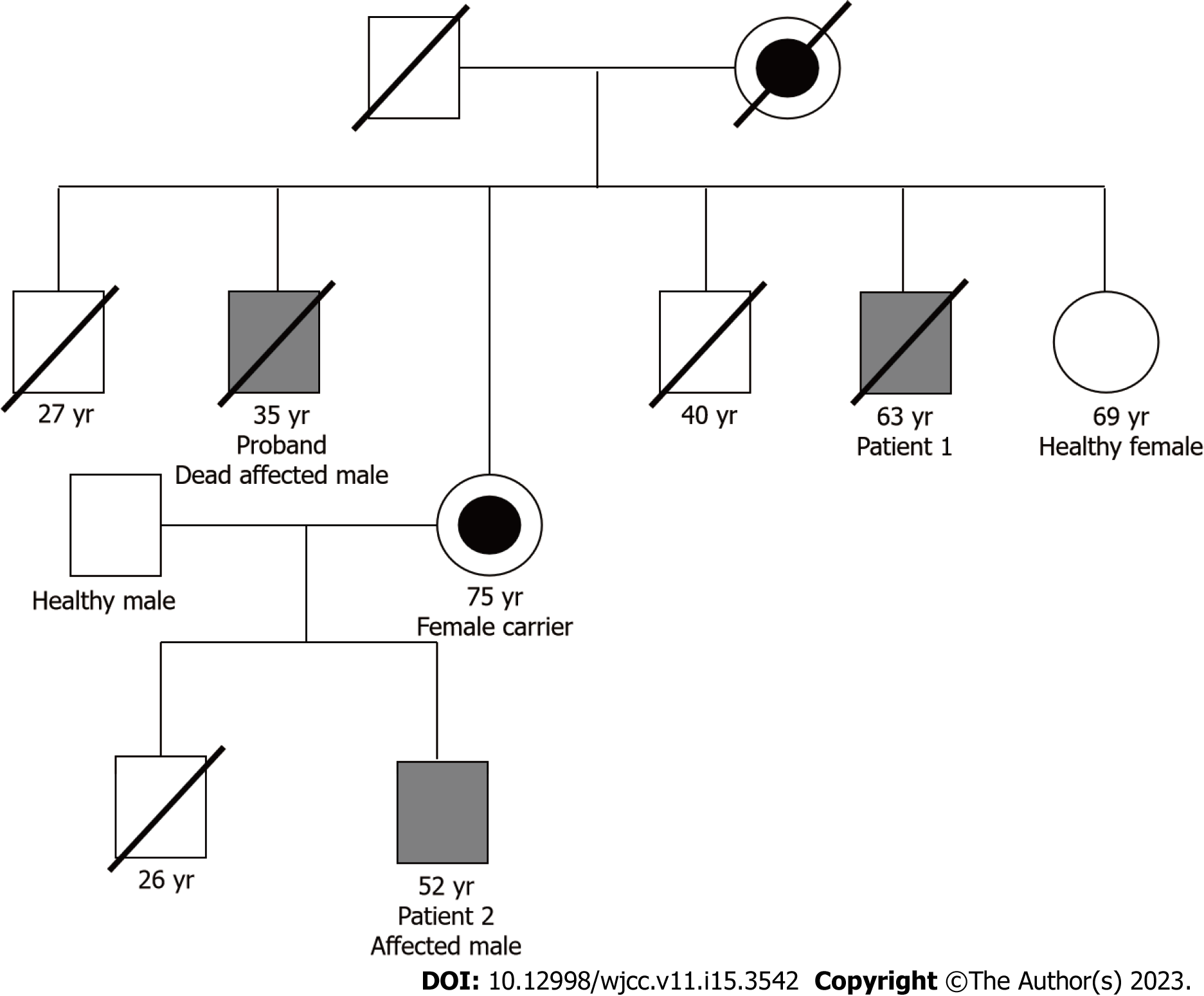
Cases 1 and 2: Patient 1 is the uncle of patient 2, patient 1 is brother of proband, mother of patient 2 is the sister of proband and patient 1.
Cases 1 and 2: Both patient 1 and patient 2 had eruption (angiokeratoma).
Case 1: Biopsy of eruption revealed FD distinctive angiokeratoma.
Case 2: The patient displayed decreased α-galactosidase activity and α-galactosidase-A gene mutation.
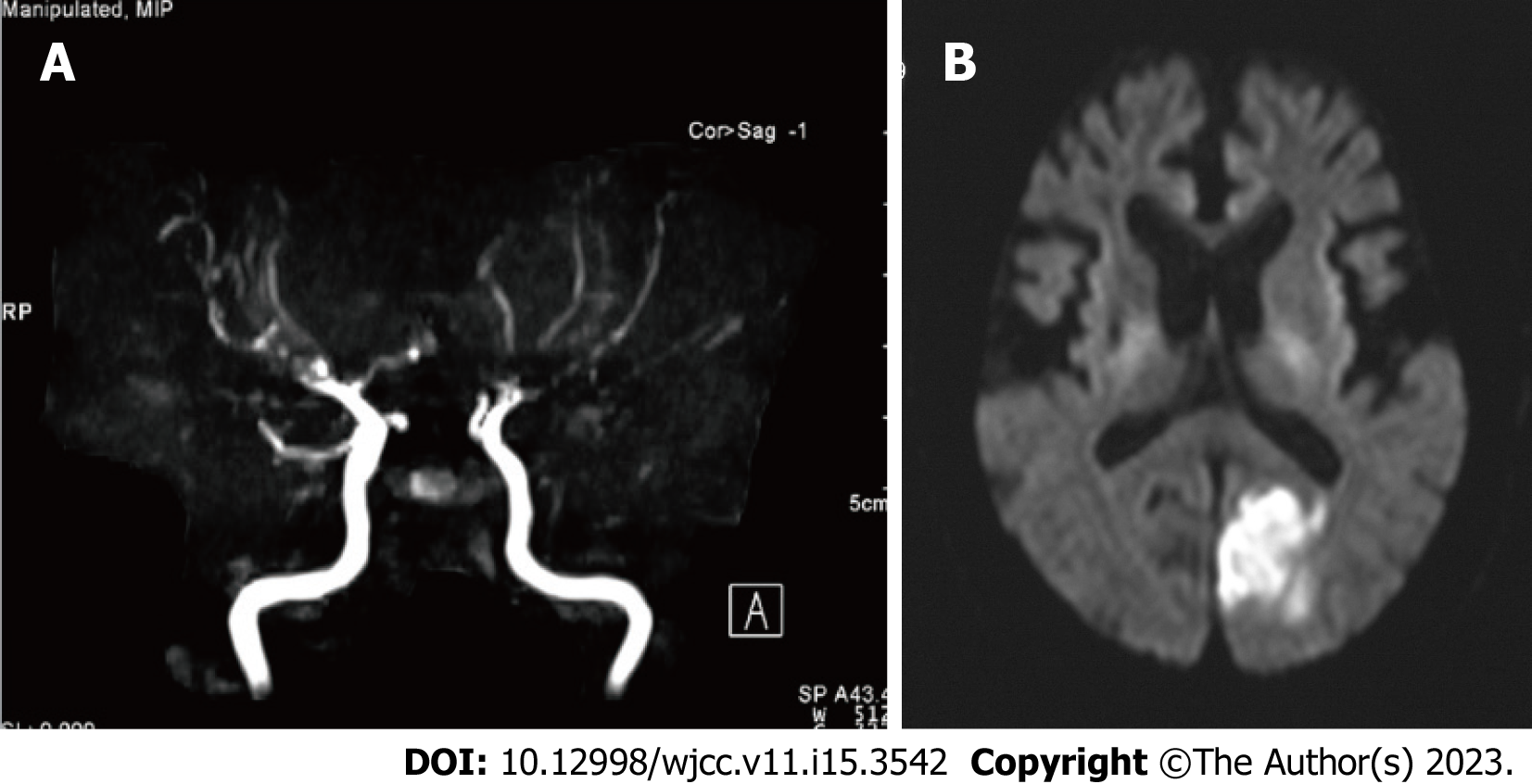
Case 1: MRI of the brain revealed damage to cerebral artery as demonstrated in Figure 1A.
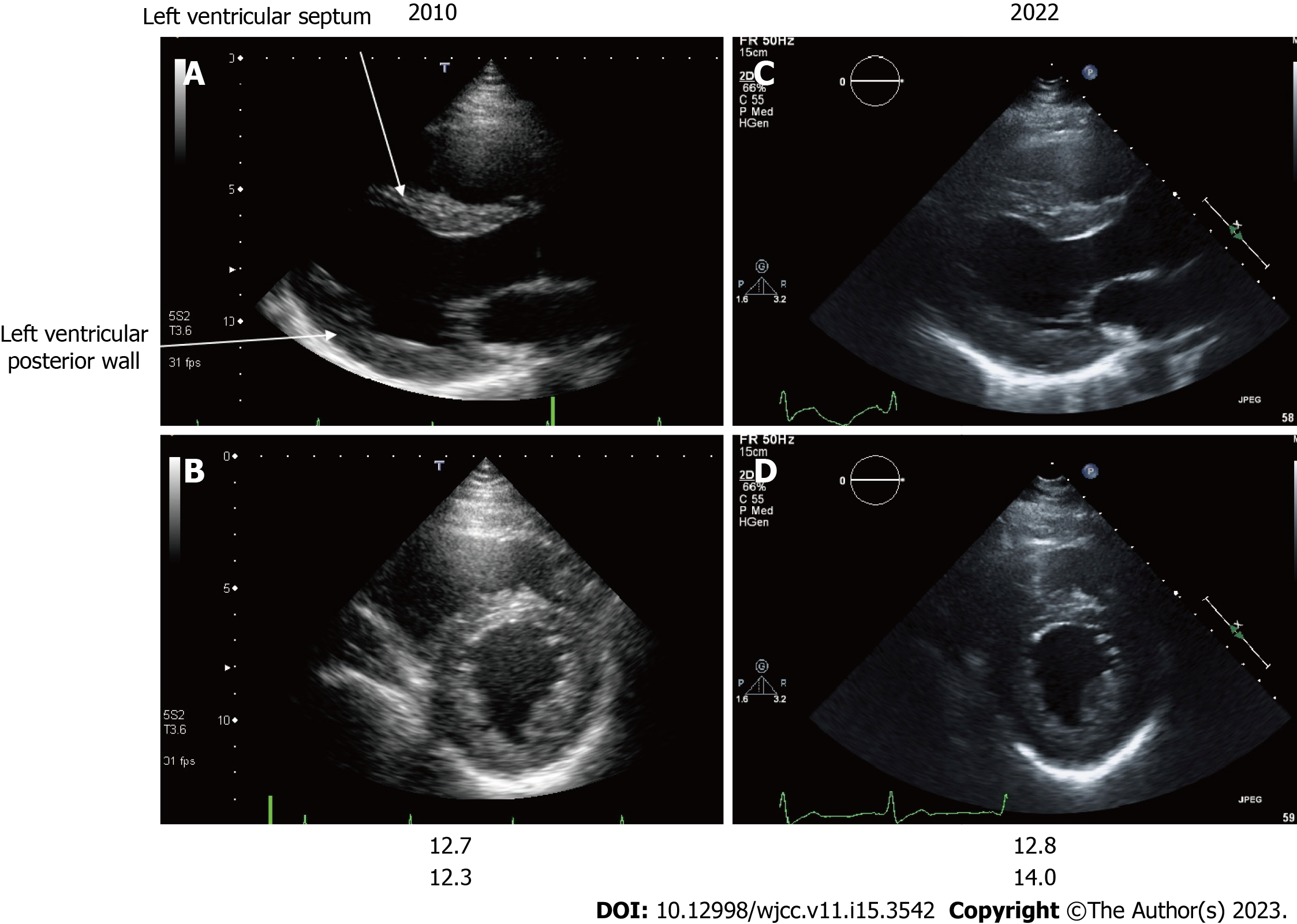
Case 2: Echocardiographic findings of left ventricular septum and left ventricular posterior wall thickness were slightly thickened.
Classic FD.
Agalsidase α (0.2 mg/kg of Replagal; Shire HGT, Inc., Cambridge, MA, United States) or agalsidase β (1 mg/kg of Fabrazyme; Sanofi Genzyme, Cambridge, MA, United States) administered bi-weekly are the commonly used drugs for ERT.
Both patients were initially treated with 1 mg/kg of agalsidase β. However, patient 2 was administered varying doses and isoforms of agalsidase over the course of 18 years. The dose of agalsidase β was decreased to 0.36-0.63 mg/kg between March and October 2010 because of a manufacturing supply shortage. Accordingly, patient 2 was administered 0.2 mg/kg of agalsidase α between October 2010 and May 2017, and 1 mg/kg of agalsidase β thereafter.
GL-3 was extracted from plasma using chloroform/methanol and purified using solid-phase chromatography. Total GL-3 levels were measured using liquid chromatography/tandem mass spectrometry[8].
The plasma antibody status was determined using ELISA. Antibody titers were measured as the reciprocal of the highest sample dilution, where antibodies were detected by ELISA. Changes in antibody status (positive or negative seroconversion) were confirmed by an immunoprecipitation assay.
The proband presented to the First Department of Internal Medicine in the Fukushima Medical University Hospital late in his teens (1975) with severe pain in the extremities and fever (37 °C). Abdominal angiokeratoma was observed, and urinary GL-3 levels were significantly increased on admission. FD was diagnosed based on clinical symptoms, including pain in the extremities, anhidrosis, angiokeratoma, corneal opacification, and sensorineural hearing loss; FD had also been previously documented in his family tree[9]. He died at the age of 35.
In 1977, when patient 1 was 32 years old, he was hospitalized at First Department of Fukushima Medical College because of eruptions, pain in the extremities, and anhidrosis. He had history of schizophrenia and manic depression at the ages of 22 and 23 years, respectively. He was diagnosed with FD based on a skin biopsy that showed the eruptions to be angiokeratoma and a family history of FD, as shown in Figure 2. Subsequently, he was transferred to a psychiatric hospital.
In 2005, he attempted suicide in the psychiatric hospital. However, he survived the suicide attempt and was then transferred to Fukushima Red Cross Hospital.
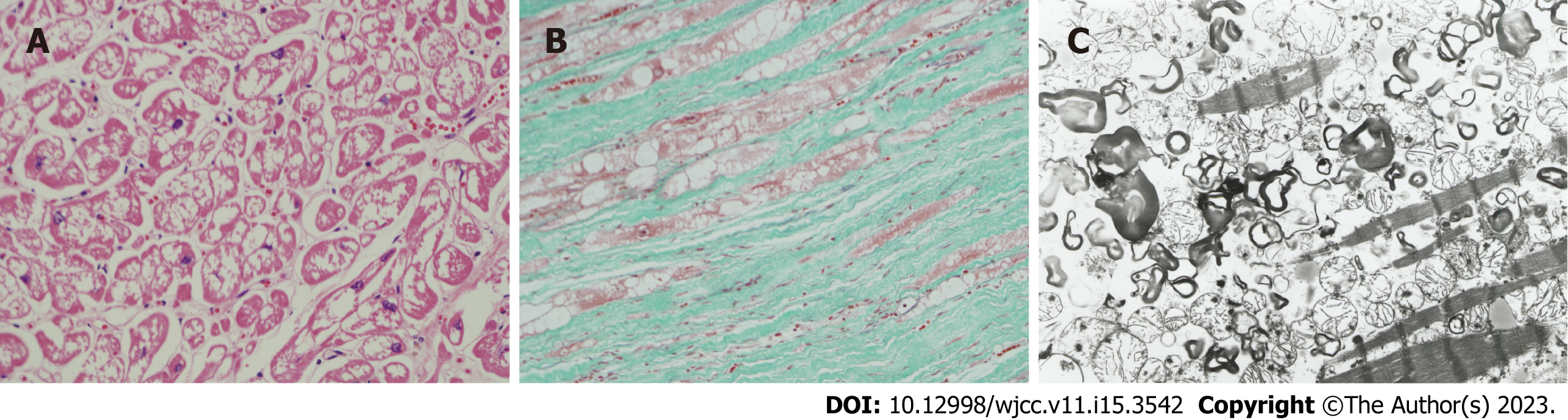
ERT, approved for clinical use in 2004 in Japan, was initiated for patient 1 at the age of 59 years. Around that period, he started experiencing dysarthria and dysphagia. Although ERT was initiated in this patient, repeated cerebral infarctions complicated the clinical course. Magnetic resonance angiography findings are shown in Figure 1A. The patient underwent ERT for four years; however, he then developed a cerebral infarction, as imaged by diffusion-weighted magnetic resonance imaging (Figure 1B). He died of sudden cardiac arrest on the third day of hospitalization. Histopathological analysis using light microscopy revealed myocardial muscle cell vacuolation, which transformed into marked fibrosis. We observed cardiac muscle vacuolation in the atrioventricular node, endothelial and epithelial cell swelling, and vacuolation in the glomeruli and smooth muscle cells of arterioles in both kidneys. Similarly, vacuolation affected cells in the submucosa of both small and large intestines as well as the arteriolar smooth muscle cells of the cerebrum. Numerous lamellar structures were observed on electron microscopy. Histopathology and electron microscopy findings of the heart are shown in Figure 3A-C. These findings are unique to FD[10].
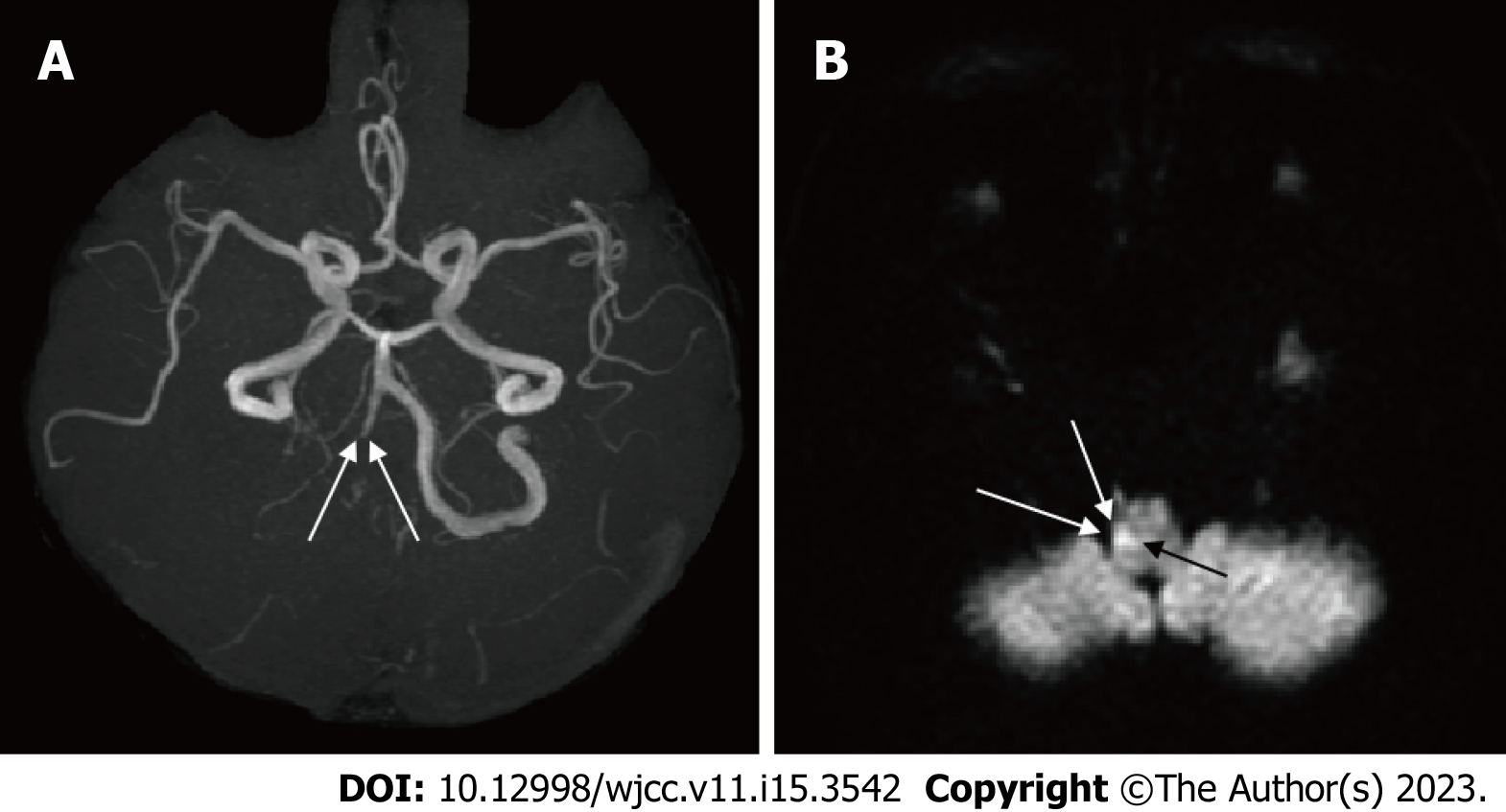
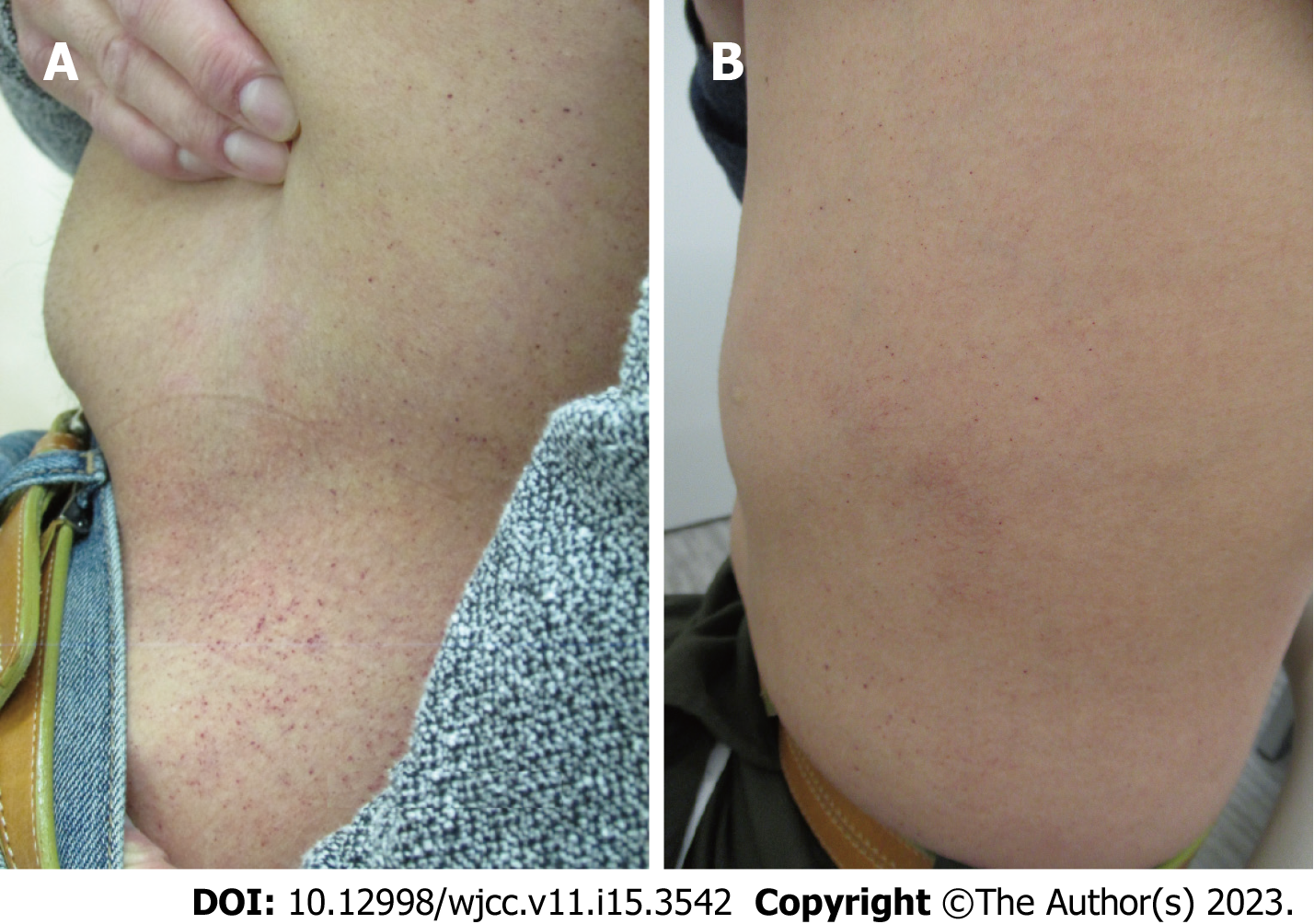
Patient 2 developed pain in the extremities, anhidrosis, and angiokeratoma at the start of puberty. This patient was noted to have experienced diarrhea in his mid-20s and psychiatric symptoms, such as depression, in his 30s. The mother of patient 2 was the sister of the proband and patient 1 (Figure 2). Patient 2 was diagnosed with FD due to decreased α-galactosidase activity (0.3 nmol/mL/2 h; baseline, 4 nmol/mL/2 h) and α-galactosidase-A gene mutation. Shortly thereafter, ERT was initiated when the patient was 34 years old. Whole body screening for FD indicated bilateral sensorineural hearing loss, corneal opacity, and cardiac hypertrophy. He developed Wallenberg syndrome, including dysphagia, balance deficit, and Horner’s triad, due to right vertebral artery dissection in his late 40s (Figure 4). The patient’s Wallenberg syndrome improved completely within one month. Angiokeratoma improved significantly during the clinical course (Figure 5A and B). For 18 years, cardiac hypertrophy was limited to minimal progression (Figure 6A-D; Table 1), and this was reflected in the brain natriuretic peptide level that did not change significantly (Table 1).
| LVS/PW | BNP | |
| November 17, 2010 | 12.7/12.3 | 5.9 |
| November 24, 2011 | 12.0/12.4 | |
| November 9, 2012 | 11.9/12.5 | 6.5 |
| October 13, 2013 | 12.1/12.0 | |
| June 19, 2014 | 12.9/12.1 | 6.9 |
| June 4, 2015 | 12.2/12.4 | |
| June 23, 2016 | 12.3/12.3 | |
| May 25, 2017 | 12.3/12.6 | 5.8 |
| November 16, 2018 | 12.8/13.0 | 5.8 |
| May 7, 2020 | 15.3/15.9 | |
| June 8, 2021 | 12.1/14.2 | |
| July 7, 2022 | 12.8/14.0 | 12.3 |
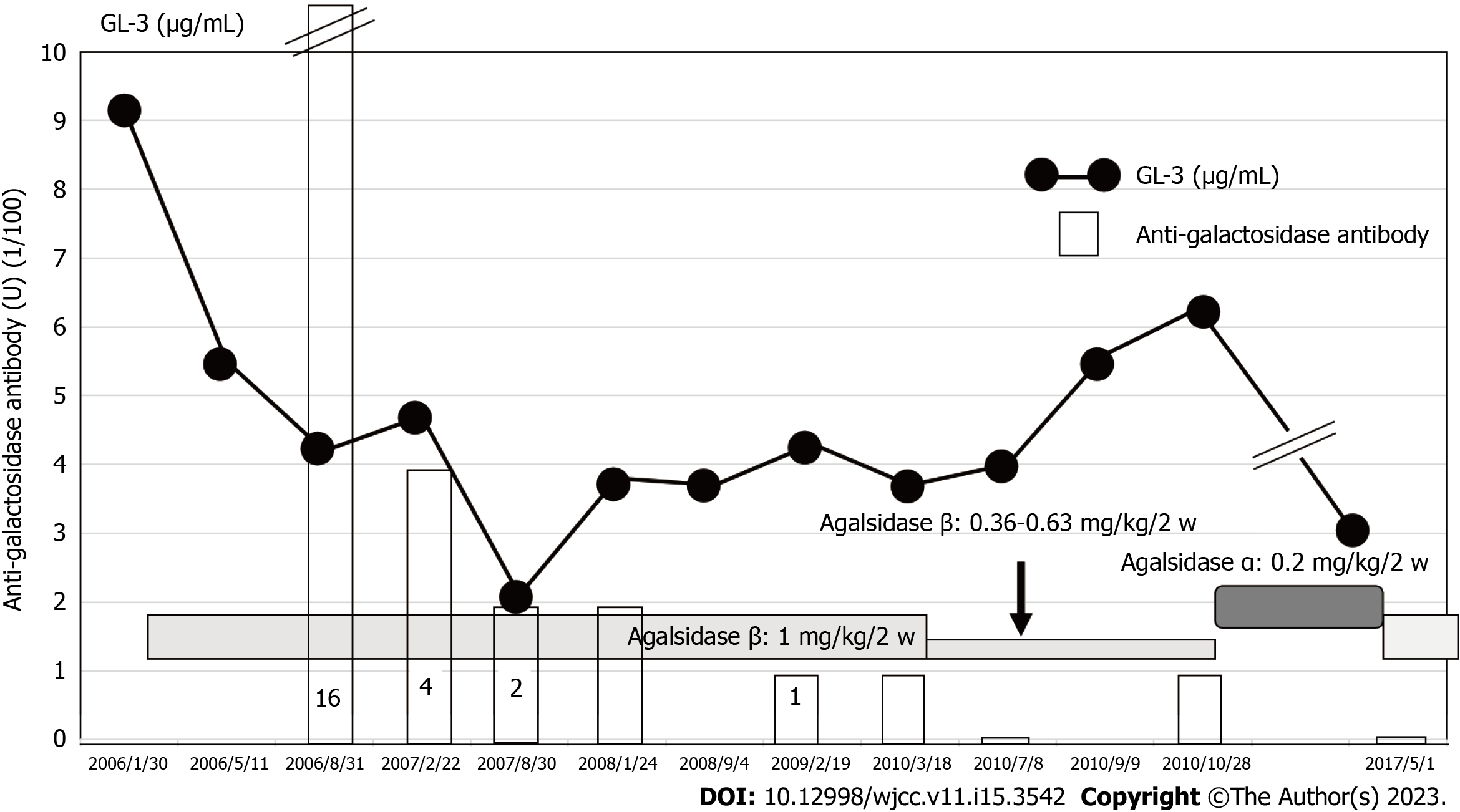
In the clinical course of patient 2, following treatment with 1 mg/kg of agalsidase β, GL-3 levels gradually decreased from > 9 μg/mL and normalized to ≤ 7 μg/mL. However, GL-3 levels gradually increased following the reduction of the dose of agalsidase β to 0.36-0.63 mg/kg between March and October 2010. Between October 2010 and 2017, the patient was treated with 0.2 μg/mL of agalsidase α instead of agalsidase β. GL-3 level was normal at 3.1 μg/mL in 2017 when agalsidase β administration resumed. The anti-agalsidase β antibody titer was 1600 U in July 2006, which then gradually decreased to ≤ 100 U in March 2010. When the agalsidase β dose was reduced to 0.36 mg/kg in March 2010, the anti-agalsidase β antibody titer increased to 100 U in October 2010 (Figure 7).
Two years after initiation of ERT in patient 2, his mother, the sister of the proband, developed fever and myocarditis at the age of 59 years. She was transferred to Fukushima Medical University Hospital and diagnosed with cardiac FD. She was treated with ERT, and her cardiac condition improved. Although continuation of ERT was recommended by the doctor in charge, she discontinued ERT after two years because she did not have any symptoms and did not want to endure the cost of medical resources. After years of living with normal liver function, she underwent surgery for a cataract, that was likely due to FD, in the department of ophthalmology at Fukushima Red-Cross hospital.
The pathophysiology of FD-induced progressive end-organ damage is irreversible; however, disease progression can be delayed using ERT. Nonetheless, complete recovery of organs in patients with advanced FD is challenging.
Despite ERT, cerebral infarctions frequently occurred during adulthood in patient 1, possibly progressing due to irreversible angiopathy. Moreover, cardiac muscle cell vacuolation and transformation into marked fibrosis were observed in the atrioventricular node and cardiac muscles. These cardiac lesions may have caused sudden cardiac arrest following the onset of cerebral infarction.
In patient 2, limb pain occurred during childhood, and anhidrosis, angiokeratoma, other gastroin
During the clinical course, patient 2 developed Wallenberg syndrome. Considering his age and right occipital pain at the onset of this attack, Wallenberg syndrome was considered to be caused by vertebral artery dissection rather than primary thrombosis. Vertebral artery dissection may have been caused by FD-induced endothelial fragility. The patient recovered completely within a month of therapy and did not experience similar complaints while on treatment with clopidogrel.
Throughout ERT for patients with FD, α-galactosidase antibodies may appear in male patients because of a complete absence of α-galactosidase in the blood[11]. In a study conducted by Vedder et al[12], 52 patients with FD (28 men and 24 women) were treated with 0.2 mg/kg of agalsidase α or agalsidase β for 1 year, and 66% had the corresponding antibodies at 6 mo of therapy. After a year of treatment, urinary GL-3 levels decreased in both groups who received 0.2 mg/kg of agalsidase α and 0.2 mg/kg of agalsidase β in antibody-negative patients; urinary GL-3 levels increased in antibody-positive patients in both groups. In contrast, 1 mg/kg of agalsidase β decreased urinary GL-3 levels in both antibody-negative and antibody-positive patients, indicating that 1 mg/kg of agalsidase β is sufficient to reduce urinary GL-3 levels. In contrast, at 0.2 mg/kg of agalsidase β, the dose becomes insufficient to reduce GL-3.
In patient 2, agalsidase dosing and isoform modification were performed as described, and GL-3 levels subsequently increased, demonstrating that GL-3 levels decreased in response to a baseline dose of 1 mg/kg rather than 0.36-0.63 mg/kg of agalsidase β. Conversely, 0.2 μg/mL of agalsidase α sufficiently decreased GL-3 levels to baseline value at 3.1 μg/mL in 2017.
Both patients had classic FD. Patient 2 was examined for GL-3 and anti-agalsidase β antibody concentrations. The patient developed anti-agalsidase antibodies after ERT initiation; throughout the course of ERT, his GL-3 levels decreased, and the antibodies disappeared, suggesting that excess agalsidase β neutralized the anti-agalsidase β antibodies and suppressed their production. Agalsidase β decreased GL-3 levels, and GL-3 disappeared in the plasma.
In patients with classic FD, sporadic accumulation of GL-3 in the heart and kidneys begins in utero; however, until childhood, GL-3 accumulation is mild, reversible, and can be restored by ERT[13]. ERT is optimal at such early disease stages. Accordingly, it is recommended to test all families with known genetic abnormalities to identify the affected children and neonates. However, due to ethical issues, such as privacy protection, it is necessary to modify treatment approaches based on the intentions and wishes of the family.
In patient 1, ERT was initiated following end-organ damage and was subsequently ineffective. In patient 2, ERT was initiated when the patient was diagnosed in his mid-30s, during which the damage to vital organs was not apparent . Following the initiation of ERT, severe progressive damage to the vital organs was not observed for 18 years.
Germain et al[14] reported on the ten-year outcomes of ERT with agalsidase β in patients with FD. Disease progression rates in patients with renal involvement at low or high baseline values were assessed, and they concluded that patients, in whom ERT was initiated at a younger age and with less kidney involvement, mostly benefited from ERT.
The current consensus is that ERT initiation during early childhood is paramount, and the value of early ERT initiation has been highlighted in guidelines for pediatric patients with FD (developed by an FD expert panel consisting of specialists from the United States)[15]. The guidelines of the European Fabry Working Group, consistent with the recommendations for stopping ERT, indicate that ERT should be considered in asymptomatic men with classic FD prior to adulthood[16].
We presented cases of two patients, wherein the clinical course of patient 2 demonstrated that end-organ damage could be treated if the damage is minor. These findings indicate that ERT, prior to severe vital organ damage, should be mandatory even among adults. Since ERT initiation during the optimal period is often missed in many asymptomatic cases of FD, long-term observational studies are warranted to help optimize the timing for ERT initiation. This will also ensure that ERT initiation has a lower economic burden on the social security system and patients.
The clinical course of patient 2 showed that end-organ damage could be treated if the damage is minor. Therefore, even among adults, ERT prior to severe vital organ damage should be mandatory. As many asymptomatic adult patients with FD have missed the optimal period for ERT initiation, long-term observational studies are necessary for economic and medical purposes, to determine a strategy of when to initiate ERT in adults.
We would like to thank all the nurses at Fukushima Red Cross Hospital for their good care of patients receiving ERT. We are grateful to Mr. T Mine for his technical assistance in performing echocardiography. We would also like to thank Sanofi and the Genzyme Company for their assistance in the measurement of GL-3 and anti-agalsidase β antibody levels.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Genetics and heredity
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koukoulaki M, Greece; Papazafiropoulou A, Greece S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Bernstein HS, Bishop DF, Astrin KH, Kornreich R, Eng CM, Sakuraba H, Desnick RJ. Fabry disease: six gene rearrangements and an exonic point mutation in the alpha-galactosidase gene. J Clin Invest. 1989;83:1390-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Kint JA. Fabry's disease: alpha-galactosidase deficiency. Science. 1970;167:1268-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 320] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry's disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276:1163-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 750] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 4. | Schiffmann R, Kopp JB, Austin HA 3rd, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 933] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 5. | Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ; International Collaborative Fabry Disease Study Group. Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1149] [Cited by in RCA: 1114] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 6. | Rombach SM, Smid BE, Bouwman MG, Linthorst GE, Dijkgraaf MG, Hollak CE. Long term enzyme replacement therapy for Fabry disease: effectiveness on kidney, heart and brain. Orphanet J Rare Dis. 2013;8:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Weidemann F, Niemann M, Störk S, Breunig F, Beer M, Sommer C, Herrmann S, Ertl G, Wanner C. Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: evidence for disease progression towards serious complications. J Intern Med. 2013;274:331-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Roddy TP, Nelson BC, Sung CC, Araghi S, Wilkens D, Zhang XK, Thomas JJ, Richards SM. Liquid chromatography-tandem mass spectrometry quantification of globotriaosylceramide in plasma for long-term monitoring of Fabry patients treated with enzyme replacement therapy. Clin Chem. 2005;51:237-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Machii K, Asaki T, Igarashi K, Ikeda K, Ikeda Y, Sato Y, Owada K, Iwaya K, Amano K, Tsuda F. A pedigree of Fabry’s disease with special reference to clinical symptoms of a proband and his brothers. Fukushima J Med Sci. 1975;25:141-151. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Suzuki O, Abe M. An autopsy case of Fabry's disease with cardiac manifestations. J Clin Exp Hematop. 2009;49:121-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Wilcox WR, Linthorst GE, Germain DP, Feldt-Rasmussen U, Waldek S, Richards SM, Beitner-Johnson D, Cizmarik M, Cole JA, Kingma W, Warnock DG. Anti-α-galactosidase A antibody response to agalsidase beta treatment: data from the Fabry Registry. Mol Genet Metab. 2012;105:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Vedder AC, Breunig F, Donker-Koopman WE, Mills K, Young E, Winchester B, Ten Berge IJ, Groener JE, Aerts JM, Wanner C, Hollak CE. Treatment of Fabry disease with different dosing regimens of agalsidase: effects on antibody formation and GL-3. Mol Genet Metab. 2008;94:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Eng CM, Fletcher J, Wilcox WR, Waldek S, Scott CR, Sillence DO, Breunig F, Charrow J, Germain DP, Nicholls K, Banikazemi M. Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J Inherit Metab Dis. 2007;30:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 257] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Germain DP, Charrow J, Desnick RJ, Guffon N, Kempf J, Lachmann RH, Lemay R, Linthorst GE, Packman S, Scott CR, Waldek S, Warnock DG, Weinreb NJ, Wilcox WR. Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet. 2015;52:353-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 255] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 15. | Hopkin RJ, Jefferies JL, Laney DA, Lawson VH, Mauer M, Taylor MR, Wilcox WR; Fabry Pediatric Expert Panel. The management and treatment of children with Fabry disease: A United States-based perspective. Mol Genet Metab. 2016;117:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Biegstraaten M, Arngrímsson R, Barbey F, Boks L, Cecchi F, Deegan PB, Feldt-Rasmussen U, Geberhiwot T, Germain DP, Hendriksz C, Hughes DA, Kantola I, Karabul N, Lavery C, Linthorst GE, Mehta A, van de Mheen E, Oliveira JP, Parini R, Ramaswami U, Rudnicki M, Serra A, Sommer C, Sunder-Plassmann G, Svarstad E, Sweeb A, Terryn W, Tylki-Szymanska A, Tøndel C, Vujkovac B, Weidemann F, Wijburg FA, Woolfson P, Hollak CE. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: the European Fabry Working Group consensus document. Orphanet J Rare Dis. 2015;10:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 248] [Article Influence: 24.8] [Reference Citation Analysis (0)] |