Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3434
Peer-review started: February 4, 2023
First decision: March 28, 2023
Revised: March 29, 2023
Accepted: April 20, 2023
Article in press: April 20, 2023
Published online: May 26, 2023
Processing time: 110 Days and 3.8 Hours
Muscle fatigue is common in many populations, particularly elderlies. Aging increases the incidence of muscle fatigue and delays its recovery. There is a huge debate about the current treatments for muscle fatigue, particularly in elderlies. Recently, it has been discovered that mechanoreceptors have an important role as a sensory system in sensing muscle fatigue which could enhance the body's response to muscle fatigue. The function of mechanoreceptors could be enhanced by applying either suprathreshold or subthreshold vibration. Although suprathreshold vibration improves muscle fatigue, it can cause desensitization of cuta
Core Tip: Subthreshold vibration may be a safe and effective treatment in the treatment of muscle fatigue. this review discusses the possible effects of subthreshold in treatment of muscle fatigue which include: (1) Enhancing the function of mechanoreceptors themselves; (2) Increasing the firing rate and function of alpha motor neurons; (3) Increasing blood flow to fatigued muscles; (4) Decreasing the rate of muscle cell death in elderlies (sarcopenia); and (5) Driving motor commands and allow better performance of muscles to decrease fatigue incidence. In conclusion, the use of subthreshold vibration could be a safe and effective treatment for muscle fatigue in elderlies. Also. It could enhance recovery from muscle fatigue. Finally, Subthreshold Vibration is safe and effective in treating muscle fatigue in comparison to suprathreshold vibration.
- Citation: Mohamed AA, Khaled E, Hesham A, Khalf A. Effectiveness and safety of subthreshold vibration over suprathreshold vibration in treatment of muscle fatigue in elderly people. World J Clin Cases 2023; 11(15): 3434-3443
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3434.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3434
Muscle fatigue is common among elderly people. Muscle fatigue usually begins at the age of 40 years and continues to increase afterward[1]. Muscle fatigue in elderly people commonly arises due to prolonged abnormality in their neuromuscular systems, which disrupts the capability of elderly people to resist muscle fatigue. These abnormalities happen in all regions of the neuromuscular system. Also, muscle fatigue in elderly people can occur due to a depletion in adenosine triphosphate (ATP) and calcium ions (Ca2+)[2]. Muscle fatigue has a close relationship to the rate of sarcopenia. The rate of muscle loss ranges from 1 to 2% per year past the age of 50 years. Thus, 25% of people under the age of 70 years and 40% of those over the age of 80 years have sarcopenia[3].
Existing rehabilitative procedures to decrease the incidence of or treat muscle fatigue are few and their attribute is minimal. Thus, it is difficult to make clear decisions on the effect of these procedures to reduce the incidence of muscle fatigue[4]. The existing rehabilitative procedures to reduce the incidence of muscle fatigue were built on the basis that muscle fatigue arises due to dysfunction in the neuromotor system. This dysfunction can happen in any region in the motor control system, involving, actin-myosin links, neuromuscular junction coupling in muscle, signals from motoneurons to muscle, signals from cortex to motoneurons, or motor cortex[5].
Existing rehabilitative procedures developed to enhance the fatigability and decrease the occurrence of muscle fatigue, focused mainly on increasing the rest periods[6], using mild training intensity[7], or grading the exercise intensity[8] to recover muscle force or allow more amount of Ca2+ ions and ATP. Other studies reported that usual participation in physical activity and grading the exercise have little effect on decreasing the occurrence of muscle fatigue or enhancing fatigability in seniors[9,10].
Numerous studies demonstrated that sensory receptors play a critical role in the development and perception of muscle fatigue[11-15]. Interestingly, muscle fatigue also has sensory receptors, which are the mechanoreceptors and metaboreceptors[5,16]. Mechanoreceptors are the most important receptors of muscle fatigue and they are sensitive to variations in muscle strain[11,16]. Metaboreceptors are the secondary receptors of muscle fatigue and they sense fluctuations in the number of metabolites produced by muscle contraction[11,16].
A large change in strain sensation in working muscles and/or joints, accumulation of muscle metabolites, and depletion of substrates during physical activity are the triggers of sensing muscle fatigue[16-20]. These peripheral changes are mainly sensed by the mechanoreceptors[21,22] and to a lesser extent by the metaboreceptors[23,24]. This sensory data then reaches the brain, informing it about the exertion or fatigue level in working muscles[11], which in turn could help in improving body response to muscle fatigue.
The sensory system has a vital role in driving motor signals[25], thus any exercise or intervention that targets the proprioception (sensory receptors of muscle fatigue) may have a superior role over the previous interventions that focused only on improving the motor system. Changing the temperature applied to the foot affected the balance and hyperemia[26]. Different walking speeds affects plantar pressure patterns at the sole[27]. Proprioceptive training accomplished significant enhancements in neuromotor function in almost conditions applied in[28-32]. Vibration is a type of proprioceptive training that directly trains the mechanoreceptors. Vibration can be either suprathreshold or subthreshold. The effect of suprathreshold vibration is still in debate and could have harmful effects. Some studies found that suprathreshold vibration has a positive effect on decreasing the incidence of muscle fatigue[33-35]. Other studies found that suprathreshold vibration has a nonsignificant effect[36,37] and may lead to harmful effects. Furthermore, a recent meta-analysis reported that the available studies on suprathreshold muscle tendon vibration after stroke are low in their methodological qualities and additional studies of high methodological quality are required to achieve a strong agreement concerning muscle tendon vibration intervention protocols and their recommendation in clinical settings[38]. Another systematic analysis reported that the studies that used focal mechanical suprathreshold vibration have several constraints[39]. Other studies found that prolonged exposure to high vibration is not safe and causes adverse effects. Adamo et al[40] reported that local suprathreshold vibration can cause a decrease in muscle force and the early development of muscle fatigue and musculoskeletal disorders. Also, long-term exposure to suprathreshold vibration causes an increased health risk to the spine and the peripheral nervous system[41]. Furthermore, suprathreshold vibration applied for 7 d or more causes significant damage to peripheral nerves when no recovery time was given[42]. Furthermore, high-frequency whole-body vibration may result in harmful effects, including intervertebral disc shift, visual impairment, and hearing loss[43].
Subthreshold vibration was applied in several studies and produced significant positive effects on neuromotor function in almost all of them with no recorded harmful effect; however, its effect in decreasing the incidence of muscle fatigue in elderly people is unclear yet. Thus, this review summarizes the possible physiological effects of subthreshold training in decreasing the incidence of muscle fatigue as an attempt to advance the rehabilitation of muscle fatigue. The goal of any rehabilitative intervention is to be applied safely and effectively for prolonged periods. Thus, this review discusses the effectiveness of subthreshold vibration in treating muscle fatigue and its safety over suprathreshold vibration for prolonged use. This review includes seven main sections including what is the subthreshold vibration, the effect of subthreshold vibration on the mechanoreceptors themselves, the effect of subthreshold vibration on alpha motor neurons, the effect of subthreshold vibration on blood flow to working muscles, the effect of subthreshold vibration on muscle cell death rate (sarcopenia), the effect subthreshold vibration as a sensory stimulation in driving motor commands, and the safety of subthreshold vibration in treating muscle fatigue in elderly people.
Subthreshold vibration can be defined as the application of vibration below the conscious level. Most studies used a 75% to 90% subsensory threshold. They used 90% subsensory amplitude and their rationale was decreasing the subsensory threshold by 10% to achieve the term of subsensory amplitude[44]. Sensory feedback is crucial in driving various motor commands including posture, balance, muscle action, gait, muscle spasm, etc[5].
Subsensory vibration depends on stochastic resonance theory. In the clinical field, stochastic resonance theory was used to train somatosensory systems. The stochastic resonance theory states that if the optimal low level of noise was applied, signal detection enhances[45,46]. The noise was accidentally discovered by Albert Einstein in 1905 when he noticed that atoms move consistent with the Brownian molecular motion[45]. After his detection, several studies, related to noise, were conducted in biological and physical systems, without identifying its vital influence on these systems[45]. However, this noise is frequently observed as an undesirable component or trouble to a system. Nowadays, targeting the optimal low level of noise for biological tissues offers incredible effects on numerous aspects of medicine[45]. Stochastic resonance theory arises in both artificial and naturally occurring non-linear systems. For example, paddlefish were shown to use stochastic resonance theory to locate and catch their targets[47]. It was found that using this small noisy input can improve the firing patterns of squid axons[48], the breathing stability in preterm infants[49], the postural control in older adults, stroke or peripheral neuropathy[44,46,50].
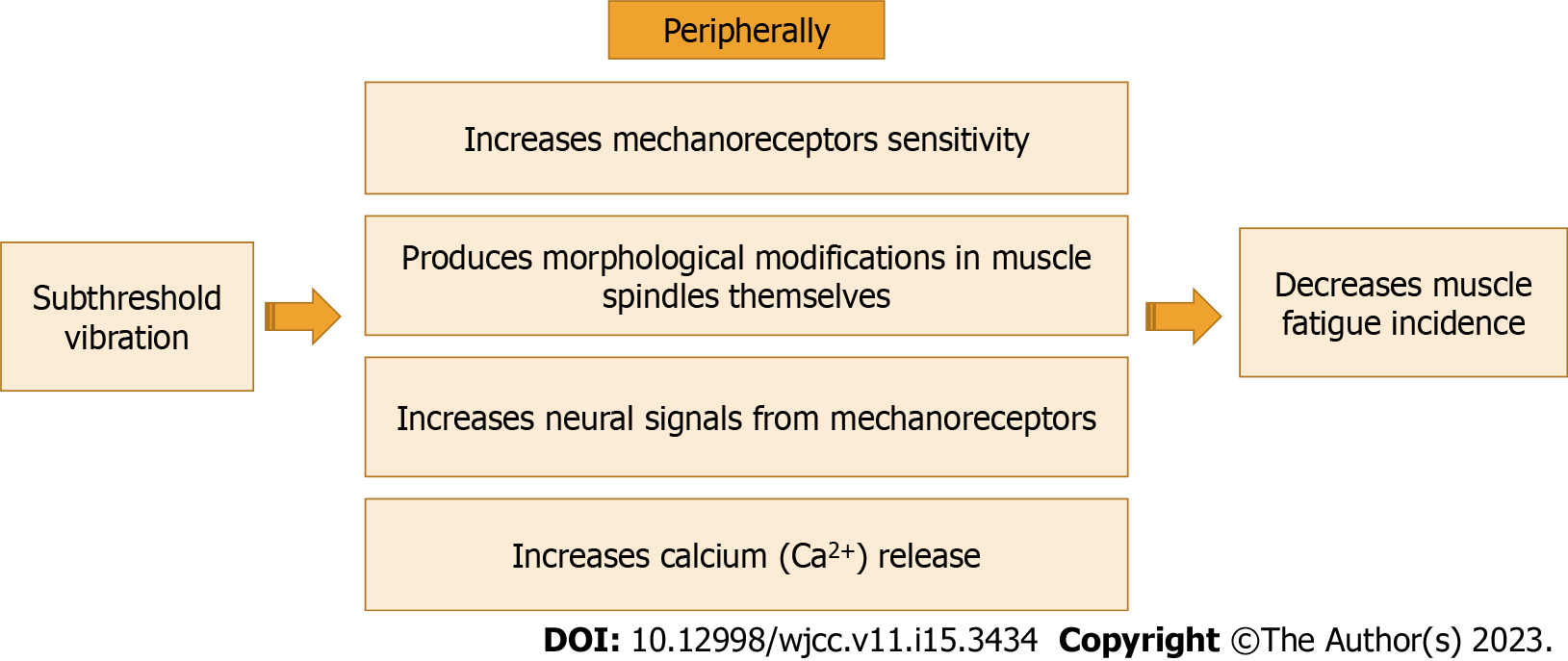
Subthreshold vibration is a proprioceptive training that can improve the function and sensitivity of mechanoreceptors themselves. Subthreshold vibration could improve the function and sensitivity of mechanoreceptors both neurologically and morphologically[51]. Subthreshold vibration could improve fatigability by improving neural signals that arise from muscle spindles and other mechanoreceptors[52]. Subthreshold vibration could produce morphological modifications in muscle spindles themselves. These morphological modifications can happen as a result of both micro-adaptations including changes in intrafusal muscle fibers as a result of metabolic modifications, and macro-adaptations including a decrease in the response latency of the stretch reflex and an increase in its amplitude[53-55]. Also, it can enhance the firing rate of α-motoneurons and reduce the disruption of the function of α-motoneurons via increasing the sensitivity of mechanoreceptors[5].
Subthreshold vibration could produce central modifications. Subthreshold vibration as a proprioceptive training can enhance the muscle spindle signals, causing plastic modifications in the central nervous system (CNS), such as improving synaptic network strength and/or normalizing the structure and numbers of networks amid neurons. Consequently, plastic modifications in the cortex occur leading to an enhancement in cortical maps of the body, and cortical representation of the joints[51].
The effect of subthreshold vibration can extend to remote areas from the application site. Plater et al[56] investigated the effect of subsensory vibration applied away from the testing site. They applied subthreshold vibration at the posterior thigh and measured the vibrotactile threshold at the calf. Also, they applied subthreshold vibration at the calf and measured the vibrotactile threshold at the sole. They found that subthreshold vibration can enhance the perception of vibrotactile inputs in hairy skin in neighborhood areas. The effect of subthreshold vibration on the mechanoreceptors themselves is shown in Figure 1.
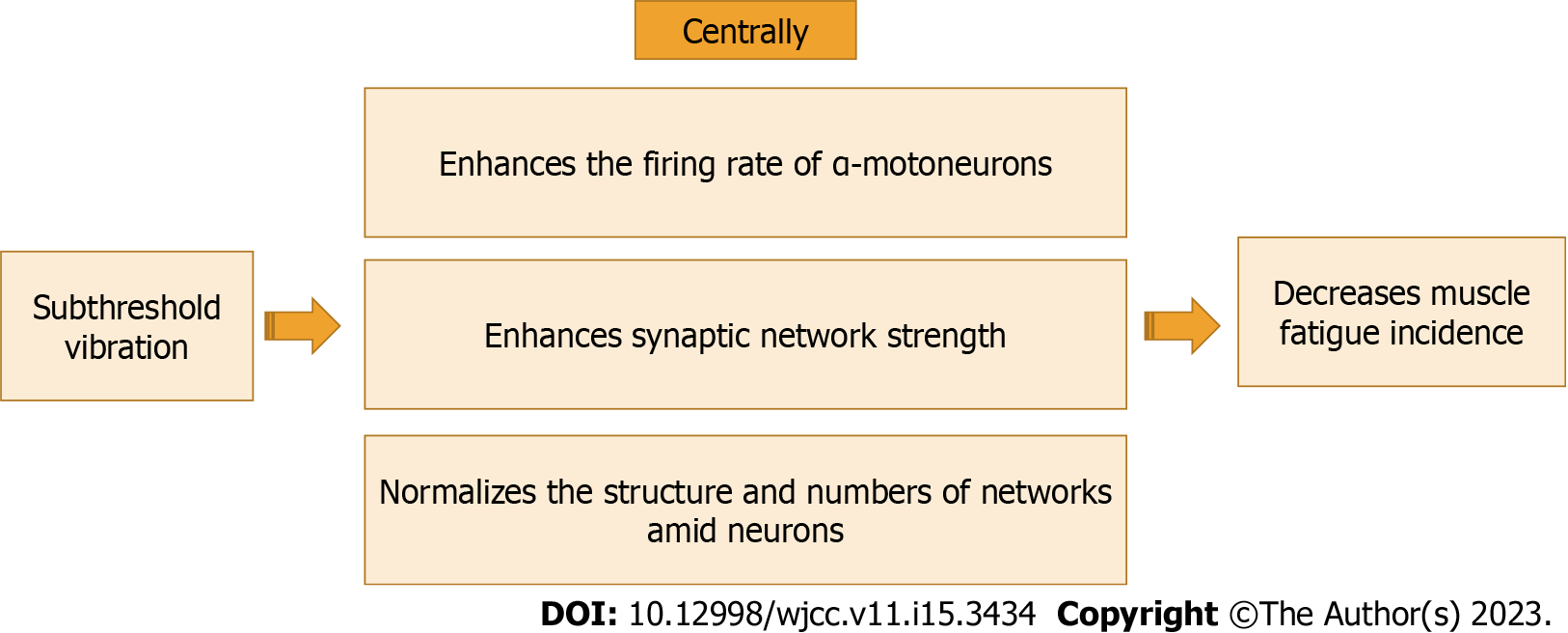
Subthreshold vibration can adjust and normalize motor neurons firing rate[5]. Normalization of motor neurons' firing rate subsequently helps in normalizing calcium secreted from calcium networks in the sarcoplasmic reticulum causing a decrease in the incidence of muscle fatigue[5]. Subthreshold vibration can decelerate the decline in the function of α-motoneurons[57-59].
This previous suggestion could be reinforced by Hospod et al[60]’s study, who found that Ia afferents significantly enhanced after proprioceptive attention task. These significant enhancements in Ia afferents occurred in the form of an enhancement in the adaptability of discharge, a reduction in neural modulation depth, and an adjustment in random activity. These previous changes caused a renormalization in the firing of α-motor neurons which consequently produced an increase in muscle performance and a reduction in the incidence of muscle fatigue.
Renormalization of α-motor neuron's firing rate can improve the secretion of Ca2+ from its channels. In-depth, it is well-established that the activation of muscle spindles happens because of increased muscle strain. Thus, the increase in muscle spindle signals causes an increase in α-motor neuron firing rate via a reflex action mediated by Ia nerve afferents. Finally, activation of extrafusal muscle fibers and muscle contraction occur[2,5]. The contraction of muscle fibers begins with acetylcholine secretion at the neuromuscular junction which extends to the synaptic cleft and activates nicotinic acetylcholine receptors within the endplate. These nicotinic acetylcholine receptors stimulate the release of calcium and the influx of cations (sodium and calcium) leading to a depolarization of the muscle cell membrane and the occurrence of muscle contraction[2]. Thus, subthreshold vibration could improve the secretion of Ca2+ ions in the neuromuscular junction, which significantly helps in decreasing the incidence of muscle fatigue[2].
Several studies found that there is a strong effect of subthreshold vibration on alpha motor neurons. Sharma et al[61] investigated the effect of applying subthreshold vibration to the sole on the cutaneous reflex generation of the lower limb. They used varying subsensory intensities (0%, 20%, 40%, 60%, 80%, or 100%) for 120 s each. They found that subthreshold vibration improved cutaneous reflex generation of the lower limb and the intensity of 20% was the best intensity among them. Seo et al[62] investigated the effect of subthreshold vibration applied to the wrist joint on the sensorimotor activity of the cortex and grip-associated desynchronization. They found that subthreshold vibration applied to the wrist joint at rest reduced electroencephalogram power and transcranial magnetic stimulation short-interval intracortical inhibition (i.e., disinhibition) compared with no vibration. Also, subthreshold vibration applied to the wrist joint at rest increased grip-associated desynchronization during vibration, compared to no vibration. The effect of subthreshold vibration on alpha motor neurons is shown in Figure 2.
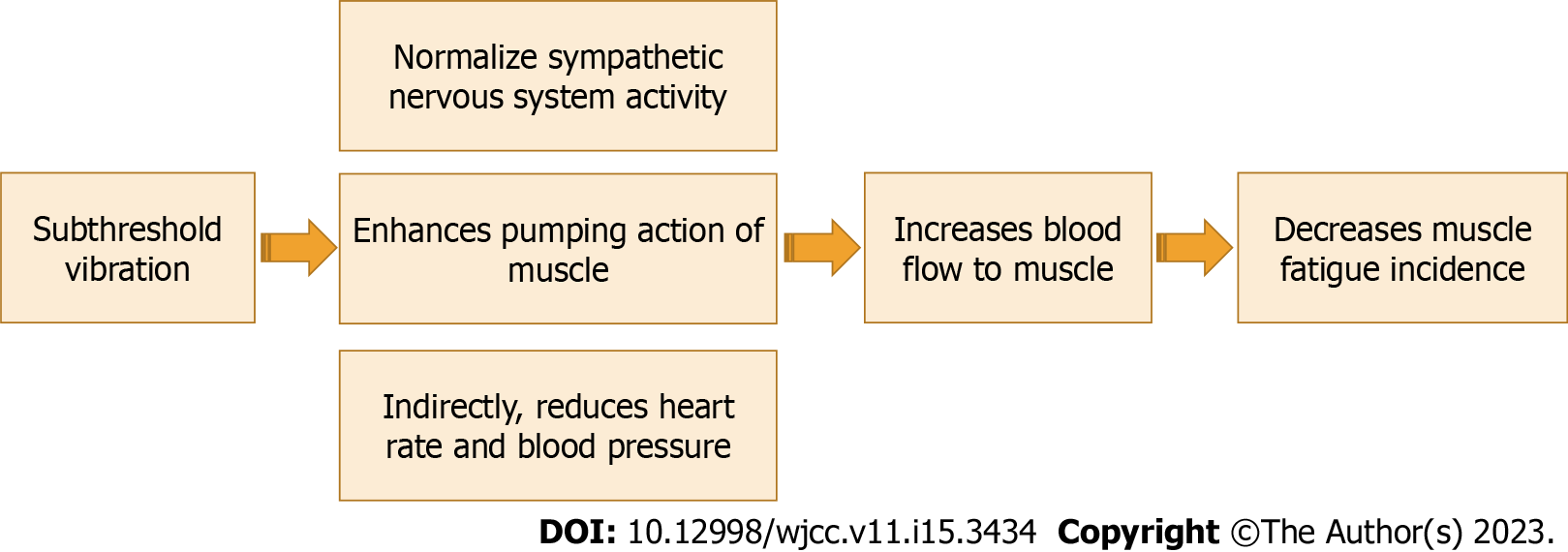
To the best of our knowledge, the effect of subthreshold vibration on blood flow to working muscles has not been demonstrated yet. The effect of subthreshold vibration on blood flow to working muscles may occur through two main mechanisms. First, muscle spindles have a connection with the sympathetic nervous system (SNS). Strong proof exists on the effect of stimulation of high threshold skin mechanoreceptors sensitive to noxious stimuli and its influence on heart rate, blood pressure, and efferent sympathetic outflow to skeletal muscle[5,63]. Thus, improving the sensitivity and function of muscle spindles might cause a decrease in the sympathetic blood flow; this would improve blood flow. Afferents from muscle spindles provide low-threshold information on muscle length. The influence of the SNS on muscle spindle receptors has been studied and concluded that stimulation of SNS decreases the activity of muscle spindles[64]. Cutaneous mechanoreceptor feedback from feet and hands can decrease the sympathetic nerve activity of muscles, causing an improvement in the blood flow to the muscle. Second, muscle spindle and Golgi tendon organs have a strong control over extrafusal muscle extrafusal muscle fibers. Proper function and sensitivity of muscle spindles cause a better contraction of the extrafusal muscle fibers, which in turn causes an increase in blood flow to working muscles (muscle pumping effect)[65,66]. Muscle spindles and Golgi tendon organs are sensitive to changes in muscle strain. Enhancing the function of muscle spindles and Golgi tendon organs functions could enhance muscle response and their pumping role in increasing the blood flow to muscles.
Studies on the effect of subthreshold vibration on blood flow to muscle are very lacking. One study conducted by Hidaka et al[67] investigated the effect of noise on heart rate and sympathetic nerve reactions to oscillatory lower body negative pressure in normal people. They found that noise significantly improved heart rate, cardiac interbeat interval, and total muscle sympathetic nerve activity. Future studies are strongly recommended to investigate this effect in both animals and humans. The effect of subthreshold vibration on blood flow to working muscles is shown in Figure 3.
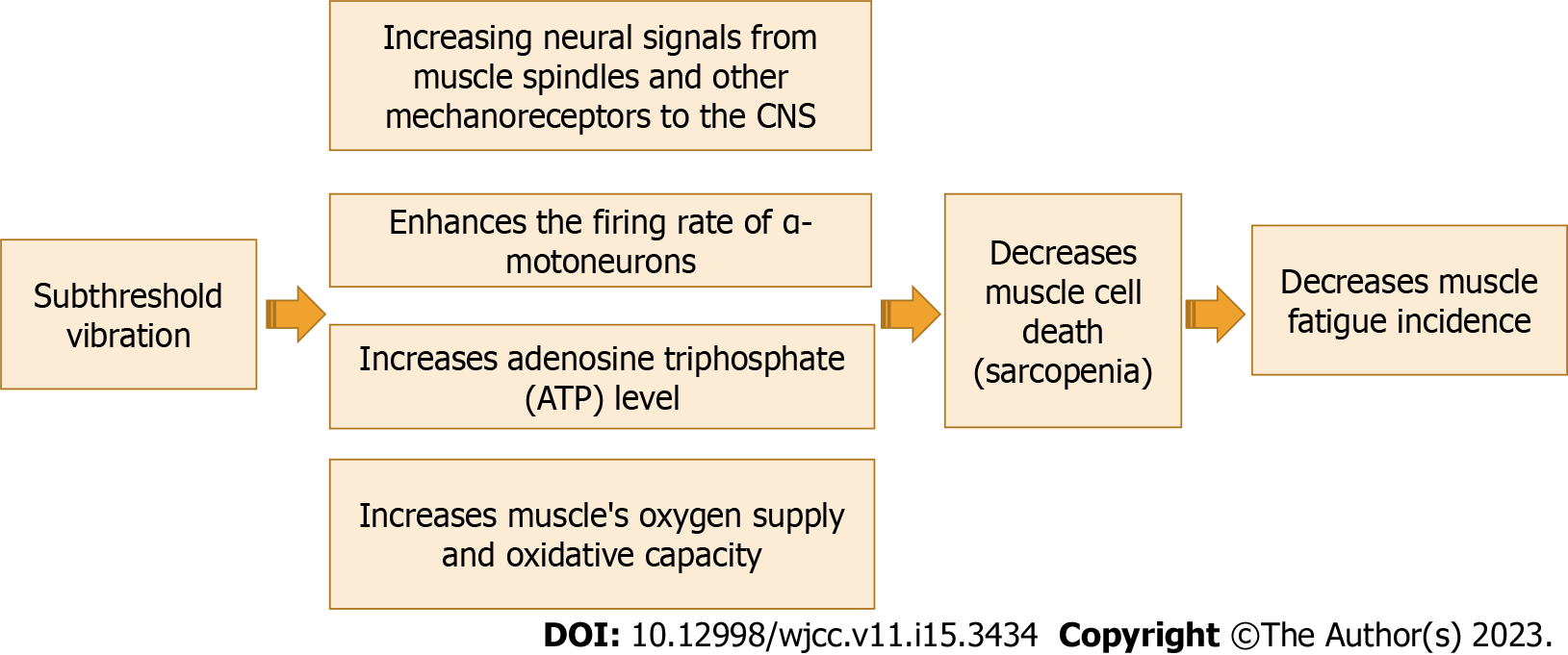
Decreased activity of muscle spindles is one of the primary mechanisms that cause a decrease in the firing rate of α -motor neurons. Subthreshold vibration as a proprioceptive training can improve the activity of muscle spindles to renormalize the firing rate of group Ia muscle afferents, presynaptic inhibition, and the firing rate of α-motor neurons[21,68,69]. Subthreshold vibration may improve fatigability and slow sarcopenia progression by improving neural signals from muscle spindles and other mechanoreceptors to the CNS leading to an increase in the firing rate of α -motor neurons and a reduction in muscle fibers loss (sarcopenia).
To the best of our knowledge, rare studies have been conducted to study the effect of subthreshold vibration on muscle strength. Kim et al[70] demonstrated that subthreshold stimulation with motor training improves functional recovery after stroke through normalizing neural reconstruction, showed by advanced neurite expression in the activated areas and associated alteration in behavior and neural spike firing rate throughout the rehabilitation after stroke. Previously, we reported that proprioceptive training might be an efficient treatment in reducing the progression rate of sarcopenia and improving the fatigability within elderly people[2]. The effect of subthreshold vibration on muscle cell death rate (sarcopenia) is shown in Figure 4.
According to the theory of motor control, sensory information has a key role in controlling motor actions[71,72]. The muscle spindle is the most important source of proprioceptive information to the CNS about limb position, movement, and velocity, as well as a sense of effort[5]. Proprioceptive feedback influences motor actions and movement accuracy in several ways, including the timing of motor command onset and coordination[73].
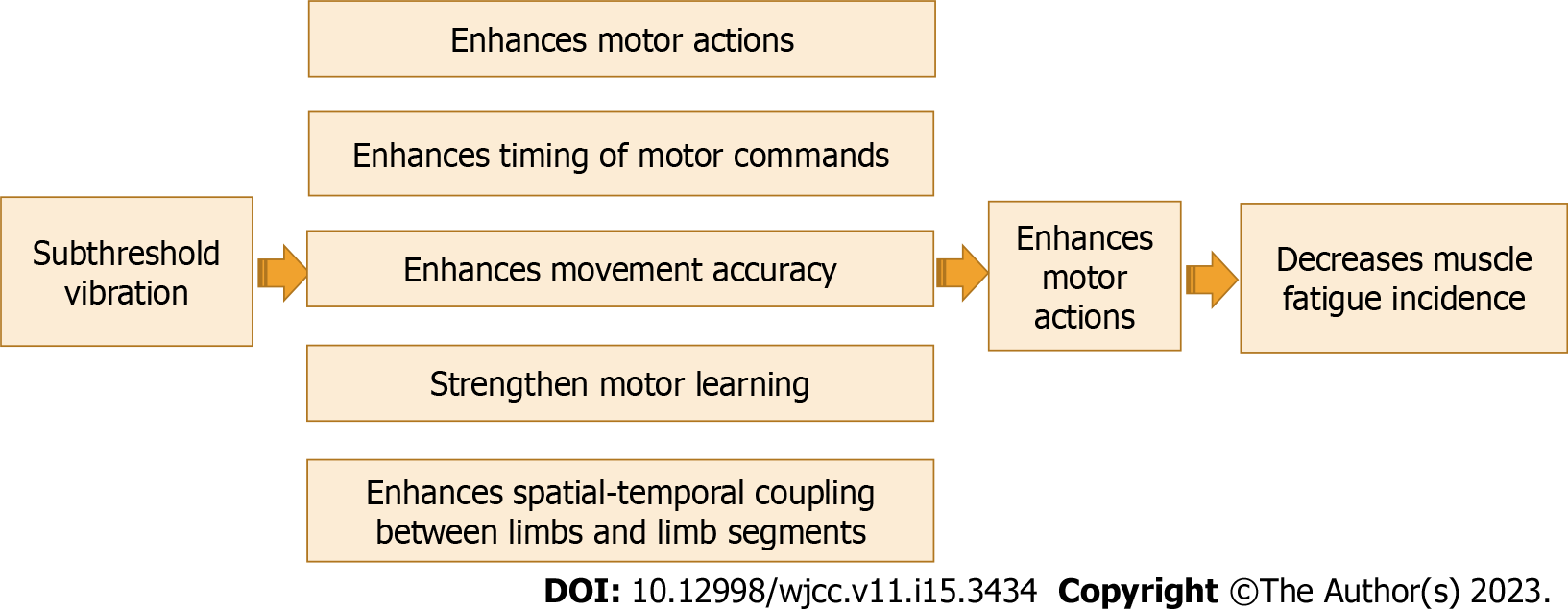
The research documented the important role of improving proprioceptive training in improving motor function and learning. Barbieri et al[74] explained the role of proprioception in controlling posture and spatial-temporal coupling among limb segments. Wong et al[75] reported that there is a connection between sensory function and motor learning and adding proprioceptive training can strengthen motor learning. Recently, Winter et al[76] demonstrated that proprioceptive training can cause significant enhancements in proprioceptive and motor function in several healthy and clinical individuals, and rehabilitative programs that aim to improve active motion are most effective in improving sensorimotor performance. A previous systematic analysis conducted by us[30] to investigate the effect of adding proprioceptive exercise to balance training in elderly people with diabetes mellites. We found that proprioceptive exercise is a crucial element in balance training to achieve short-term enhancement of balance control in elderly people with diabetes mellites. The effect of subthreshold vibration as a sensory stimulation in driving motor commands is shown in Figure 5.
The optimal role of any rehabilitative technology is to be both effective and safe[77]. Seo et al[78] reported that prolonged application of vibration for hours and days, as in rehabilitative settings, might cause modification of mechanoreceptors’ sensitization, which requires to be investigated before application of vibration in a prolonged rehabilitation setting.
Almost all studies reported no harmful effects for subthreshold vibration. Subthreshold vibration is a safe rehabilitative method that can be applied for hours and days because it is subsensory and the patient will not feel it. Regueme et al[79] investigated the effect of subthreshold vibration insole on postural stability in elderly people with Type 2 Diabetes mellites. They demonstrated that there were no adverse reactions connected to vibrating insoles. Seo et al[78] investigated the effect of subsensory vibratory vibration conducted to the skin of the wrist joint on fingertip touch evoked potentials. they used a 500 Hz, and 60% intensity of participants’ sensory threshold at the wrist. They reported no harmful effects for the immediate application of subthreshold vibration. Child et al[80] investigated the effect of prolonged subthreshold stimulation applied to subdural cortical stimulation. They use a constantly implantable 4 × 4 grid with 4-contact electrodes. They reported no harmful effects for subthreshold stimulation.
The use of subthreshold vibration could be a safe and effective treatment for muscle fatigue in elderly people over suprathreshold vibration. Subthreshold vibration could enhance the function of mechanoreceptors themselves, increase the firing rate and function of alpha motor neurons, increase blood flow to fatigued muscles, decrease the rate of muscle cell death in elderly people (sarcopenia), and drive motor commands. Subthreshold vibration also could enhance recovery from muscle fatigue.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bernardes A, Portugal; Dye DC, United States S-Editor: Liu XF L-Editor: A P-Editor: Chen YX
| 1. | Aman JE, Elangovan N, Yeh IL, Konczak J. The effectiveness of proprioceptive training for improving motor function: a systematic review. Front Hum Neurosci. 2014;8:1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 2. | Mohamed AA. Can Proprioceptive Training Enhance Fatigability and Decrease Progression Rate of Sarcopenia in Seniors? A Novel Approach. Curr Rheumatol Rev. 2021;17:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. |
Seene T, Kaasik P.
Muscle weakness in the elderly: Role of sarcopenia, dynapenia, and possibilities for rehabilitation |
| 4. | Gibbons C, Pagnini F, Friede T, Young CA. Treatment of fatigue in amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2018;1:CD011005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Mohamed AA. Can Proprioceptive Training Reduce Muscle Fatigue in Patients With Motor Neuron Diseases? A New Direction of Treatment. Front Physiol. 2019;10:1243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Nogueira DV, Silva SB, de Abreu LC, Valenti VE, Fujimori M, de Mello Monteiro CB, Tortoza C, Ribeiro W, Lazo-Osório RA, Tierra-Criollo CJ. Effect of the rest interval duration between contractions on muscle fatigue. Biomed Eng Online. 2012;11:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Dennett AM, Peiris CL, Shields N, Prendergast LA, Taylor NF. Moderate-intensity exercise reduces fatigue and improves mobility in cancer survivors: a systematic review and meta-regression. J Physiother. 2016;62:68-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Wallman KE, Morton AR, Goodman C, Grove R, Guilfoyle AM. Randomised controlled trial of graded exercise in chronic fatigue syndrome. Med J Aust. 2004;180:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Black CD, McCully KK. Time course of exercise induced alterations in daily activity in chronic fatigue syndrome. Dyn Med. 2005;4:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Oh SM, Bae WK, Choo SR, Kim HT, Kim HH, Lee SH, Jeong HS. Relationship between Changes in Fatigue and Exercise by Follow-Up Period. Korean J Fam Med. 2016;37:78-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Light AR, Vierck CJ, Light KC. Myalgia and Fatigue: Translation from Mouse Sensory Neurons to Fibromyalgia and Chronic Fatigue Syndromes. In: Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. [PubMed] |
| 12. | Boyas S, Guével A. Neuromuscular fatigue in healthy muscle: underlying factors and adaptation mechanisms. Ann Phys Rehabil Med. 2011;54:88-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Staud R. Peripheral and central mechanisms of fatigue in inflammatory and noninflammatory rheumatic diseases. Curr Rheumatol Rep. 2012;14:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Nunes GS, Bender PU, de Menezes FS, Yamashitafuji I, Vargas VZ, Wageck B. Massage therapy decreases pain and perceived fatigue after long-distance Ironman triathlon: a randomised trial. J Physiother. 2016;62:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Kuppuswamy A. The fatigue conundrum. Brain. 2017;140:2240-2245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 16. | St Clair Gibson A, Baden DA, Lambert MI, Lambert EV, Harley YX, Hampson D, Russell VA, Noakes TD. The conscious perception of the sensation of fatigue. Sports Med. 2003;33:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 906] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 18. | Green HJ. Mechanisms of muscle fatigue in intense exercise. J Sports Sci. 1997;15:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 161] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Balsom PD, Gaitanos GC, Söderlund K, Ekblom B. High-intensity exercise and muscle glycogen availability in humans. Acta Physiol Scand. 1999;165:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | McConell G, Snow RJ, Proietto J, Hargreaves M. Muscle metabolism during prolonged exercise in humans: influence of carbohydrate availability. J Appl Physiol (1985). 1999;87:1083-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Pandolf KB, Burse RL, Goldman RF. Differentiated ratings of perceived exertion during physical conditioning of older individuals using leg-weight loading. Percept Mot Skills. 1975;40:563-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Mihevic PM. Sensory cues for perceived exertion: a review. Med Sci Sports Exerc. 1981;13:150-163. [PubMed] |
| 23. | Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol (1985). 1988;64:2306-2313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 396] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Bongiovanni LG, Hagbarth KE. Tonic vibration reflexes elicited during fatigue from maximal voluntary contractions in man. J Physiol. 1990;423:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Riemann BL, Myers JB, Lephart SM. Sensorimotor system measurement techniques. J Athl Train. 2002;37:85-98. [PubMed] |
| 26. | Liao F, Yang TD, Wu FL, Cao C, Mohamed A, Jan YK. Using Multiscale Entropy to Assess the Efficacy of Local Cooling on Reactive Hyperemia in People with a Spinal Cord Injury. Entropy (Basel). 2019;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Liau BY, Wu FL, Li Y, Lung CW, Mohamed AA, Jan YK. Effect of Walking Speeds on Complexity of Plantar Pressure Patterns. Complexity. 2021. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Mohamed AA, Alawna M. The use of passive cable theory to increase the threshold of nociceptors in people with chronic pain. Phys Ther Rev. 26:53-63. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Mohamed A. Effect of exaggerated studying stress between medical students on central somatosensory conduction time: An EMG study. Res J Pharm Biol Chem Sci. 2018;9:1671-1677. |
| 30. | Mohamed AA, Jan YK. Effect of Adding Proprioceptive Exercise to Balance Training in Older Adults with Diabetes: A Systematic Review. Curr Diabetes Rev. 2020;16:327-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Zambelli G. Clinical cases. Psicoter e Sci Um. 2021;10:511-513. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Alawna M, Mohamed AA. Short-term and long-term effects of ankle joint taping and bandaging on balance, proprioception and vertical jump among volleyball players with chronic ankle instability. Phys Ther Sport. 2020;46:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Chu Y, Wang Q, Chu M, Geng B, Jia H, Li X, Lv T, Jiang S. Long-Term Effect of Vibration Therapy for Training-Induced Muscle Fatigue in Elite Athletes. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 34. | Otadi K, Ghasemi M, Jalaie S, Bagheri H, Azizian M, Emamdoost S, Sarafraz H, Sepahvand M. A prophylactic effect of local vibration on quadriceps muscle fatigue in non-athletic males: a randomized controlled trial study. J Phys Ther Sci. 2019;31:223-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Bochkezanian V, Newton RU, Trajano GS, Vieira A, Pulverenti TS, Blazevich AJ. Effect of tendon vibration during wide-pulse neuromuscular electrical stimulation (NMES) on the decline and recovery of muscle force. BMC Neurol. 2017;17:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Gomes PSC, Campos MO, Oliveira LF, Mello RGT, Fernandes IA. Whole-Body Vibration Does Not Seem to Affect Postural Control in Healthy Active Older Women. Rehabil Res Pract. 2018;2018:5798265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Dincher A, Wydra G. Effect of Whole Body Vibration on Balance in Parkinson's Disease-A Randomized Controlled Pilot Study. Alzheimer's Dis Treat. 2021;3:21-25. |
| 38. | Sambe AY, Joyce Karla Machado da Silva, Camila Costa de Araújo Pellizzari, Paola Janeiro Valenciano. Effects of muscle tendon vibration on balance in adults with stroke: a systematic review. Fisioter Pesqui. 29:311-326. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Paolucci T, Pezzi L, La Verde R, Latessa PM, Bellomo RG, Saggini R. The Focal Mechanical Vibration for Balance Improvement in Elderly - A Systematic Review. Clin Interv Aging. 2021;16:2009-2021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 40. | Adamo DE, Martin BJ, Johnson PW. Vibration-induced muscle fatigue, a possible contribution to musculoskeletal injury. Eur J Appl Physiol. 2002;88:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Seidel H, Heide R. Long-term effects of whole-body vibration: a critical survey of the literature. Int Arch Occup Environ Health. 1986;58:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 107] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Davis J, Wang Z, Zhang LL, Agresti M, Matloub HS, Yan JG. A quantitative study of vibration injury to peripheral nerves-introducing a new longitudinal section analysis. Hand (N Y). 2014;9:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Abercromby AF, Amonette WE, Layne CS, McFarlin BK, Hinman MR, Paloski WH. Variation in neuromuscular responses during acute whole-body vibration exercise. Med Sci Sports Exerc. 2007;39:1642-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 44. | Hijmans JM, Geertzen JH, Zijlstra W, Hof AL, Postema K. Effects of vibrating insoles on standing balance in diabetic neuropathy. J Rehabil Res Dev. 2008;45:1441-1449. [PubMed] |
| 45. | Sejdić E, Lipsitz LA. Necessity of noise in physiology and medicine. Comput Methods Programs Biomed. 2013;111:459-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 46. | Lipsitz LA, Lough M, Niemi J, Travison T, Howlett H, Manor B. A shoe insole delivering subsensory vibratory noise improves balance and gait in healthy elderly people. Arch Phys Med Rehabil. 2015;96:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 47. | Collins JJ. Fishing for function in noise. Nature. 1999;402:241-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Paydarfar D, Forger DB, Clay JR. Noisy inputs and the induction of on-off switching behavior in a neuronal pacemaker. J Neurophysiol. 2006;96:3338-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Bloch-Salisbury E, Indic P, Bednarek F, Paydarfar D. Stabilizing immature breathing patterns of preterm infants using stochastic mechanosensory stimulation. J Appl Physiol (1985). 2009;107:1017-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipsitz LA, Veves A, Stein J, Bonato P, Collins JJ. Noise-enhanced balance control in patients with diabetes and patients with stroke. Ann Neurol. 2006;59:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 51. | Kaya D. Exercise and Proprioception. In Proprioception: The Forgotten Sixth Sense, Foster City, USA: OMICS Group, 2016. |
| 52. | Ackerley R, Samain-Aupic L, Ribot-Ciscar E. Passive Proprioceptive Training Alters the Sensitivity of Muscle Spindles to Imposed Movements. eNeuro. 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Hutton RS, Atwater SW. Acute and chronic adaptations of muscle proprioceptors in response to increased use. Sports Med. 1992;14:406-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Mohamed AA, Jan YK, El Sayed WH, Wanis MEA, Yamany AA. Dynamic scapular recognition exercise improves scapular upward rotation and shoulder pain and disability in patients with adhesive capsulitis: a randomized controlled trial. J Man Manip Ther. 2020;28:146-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 55. | Mohamed AA, Alawna M. Effect of Adding Vertical Correction to Dynamic Scapular Recognition on Scapular Dyskinesis and Shoulder Disability in Patients With Adhesive Capsulitis: A Randomized Clinical Study. J Chiropr Med. 2022;21:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 56. | Plater EB, Seto VS, Peters RM, Bent LR. Remote Subthreshold Stimulation Enhances Skin Sensitivity in the Lower Extremity. Front Hum Neurosci. 2021;15:789271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Shumaker RG. The response of manual motor functioning in Parkinsonians to frontal EMG biofeedback and progressive relaxation. Biofeedback Self Regul. 1980;5:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | Bieńkiewicz MM, Rodger MW, Young WR, Craig CM. Time to get a move on: overcoming bradykinetic movement in Parkinson's disease with artificial sensory guidance generated from biological motion. Behav Brain Res. 2013;253:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Shih MC, Wang RY, Cheng SJ, Yang YR. Effects of a balance-based exergaming intervention using the Kinect sensor on posture stability in individuals with Parkinson's disease: a single-blinded randomized controlled trial. J Neuroeng Rehabil. 2016;13:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 60. | Hospod V, Aimonetti JM, Roll JP, Ribot-Ciscar E. Changes in human muscle spindle sensitivity during a proprioceptive attention task. J Neurosci. 2007;27:5172-5178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 61. | Sharma T, Peters RM, Bent LR. Subthreshold Electrical Noise Applied to the Plantar Foot Enhances Lower-Limb Cutaneous Reflex Generation. Front Hum Neurosci. 2020;14:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Seo NJ, Lakshminarayanan K, Lauer AW, Ramakrishnan V, Schmit BD, Hanlon CA, George MS, Bonilha L, Downey RJ, DeVries W, Nagy T. Use of imperceptible wrist vibration to modulate sensorimotor cortical activity. Exp Brain Res. 2019;237:805-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Burton AR, Fazalbhoy A, Macefield VG. Sympathetic Responses to Noxious Stimulation of Muscle and Skin. Front Neurol. 2016;7:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 64. | Hellström F, Roatta S, Thunberg J, Passatore M, Djupsjöbacka M. Responses of muscle spindles in feline dorsal neck muscles to electrical stimulation of the cervical sympathetic nerve. Exp Brain Res. 2005;165:328-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 65. | Park SY, Son WM, Kwon OS. Effects of whole body vibration training on body composition, skeletal muscle strength, and cardiovascular health. J Exerc Rehabil. 2015;11:289-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 66. | Ren W, Pu F, Luan H, Duan Y, Su H, Fan Y, Jan YK. Effects of Local Vibration With Different Intermittent Durations on Skin Blood Flow Responses in Diabetic People. Front Bioeng Biotechnol. 2019;7:310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Hidaka I, Ando S, Shigematsu H, Sakai K, Setoguchi S, Seto T, Hirooka Y, Takeshita A, Yamamoto Y. Noise-enhanced heart rate and sympathetic nerve responses to oscillatory lower body negative pressure in humans. J Neurophysiol. 2001;86:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Patino-Hernandez D, David-Pardo DG, Borda MG, Pérez-Zepeda MU, Cano-Gutiérrez C. Association of Fatigue With Sarcopenia and its Elements: A Secondary Analysis of SABE-Bogotá. Gerontol Geriatr Med. 2017;3:2333721417703734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Potvin JR, Fuglevand AJ. A motor unit-based model of muscle fatigue. PLoS Comput Biol. 2017;13:e1005581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 70. | Kim K, Yoo SJ, Kim SY, Lee T, Lim SH, Jang JE, Je M, Moon C, Choi JW. Subthreshold electrical stimulation as a low power electrical treatment for stroke rehabilitation. Sci Rep. 2021;11:14048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 71. | Chen X, Liu F, Yan Z, Cheng S, Liu X, Li H, Li Z. Therapeutic effects of sensory input training on motor function rehabilitation after stroke. Medicine (Baltimore). 2018;97:e13387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 72. | Azim E, Seki K. Gain control in the sensorimotor system. Curr Opin Physiol. 2019;8:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 73. | Dean JC. Proprioceptive feedback and preferred patterns of human movement. Exerc Sport Sci Rev. 2013;41:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Barbieri G, Gissot AS, Fouque F, Casillas JM, Pozzo T, Pérennou D. Does proprioception contribute to the sense of verticality? Exp Brain Res. 2008;185:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Wong JD, Kistemaker DA, Chin A, Gribble PL. Can proprioceptive training improve motor learning? J Neurophysiol. 2012;108:3313-3321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 76. | Winter L, Huang Q, Sertic JVL, Konczak J. The Effectiveness of Proprioceptive Training for Improving Motor Performance and Motor Dysfunction: A Systematic Review. Front Rehabil Sci. 2022;3:830166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 77. | Nelson A, Harwood KJ, Tracey CA, Dunn KL. Myths and facts about safe patient handling in rehabilitation. Rehabil Nurs. 2008;33:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Seo NJ, Lakshminarayanan K, Bonilha L, Lauer AW, Schmit BD. Effect of imperceptible vibratory noise applied to wrist skin on fingertip touch evoked potentials - an EEG study. Physiol Rep. 2015;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Regueme SC, Cowtan C, Sedgelmaci MY, Kelson M, Poustis J, Rodriguez-Mañas L, Sinclair AJ, Dallaudière B, Bourdel-Marchasson I. A Therapeutic Insole Device for Postural Stability in Older People With Type 2 Diabetes. A Feasibility Study (SENSOLE Part I). Front Med (Lausanne). 2019;6:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Child ND, Stead M, Wirrell EC, Nickels KC, Wetjen NM, Lee KH, Klassen BT. Chronic subthreshold subdural cortical stimulation for the treatment of focal epilepsy originating from eloquent cortex. Epilepsia. 2014;55:e18-e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |