Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3502
Peer-review started: January 7, 2023
First decision: January 30, 2023
Revised: February 1, 2023
Accepted: April 14, 2023
Article in press: April 14, 2023
Published online: May 26, 2023
Processing time: 138 Days and 0.3 Hours
Methanol is a highly toxic, non-potable alcohol. Outbreaks of methanol toxicity occur due to its fraudulent addition to alcoholic beverages as a cheaper substitute for ethanol. Recently, alongside the coronavirus disease 2019 (COVID-19) pandemic, rumors circulated on social media that consuming alcohol can prevent or cure the virus, leading to a COVID-19 and methanol-induced optic neuropathy (MON) syndemic.
To investigate the impact of erythropoietin (EPO) on the outcomes of patients diagnosed with MON.
In this prospective study, 105 patients presenting with acute bilateral visual loss secondary to methanol intoxication were enrolled from March to May 2020 at Farabi Eye Hospital. A comprehensive ocular examination was conducted for all participants. Recombinant human EPO and methylprednisolone were admini
The mean age of the participants was 39.9 years (± 12.6). Ninety-four patients were male and eleven were female. The mean pre-treatment best corrected visual acuity (BCVA) improved from 2.0 ± 0.86 to 1.39 ± 0.69 logarithm of the minimum angle of resolution post-treatment (P < 0.001), with significant improvement observed in all age categories and genders (P < 0.001). Visual acuity improvement was also significant regardless of whether the patient presented before or after 72 h (P < 0.001), and the post-treatment BCVA remained significant at all monthly follow-up visits (P < 0.001).
EPO and methylprednisolone therapy have been shown to be effective in improving visual outcomes in patients with MON when administrated within the first month of exposure. Public awareness efforts are necessary to prevent further outbreaks of methanol toxicity in the current COVID-19 era.
Core Tip: This is a prospective cohort study that assessed the impact of erythropoietin (EPO) and methylprednisolone treatment on 105 patients diagnosed with acute bilateral visual loss due to methanol-induced optic neuropathy. The results suggest that administering EPO within the first month of methanol exposure can significantly improve visual outcomes.
- Citation: Tabatabaei SA, Amini M, Haydar AA, Soleimani M, Cheraqpour K, Shahriari M, Hassanian-Moghaddam H, Zamani N, Akbari MR. Outbreak of methanol-induced optic neuropathy in early COVID-19 era; effectiveness of erythropoietin and methylprednisolone therapy. World J Clin Cases 2023; 11(15): 3502-3510
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3502.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3502
Methanol (CH3OH) is a type of non-drinking alcohol used in the production of fuel, acetic acid, antifreeze, dyes, and cologne. Unlike ethanol, it is highly toxic to humans and is a colorless, volatile, and flammable fluid. In the body, methanol is slowly metabolized into formaldehyde and formic acid, which cause its toxic effects[1]. The lethal dose of methanol ranges from 30-240 mL[2]. The minimal amount required to cause permanent visual damage in adults is not known, but it is thought to be greater than 30 mL[3]. Symptoms of toxicity, including metabolic acidosis, usually appear 12-24 h after exposure and can include weakness, anorexia, headache, nausea, vomiting, and hyperventilation[2]. Progression to coma and respiratory and circulatory arrest may also occur[4], as well as cerebral hemorrhage[5]. Optic neuropathy, causing blurred vision, tunnel vision, visual loss, and photophobia, may appear first or simultaneously with systemic symptoms[3].
The prognosis of methanol-induced optic neuropathy (MON) is generally poor. Currently, two antidotes are available for methanol toxicity: ethanol and fomepizole. Ethanol has potential adverse effects and limited success in restoring vision[6]. Fomepizole is deemed safe but is expensive and unavailable in many undeveloped countries where methanol toxicity occurs frequently[7]. Erythropoietin (EPO), a hormone produced mainly in the kidneys to respond to hypoxia, stimulates the production of red blood cells in the bone morrow. The investigation of EPO’s neuroprotective and neuroregenerative properties is an active area of research. Several studies have reported improvement in visual acuity (VA) when EPO was used to treat patients with optic nerve disorders[8-10].
Epidemics of methanol toxicity occur when it is fraudulently added to alcoholic beverages as a cheaper substitute to ethanol. In Iran, where the consumption of alcoholic beverages is prohibited by law, hundreds of cases of fatalities, permanent blindness, and irreversible neurological sequalae are reported annually following ingestion of methanol[11-13]. Most recently, in parallel with the coronavirus disease 2019 (COVID-19) pandemic, a methanol toxicity outbreak emerged in Iran, with the Iranian Ministry of Health documenting 5876 hospitalizations and over 700 deaths from February 23 (the first documented COVID-19 case in Iran) until May 2, 2020[14]. This is the largest recorded methanol toxicity outbreak in history, with the second-largest documented in Libya in March 2013, with 1066 cases[15].
During the COVID-19 pandemic, hospitals in Iran have seen a surge in the number of patients with MON. Reports of a higher incidence of MON during the COVID-19 era than the total number of patients seen in the past 15 years have been recorded in Shiraz[16]. Our emergency department of Farabi Eye Hospital has similarly observed a significant increase in the number of MON patients. We conducted a prospective study to investigate the effects of EPO on visual outcomes in MON patients.
In this prospective study, 105 patients with acute bilateral visual loss secondary to methanol intoxication who presented at Farabi Eye Hospital between March and May 2020 were enrolled. The study was approved by the Institutional Review Board of Tehran University of Medical Sciences and all procedures conformed to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants. Screening for eligibility was performed in the emergency room and data was collected from the patient’s medical records.
The inclusion criteria for this study were acute visual loss secondary to methanol poisoning, detoxification or dialysis, and consumption of homemade alcoholic beverages within one month. Exclusion criteria included a history of ophthalmic disease, neurologic disorder, hemoglobin concentration ≥ 16 gm/dL, pregnancy, lactation, and uncontrolled hypertension.
All patients underwent toxicology screening and received hemodialysis when deemed necessary at our affiliated or nearby general hospitals. An ophthalmic examination was performed, including best-corrected VA (BCVA), assessment of relative afferent pupillary defect, evaluation of extraocular movements, slit-lamp examination, and funduscopy.
In this study, recruited patients were hospitalized for a three-day period. They underwent a comprehensive history and physical examination, and a complete blood count test. Intravenous recombinant human EPO (10000 IU/mL) was administered every 12 h for all three consecutive days. In addition, all patients received intravenous methylprednisolone (250 mg) every 6 h for three days. A conventional therapy regimen, consisting of vitamin B12 (100 mg/d), vitamin B6 (100 mg/d), and folic acid (10 mg/d), was added for a duration of three months. During hospitalization, patients were evaluated daily for any potential adverse effects. The treatment protocol was repeated once a month for a period of six months, with EPO and methylprednisolone being administrated in a single dose, but not for three days.
Vision was measured using the logarithm of the minimum angle of resolution (LogMAR) and categorized as counting fingers equal to 1.9, hand motion equal to 2.3, light perception (LP) equal to 2.7, and no LP (NLP) equal to 3.0[17]. Data was presented as mean, standard deviation, median, and range, and the trend of vision during the follow-up periods were demonstrated using a line-error bar. Generalized estimating equation was used to evaluate visual changes during follow-ups and Wilcoxon signed-rank test was used to compare the pre- and final VA. Multiple comparisons were handled using the Sidak method. Statistical analysis was performed using SPSS v.26 (IBM Corp., Armonk, NY, Untied States). A P value less than 0.05 was considered statistically significant.
A total of 105 patients with recent MON were enrolled in our prospective study. The mean age was 39.9 years (± 12.6, range: 15-53 years). Ninety-four (89.5%) of the patients were male and eleven (10.5%) were female, yielding a male-to-female ratio of 8.55. The mean interval between methanol intoxication and initiation of EPO treatment was 6.1 d (± 4.96, range: 1-29 d). The mean follow-up time was 4.5 ± 1.7 mo.
The baseline BCVA in the right and left eyes was 2.02 ± 0.91 and 2.0 ± 0.95 LogMAR, respectively, and significantly improved to 1.38 ± 0.82 and 1.40 ± 0.82 LogMAR (P < 0.001) after treatment. The results of VA changes based on gender, age, and time interval are shown in Table 1. Both male and female patients showed significant improvement in their BCVA from 2.04 ± 0.86 and 1.89 ± 1.21 to 1.16 ± 1.03 (P < 0.001) and 1.1 ± 0.85 (P = 0.0), respectively. All age categories also showed significant improvement in final BCVA (P < 0.001). Patients who presented either before or after 72 h of methanol ingestion also showed significant improvement in their final BCVA (P < 0.001).
| Time | Gender | Age (yr) | Time interval (h) | ||||
| F | M | ≤ 35 | 36-50 | ≥ 51 | ≤ 72 | ≥ 73 | |
| Pre | 1.89 ± 1.21 | 2.04 ± 0.88 | 1.9 ± 0.86 | 2.2 ± 0.88 | 1.91 ± 1.04 | 1.81 ± 0.89 | 2.16 ± 0.88 |
| Month 1 | 0.13 ± 0.04 | 1.19 ± 1.08 | 0.5 ± 0.85 | 1.9 ± 0.9 | 0.54 ± 0.68 | 1.64 ± 0.72 | 1.44 ± 1.18 |
| Month 2 | 1.21 ± 0.91 | 1.08 ± 0.98 | 0.89 ± 0.89 | 1.29 ± 1.02 | 0.98 ± 0.93 | 0.83 ± 0.93 | 1.22 ± 1 |
| Month 3 | 0.91 ± 0.89 | 1.28 ± 0.95 | 0.91 ± 0.83 | 1.5 ± 0.97 | 1.41 ± 0.97 | 1.11 ± 0.96 | 1.39 ± 0.91 |
| Month 4 | 0.29 ± 0.32 | 1.37 ± 1.04 | 1.04 ± 1.01 | 1.64 ± 1.01 | 1.07 ± 1.05 | 0.93 ± 0.95 | 1.5 ± 1.04 |
| Month 5 | 1.05 ± 0.85 | 1.18 ± 1.01 | 0.96 ± 0.96 | 1.26 ± 0.97 | 1.33 ± 1.03 | 1.07 ± 1 | 1.19 ± 1 |
| Month 6 | 0.96 ± 0.85 | 1.4 ± 0.97 | 1.15 ± 0.95 | 1.53 ± 0.87 | 1.73 ± 1.05 | 1.39 ± 1.02 | 1.27 ± 0.96 |
| Final evaluation | 1.1 ± 0.85 | 1.16 ± 1.03 | 0.88 ± 0.93 | 1.37 ± 1.03 | 1.27 ± 1.01 | 0.95 ± 0.94 | 1.3 ± 1.02 |
| P valuea | 0.01 | < 0.001 | < 0.001 | < 0.001 | 0.004 | < 0.001 | < 0.001 |
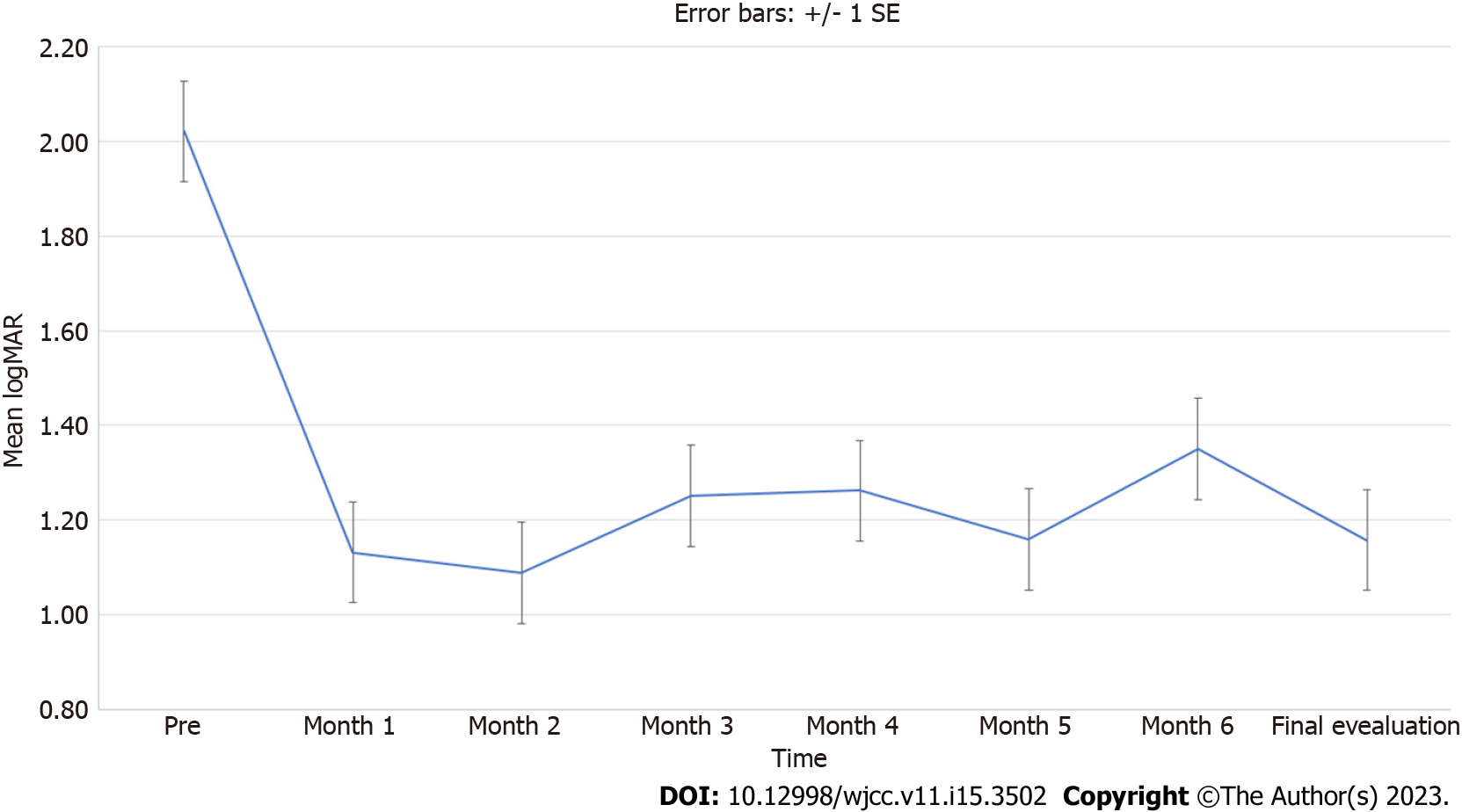
Comparison of the post-BCVA of the study population at each monthly follow-up with the pre-BCVA showed a significant improvement (P < 0.001). However, when stratified by gender, age, and time interval, the comparisons between post-BCVA of monthly follow-up visits and pre-BCVA were not always statistically significant. Figure 1 displays the trend of VA during the follow-up periods using a line plot with error bars.
Methanol toxicity remains a major health concern in low-income areas and is more prevalent among those with low socio-economic status. In the body, methanol is converted to formaldehyde by oxidation and then further metabolized in the liver by alcohol dehydrogenase to formic acid, which is responsible for the toxic effects seen with methanol exposure[1]. The buildup of formic acid leads to metabolic acidosis, inhibits mitochondrial cytochrome C oxidase, and depletes adenosine triphosphate, causing tissue hypoxia and ultimately resulting in axonal cell death[18,19]. This can lead to optic nerve hyperemia, mild edema, and eventually optic atrophy, as well as cystoid macular edema, a pseudo-cherry red spot, retinal hemorrhage, and retinal vascular tortuosity. Studies have documented damage to photoreceptors, Müller cells, and the retrolaminar optic nerve[20,21].
The treatment of methanol toxicity involves correcting metabolic acidosis with sodium bicarbonate and inhibiting further methanol metabolism to formic acid using fomepizole or ethanol antidotes[1]. Although fomepizole is preferred because it does not induce central nervous system (CNS) depression or hypoglycemia, it is expensive and unavailable in many developing countries. Early administration of fomepizole is crucial for effective treatment, but it offers no additional benefits after hemodialysis or when ocular damage has occurred[1]. Hemodialysis is indicated in cases of visual impairment with metabolic acidosis[22]. Folinic acid is used to promote oxidation of formic acid to carbon dioxide and water[1]. High-dose systemic steroids are sometimes used to improve vision in methanol toxicity, but their effectiveness has not been supported by clinical trials and results are inconsistent[23-25]. Systemic steroids are believed to alleviate the pressure on the swollen optic nerve head and prevent secondary damage to axons, as they are used in similar scenarios for other eye conditions such as thyroid eye disease, anterior ischemic optic neuropathy, and papilledema[26,27].
We showed that EPO infusion may improve vision in patients with MON. EPO is a glycoprotein cytokine mainly produced by the kidneys in response to tissue hypoxia, which stimulates the production of red blood cells. In clinical practice, EPO is used to correct anemia. Experimental studies have shown that EPO has multiple functions, including antiapoptotic and angiogenic activities, anti-inflammatory and antioxidant effects, and potential neuroprotective and neurogenetic roles against ischemia[28]. Additionally, EPO is involved in neurogenesis and can prevent neuronal degeneration, such as in retinal ganglion cells and axons[29-31]. As a result, EPO has been investigated for therapeutic use in various fields, including ophthalmology, where it has been studied for the treatment of several ocular diseases, such as diabetic macular edema[32], glaucoma[33], optic neuritis[34], traumatic optic neuropathy[9], and non-arteritic anterior ischemic optic neuropathy[8]. These human studies have shown promising results. However, it is important to note that EPO is considered an adjunctive treatment to prevent, maintain, or improve the vision loss and cannot replace hemodialysis when necessary.
In this prospective study, 105 patients with MON received EPO therapy in addition to conventional treatment. Patients received supportive care, including airway management, correction of electrolyte imbalances, and hemodialysis at affiliated general hospitals prior to admission to our facility. Results showed that EPO significantly improved the final VA in MON patients, and this improvement was maintained regardless of gender, age, and time interval. Additionally, post-BCVA at each follow-up visit from 1 to 6 mo was also significant. However, the comparison of post-BCVA between monthly follow-up visits was not always significant. No significant adverse effects were observed during or after EPO administration.
Table 2 summarizes the studies on the use of EPO in the management of MON. A total number of four case reports and series were identified in the literature review, involving 39 patients[35-38]. All the 39 patients presented within 10 d of methanol ingestion and all except one were male, with an average age in the mid-thirties Three studies employed a combination of EPO and methylprednisolone, while one report used EOP only. In 2012, Pakravan et al[35] were the first to use EOP in two MON patients[35], and they observed dramatic vision improvement, although the follow-up was short. The same authors treated 11 patients with the same protocol in 2016 and also achieved favorable results[36]. In a case-control study, Zamani et al[38] found no benefit from adding EPO to methylprednisolone and concluded that the effect of EPO on MON may be transient[38]. In 2018, Pakdel et al[37] treated 16 MON patients with EPO only and followed them for a longer period[37]. Their vision improved significantly from 3.6 to 1.0 LogMAR (P < 0.0001). Our study reports the largest cohort of MON patients treated with EPO and followed for a long period. We used a combined treatment strategy of EPO plus methylprednisolone and repeated the treatment monthly for 6 mo for all patients who presented for follow-up. However, EPO and methylprednisolone were administrated in a single dose, not for 3 d.
| Ref. | Type of study | No. of patients | Age (yr)1/Sex | Time interval | Management | No. of Courses | Pre VA | Post VA | Follow-up1 |
| Pakravan et al[35], 2012 | Case report | 2 | 30/M | 3 d | IV EPO 10000 IU BID for 3 d | 1 | OU: NLP | OU: 20/20 | 3 wk |
| 35/M | 7 d | IV Methylprednisolone 500 mg BID for 5 d followed by 2 wk of oral prednisolone 1 mg/kg/d, Vitamin B6 100 mg/d, vitamin B12 100mg/d, and folic acid 10 mg/d for 1 mo | OU: NLP | OD: CF; OS: 20/30 | 2 wk | ||||
| Pakravan et al[36], 2016 | Nonrandomized interventional comparative | 11 | 34.5/M | 4.8 ± 2.6 d | IV EPO 10000 IU BID for 3 d, IV Methylprednisolone 500 mg BID for 5 d followed by 2 wk of oral prednisolone 1 mg/kg/d, Vitamin B6 100 mg/d, vitamin B12 100 mg/d, and folic acid 10 mg/d for 1 mo | 1 | 2.93 ± 0.55 LogMAR | 1.75 ± 1.16 LogMAR | 3 mo |
| Zamani et al[38], 2018 | Case-control | 10 | 34/NA | 2 d | SQ EPO 10000 IU BID for 3 d, Methylprednisolone 250 mg QID for 3 d | 1 | 0.002 | 0.004 | 2 mo |
| Pakdel et al[38], 2018 | Case-series | 16 | 34.2/15 M | 9.1 d | IV EPO 20000 IU QD for 3 d | 3 in six cases, 2 in eight, 1 in two | Better eye: 3.6 LogMAR | Better eye: 1.0 LogMAR | 7.5 mo |
In this prospective study, no adverse effects such as hypertension were reported during or after EPO infusion, and no patient experienced post-treatment polycythemia. Our results are consistent with previous studies that found significant improvement in VA post-EPO treatment. However, the study by Zamani et al[38] found no benefits of adding EPO to methylprednisolone, but their sample size was small (10 cases and 5 controls) and the follow-up period was short. They also excluded patients who underwent hemodialysis and achieved VA improvement, which can be considered as a bias, as their vision may deteriorate shortly after. Our patients showed significantly better post-BCVA patterns at every monthly follow-up from 1 to 6 mo compared to pre-BCVA (P < 0.001) (Figure 1), contradicting the hypothesis by Zamani et al[38] that EPO’s effect may be transient.
The COVID-19 outbreak in Iran, like in other countries, caused widespread fear of infection due to the lack of effective treatments and limited access to vaccination, particularly in developing countries. This led to self-medication and the concurrent outbreak of methanol toxicity. Rumors that drinking alcohol could prevent or cure COVID-19 circulated on social media[39], leading to increased consumption of alcohol, some of which contained illegally added methanol[40]. This created a syndemic and a surge of methanol toxicity patients visiting emergency departments.
The study was limited by the absence of a control group and the lack of visual field tests and imaging such as optical coherence tomography to evaluate changes in retinal fiber nerve layer and ganglion cell layer (GCL) thickness before and after EPO treatment. Further research is needed to compare different treatment protocols, establish optimal dosages and timing of EOP through randomized clinical trials, and better understand the mechanism of EPO in ophthalmology and its potential side effects.
In conclusion, EPO was found to be effective in improving the VA outcomes for MON patients when administrated within first month of ingestion. The exact mechanism behind its neuroprotective effects on the optic nerve and retina is still unknown. Public awareness campaigns are necessary to prevent further concurrent outbreaks of methanol toxicity, especially during the COVID-19 era.
Methanol, which is a type of non-drinking alcohol, has a highly toxic profile to humans. Methanol toxicity can damage to different sites of the human body such as the optic nerve. Epidemics of methanol toxicity occur when it is fraudulently added to alcoholic beverages as a cheaper substitute for ethanol, especially where alcoholic drinks are prohibited by law. Most recently, in parallel with the coronavirus disease 2019 pandemic, a methanol toxicity outbreak emerged in Iran.
The prognosis of methanol-induced optic neuropathy (MON) is generally poor. Currently, two antidotes are available for methanol toxicity: ethanol and fomepizole. Erythropoietin (EPO), a hormone produced mainly in the kidneys, has been shown neuroprotective and neuroregenerative properties.
We conducted a prospective study to investigate the effects of EPO on visual outcomes in MON patients.
In this prospective study, patients with acute bilateral visual loss secondary to methanol intoxication from one month before presentation were enrolled. All patients underwent toxicology screening and received hemodialysis when deemed necessary. A comprehensive ophthalmic examination was performed. Patients were hospitalized for a three-day period. Intravenous recombinant human EPO (10000 IU/mL) was administered every 12 h for all three consecutive days. In addition, all patients received intravenous methylprednisolone (250 mg) every 6 h for three days.
The mean best corrected visual acuity (VA) improved significantly (P < 0.001). VA improvement was significant regardless of whether the patient presented before or after 72 h (P < 0.001).
EPO and methylprednisolone therapy have been shown to be effective in improving visual outcomes in patients with methanol-induced optic neuropathy when administrated within the first month of exposure.
Further research is needed to compare different treatment protocols, establish optimal dosages and timing of EOP through randomized clinical trials.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jovandaric MZ, Serbia; Shen ZY, China S-Editor: Li L L-Editor: A P-Editor: Yu HG
| 1. | Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA; American Academy of Clinical Toxicology Ad Hoc Committee on the Treatment Guidelines for Methanol Poisoning. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol. 2002;40:415-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 359] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 2. | Jacobsen D, McMartin KE. Methanol and ethylene glycol poisonings. Mechanism of toxicity, clinical course, diagnosis and treatment. Med Toxicol. 1986;1:309-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 293] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Hovda KE, McMartin K, Jacobsen D. Methanol and formaldehyde poisoning. In: Megarbane B, Palmer R, Hatten B, Burkhart K, editors. Critical care toxicology. NY: Springer Publishing, 2017: pp: 486. |
| 4. | Gouda AS, Khattab AM, Mégarbane B. Lessons from a methanol poisoning outbreak in Egypt: Six case reports. World J Crit Care Med. 2020;9:54-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Li J, Feng ZJ, Liu L, Ma YJ. Acute methanol poisoning with bilateral diffuse cerebral hemorrhage: A case report. World J Clin Cases. 2022;10:6571-6579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (2)] |
| 6. | Sanaei-Zadeh H, Zamani N, Shadnia S. Outcomes of visual disturbances after methanol poisoning. Clin Toxicol (Phila). 2011;49:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Brent J, McMartin K, Phillips S, Aaron C, Kulig K; Methylpyrazole for Toxic Alcohols Study Group. Fomepizole for the treatment of methanol poisoning. N Engl J Med. 2001;344:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Modarres M, Falavarjani KG, Nazari H, Sanjari MS, Aghamohammadi F, Homaii M, Samiy N. Intravitreal erythropoietin injection for the treatment of non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2011;95:992-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 9. | Kashkouli MB, Pakdel F, Sanjari MS, Haghighi A, Nojomi M, Homaee MH, Heirati A. Erythropoietin: a novel treatment for traumatic optic neuropathy-a pilot study. Graefes Arch Clin Exp Ophthalmol. 2011;249:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Borhani-Haghighi A, Ghodsi M, Razeghinejad MR, Mardani S, Mardani M, Nikseresht AR, Safari A, Bagheri MH. Erythropoietin for acute multiple sclerosis in patients with optic neuritis as a first demyelination event. Neurosciences (Riyadh). 2012;17:151-155. [PubMed] |
| 11. | Sefidbakht S, Rasekhi AR, Kamali K, Borhani Haghighi A, Salooti A, Meshksar A, Abbasi HR, Moghadami M, Nabavizadeh SA. Methanol poisoning: acute MR and CT findings in nine patients. Neuroradiology. 2007;49:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Hassanian-Moghaddam H, Nikfarjam A, Mirafzal A, Saberinia A, Nasehi AA, Masoumi Asl H, Memaryan N. Methanol mass poisoning in Iran: role of case finding in outbreak management. J Public Health (Oxf). 2015;37:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Aghababaeian H, Araghi Ahvazi L, Ostadtaghizadeh A. The Methanol Poisoning Outbreaks in Iran 2018. Alcohol Alcohol. 2019;54:128-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Hassanian-Moghaddam H, Zamani N, Kolahi AA, McDonald R, Hovda KE. Double trouble: methanol outbreak in the wake of the COVID-19 pandemic in Iran-a cross-sectional assessment. Crit Care. 2020;24:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Rostrup M, Edwards JK, Abukalish M, Ezzabi M, Some D, Ritter H, Menge T, Abdelrahman A, Rootwelt R, Janssens B, Lind K, Paasma R, Hovda KE. The Methanol Poisoning Outbreaks in Libya 2013 and Kenya 2014. PLoS One. 2016;11:e0152676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Khalili MR, Sadati MS, Jahanbani-Ardakani H. Outbreak of methanol-induced optic neuropathy amid COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2021;259:1375-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities "hand motion" and "counting fingers" can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47:1236-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 754] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 18. | Liesivuori J, Savolainen H. Methanol and formic acid toxicity: biochemical mechanisms. Pharmacol Toxicol. 1991;69:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 237] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Sejersted OM, Jacobsen D, Ovrebø S, Jansen H. Formate concentrations in plasma from patients poisoned with methanol. Acta Med Scand. 1983;213:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | McKellar MJ, Hidajat RR, Elder MJ. Acute ocular methanol toxicity: clinical and electrophysiological features. Aust N Z J Ophthalmol. 1997;25:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Moschos MM, Gouliopoulos NS, Rouvas A, Ladas I. Vision loss after accidental methanol intoxication: a case report. BMC Res Notes. 2013;6:479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Pressman P, Clemens R, Sahu S, Hayes AW. A review of methanol poisoning: a crisis beyond ocular toxicology. Cutan Ocul Toxicol. 2020;39:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Shukla M, Shikoh I, Saleem A. Intravenous methylprednisolone could salvage vision in methyl alcohol poisoning. Indian J Ophthalmol. 2006;54:68-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Abrishami M, Khalifeh M, Shoayb M, Abrishami M. Therapeutic effects of high-dose intravenous prednisolone in methanol-induced toxic optic neuropathy. J Ocul Pharmacol Ther. 2011;27:261-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Fujihara M, Kikuchi M, Kurimoto Y. Methanol-induced retinal toxicity patient examined by optical coherence tomography. Jpn J Ophthalmol. 2006;50:239-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Ruchała M, Sawicka-Gutaj N. Advances in the pharmacological treatment of Graves' orbitopathy. Expert Rev Clin Pharmacol. 2016;9:981-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Pakravan M, Sanjari N, Esfandiari H, Pakravan P, Yaseri M. The effect of high-dose steroids, and normobaric oxygen therapy, on recent onset non-arteritic anterior ischemic optic neuropathy: a randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2016;254:2043-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Lombardero M, Kovacs K, Scheithauer BW. Erythropoietin: a hormone with multiple functions. Pathobiology. 2011;78:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21:9733-9743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 458] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 30. | Tan H, Kang X, Zhong Y, Shen X, Cheng Y, Jiao Q, Deng L. Erythropoietin upregulates growth associated protein-43 expression and promotes retinal ganglion cell axonal regeneration in vivo after optic nerve crush. Neural Regen Res. 2012;7:295-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Yamasaki M, Mishima HK, Yamashita H, Kashiwagi K, Murata K, Minamoto A, Inaba T. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res. 2005;1050:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Li W, Sinclair SH, Xu GT. Effects of intravitreal erythropoietin therapy for patients with chronic and progressive diabetic macular edema. Ophthalmic Surg Lasers Imaging. 2010;41:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Aktas Z, Unlu M, Uludag K, Erten Y, Hasanreisoglu B. The effect of systemic erythropoietin treatment on retinal nerve fiber layer parameters in patients with chronic renal failure undergoing peritoneal dialysis. J Glaucoma. 2015;24:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Sühs KW, Hein K, Sättler MB, Görlitz A, Ciupka C, Scholz K, Käsmann-Kellner B, Papanagiotou P, Schäffler N, Restemeyer C, Bittersohl D, Hassenstein A, Seitz B, Reith W, Fassbender K, Hilgers R, Heesen C, Bähr M, Diem R. A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann Neurol. 2012;72:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 35. | Pakravan M, Sanjari N. Erythropoietin treatment for methanol optic neuropathy. J Neuroophthalmol. 2012;32:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Pakravan M, Esfandiari H, Sanjari N, Ghahari E. Erythropoietin as an adjunctive treatment for methanol-induced toxic optic neuropathy. Am J Drug Alcohol Abuse. 2016;42:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Pakdel F, Sanjari MS, Naderi A, Pirmarzdashti N, Haghighi A, Kashkouli MB. Erythropoietin in Treatment of Methanol Optic Neuropathy. J Neuroophthalmol. 2018;38:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Zamani N, Hassanian-Moghaddam H, Shojaei M, Rahimian S. Evaluation of the effect of erythropoietin + corticosteroid versus corticosteroid alone in methanol-induced optic nerve neuropathy. Cutan Ocul Toxicol. 2018;37:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Aghababaeian H, Hamdanieh L, Ostadtaghizadeh A. Alcohol intake in an attempt to fight COVID-19: A medical myth in Iran. Alcohol. 2020;88:29-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | World Health Organization. In Middle East COVID-19 hotspot Iran, WHO walks the talk. Dec 23, 2020. [cited 3 April 2023]. Available from: https://www.who.int/news-room/feature-stories/detail/in-middle-east-covid-19-hotspot-iran-who-walks-the-talk. |