Published online May 16, 2023. doi: 10.12998/wjcc.v11.i14.3288
Peer-review started: January 6, 2023
First decision: February 8, 2023
Revised: March 15, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: May 16, 2023
Processing time: 129 Days and 16.9 Hours
Hereditary spastic paraplegia (HSP) is a group of neurogenetic diseases of the corticospinal tract, accompanied by distinct spasticity and weakness of the lower extremities. Mutations in the spastic paraplegia type 4 (SPG4) gene, encoding the spastin protein, are the major cause of the disease. This study reported a Chinese family with HSP caused by a novel mutation of the SPG4 gene.
A 44-year-old male was admitted to our hospital for long-term right lower limb weakness, leg stiffness, and unstable walking. His symptoms gradually worsened, while no obvious muscle atrophy in the lower limbs was found. Neurological examinations revealed that the muscle strength of the lower limbs was normal, and knee reflex hyperreflexia and bilateral positive Babinski signs were detected. Members of his family also had the same symptoms. Using mutation analysis, a novel heterozygous duplication mutation, c.1053dupA, p. (Gln352Thrfs*15), was identified in the SPG4 gene in this family.
A Chinese family with HSP had a novel mutation of the SPG4 gene, which is autosomal dominant and inherited as pure HSP. The age of onset, sex distribution, and clinical manifestations of all existing living patients in this family were analyzed. The findings may extend the current knowledge on the existing mutations in the SPG4 gene.
Core Tip: It is difficult to distinguish hereditary spastic paraplegia (HSP) from other spasticity-related genetic diseases because the different affected genes lead to large differences in the pathogenic mechanisms, clinical features, and imaging abnormalities of HSP. Therefore, genetic testing is important for the diagnosis and typing of HSP. A Chinese HSP male patient was identified, and pedigree surveys of his relatives were performed. Furthermore, genomic DNA was extracted for whole-exome sequencing, and pathogenic variants were screened by bioinformatics methods and verified using Sanger sequencing. A novel heterozygous duplication mutation, c.1053dupA, p. (Gln352Thrfs*15), was identified in the SPG4 gene in this family.
- Citation: Wang J, Bu WT, Zhu MJ, Tang JY, Liu XM. Novel mutation of SPG4 gene in a Chinese family with hereditary spastic paraplegia: A case report. World J Clin Cases 2023; 11(14): 3288-3294
- URL: https://www.wjgnet.com/2307-8960/full/v11/i14/3288.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i14.3288
Hereditary spastic paraplegia (HSP) is a group of neurogenetic diseases caused by degeneration of the corticospinal tract, which is characterized by slow progressive spasms and weakness of the lower limbs. The disease has obvious clinical and genetic heterogeneity[1]. According to their clinical manifestations, HSP is divided into pure and complex types. The pure type presents with slowly progressive lower extremity weakness and spasticity, corticospinal tract signs, disturbance in the vibration sense and proprioception, and possible sphincter disturbances. Complex HSP is characterized by leg spasticity and other complications, such as ataxia, a thin corpus callosum, extrapyramidal signs, chorioretinal dystrophy, peripheral neuropathy, and mental retardation[2,3]. HSP can be inherited by autosomal dominant (AD), autosomal recessive, X-linked, and mitochondrial maternal patterns. More than 80 genes or loci involved in the inheritance of this disease have been identified. Spastic paraplegia type 4 (SPG4) is the most common cause of pure HSP, accounting for 45% of pure HSP cases. It is inherited by AD mode and is caused by the mutation of the SPAST gene[1,4,5].
Seven years before his admission to our hospital, a 44-year-old male (patient V: 4) experienced right lower limb weakness, leg stiffness, and unstable walking. The symptoms appeared without obvious inducement. The patient was from the Han Chinese population in Shandong Province, China.
Initially, he felt that his symptoms were mild. He was never treated, and his symptoms gradually worsened over time. In the 4th year after onset, he began to experience hotness in his right knee joint, his gait became increasingly slow, his legs felt stiff, and lumbago appeared. The patient’s lumbago symptoms were most severe at night during sleep and in the morning. Meanwhile, the patient felt that the range of motion of both knees was limited, which aggravated when he was tired. He could not run and had difficulty climbing stairs.
The patient was healthy before. The patient denied a history of surgery and trauma, diabetes mellitus, cardiac disease, or infectious diseases.
His family history revealed that he was born to non-consanguineous parents, but similar symptoms were found in several of his relatives.
Neurological examination showed that the cranial nerves of the proband were not abnormal. The proximal and distal muscle strength and muscle tone of double upper extremities were normal. Biceps reflexes, triceps reflexes, and radial reflexes were normal, and bilateral Hoffman signs were negative. The proximal and distal muscle strength of both lower limbs was normal. Lower limb hypermyotonia and bilateral knee reflex were enhanced. Bilateral Chaddock’s sign, Oppenheim sign, and Gordon sign were negative, while Babinski sign was positive. There was no muscle atrophy or abnormal involuntary movement.
The proband was initially suspected of having myelopathy. Further laboratory examinations, such as cerebrospinal fluid sample analysis and determination of the serum vitamin B12 level, were conducted. No inflammatory or immune lesions were detected. Neuro-electrophysiological examination suggested normal motor nerve conduction, sensory nerve conduction, and needle electromyography.
The magnetic resonance imaging of the cervical spinal cord, thoracic spinal cord, and lumbar spinal cord revealed only intervertebral disc herniation of the cervical and lumbar spine. No obvious swelling, atrophy, or compression of the spinal cord was found.
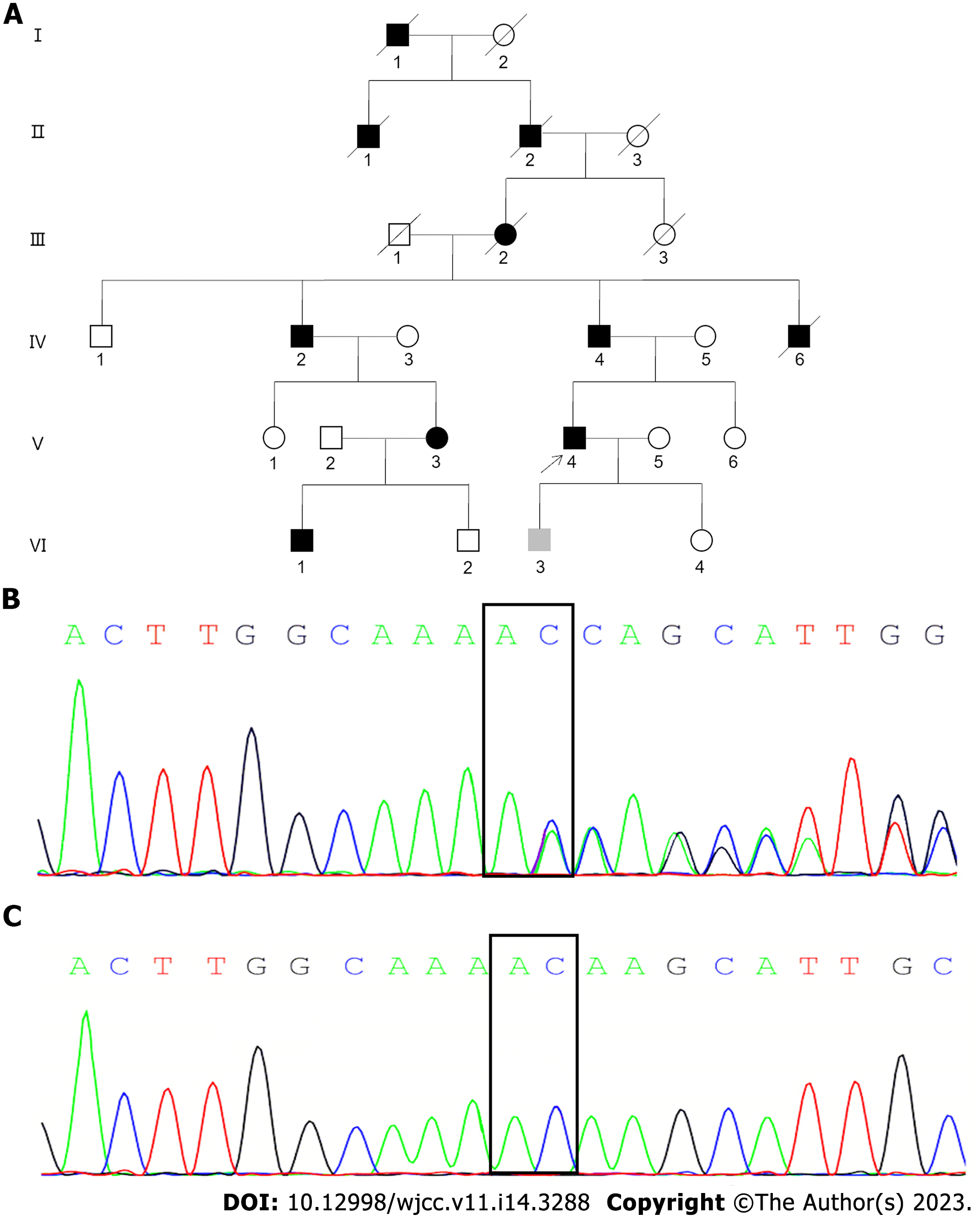
After obtaining written informed consent from the proband and his relatives, a detailed investigation of this Han Chinese family from Shandong Province, China was conducted. This family was traced back to the sixth generation, with a total of 11 people suffering from the disease, with an AD genetic pattern (Figure 1A). Five deceased individuals had spastic paraplegia in their lifetime, including four males (patients I: 1, II: 1, II: 2, and IV: 6) and one female (patient III: 2). Of the six living patients, five were male (patients IV: 2, IV: 4, V: 4, VI: 1, and VI: 3), and one was female (patient V: 3).
Other living and symptomatic patients initially showed weak legs or unstable walking. They developed different clinical symptoms as they grew older and the course of the disease progressed. The proband’s father (patient IV: 4) and uncle (patient IV: 2) were affected by the illness in middle age, with a long course of disease, severe symptoms, difficulty in lifting the legs, and a scissor gait. Neurological examination revealed the increased lower limb hypermyotonia and tendon hyperreflexia, and bilateral Babinski signs were positive. They started using crutches to assist walking approximately 20 years and 10 years after onset, respectively. The proband’s nephew (VI: 1), now 20 years old, developed a gait disturbance at the age of 10-years-old. Although there was obvious difficulty in walking, he did not need the assistance of appliances. The proband’s cousin (V: 3) was a female patient with an onset age of 46-years-old and a disease course of 2 years who presented with mild weakness of both lower limbs. The son (VI: 3) of the proband, aged 19-years-old, was an asymptomatic mutation carrier. The mean age of onset of symptoms was 36.6 ± 14.1-years-old. The disease course was 14.8 ± 10.9 years. All the surviving patients were intellectually normal with no cognitive impairment, peripheral neuropathy, bladder dysfunction, and claw-feet. No scoliosis was detected. Their age of onset, course of disease, sex, spastic paraplegia rating scale[6], and disability stage were recorded and evaluated. The spastic paraplegia rating scale is a composite measurement modality. It includes walking distance without pause, gait speed, quality of gait, climbing stairs, arising from chair, spasticity quality, weakness and contractures, pain caused by spastic paraplegia-related symptoms, and bladder dysfunction. The score for each item ranged from 0 (no dysfunction) to 4 (the most severe dysfunction), and the highest total score of all items is 52. Thus, higher spastic paraplegia rating scale scores represent heavier dysfunction. Additional data are presented in Table 1.
| Patient | Sex | Age at onset, yr | Duration of the disease, yr | Hyperreflexia | Spasticity | Decreased vibration sense | Sensory impairment | Stagesa | SPRS |
| IV: 2 | Male | 50 | 24 | + | + | - | N | 4 | 28 |
| IV: 4 | Male | 40 | 31 | + | + | - | N | 4 | 30 |
| V: 3 | Female | 46 | 2 | - | - | - | N | 1 | 3 |
| V: 4 | Male | 37 | 7 | + | + | - | N | 3 | 20 |
| VI: 1 | Male | 10 | 10 | + | + | - | N | 2 | 16 |
| VI: 3 | Male | - | - | - | - | - | N | 1 | 0 |
After obtaining written informed consent from participants, DNA was extracted from seven peripheral blood samples, including the proband (V: 4), his father (IV: 4), his mother (IV: 5), his wife (V: 5), his sister (V: 6), his son (VI: 3), and his daughter (VI: 4). Whole-exome sequencing was first performed, pathogenic variants were analyzed by bioinformatics methods, and were then verified using Sanger sequencing. It was found that the proband (V: 4) and the patient (IV: 4, VI: 3) carried a pathogenic heterozygous variant of the SPG4 gene, namely c.1053dupA, p. (Gln352Thrfs*15), located at the shear site of the SPG4 exon 7 (Figures 1B and C). This mutation has not previously been reported. It was also not registered in the Clinvar, dbSNP, and HGMD databases.
Referring to the Harding diagnostic criteria[7] and based on genetic testing, the proband and other related relatives were finally diagnosed with SPG4 HSP.
The proband was given an intravenous injection of methylcobalamin (500 μg/d) for 2 wk; then the route was changed to oral administration (1.5 mg/d) for 3 mo. He also took baclofen orally (15 mg/d at the initial stage, which then increased to 30 mg/d) for 6 wk.
Although his low back pain was resolved somewhat, his slow walking and stiffness in both lower limbs were not significantly improved. He still could not run, and he had difficulty climbing the stairs.
HSP is a nervous system disease, accompanied by diverse clinical manifestations and complex genetic etiology and pathogenesis. The onset age ranges from infancy to senility, and the functional impairment is highly variable[4]. The onset age span of patients in the present study was large, in which the largest age span between two relatives was 40 years. The majority of patients developed the disease in middle age.
According to the clinical symptoms, it was revealed that the pure HSP type was dominant in the proband’s family, including progressive weakness of the lower extremities and spasticity[1-4]. Notably, in this family, male patients were more affected by the disease than female patients, with a male to female ratio of 9:2, which is consistent with the findings of another study[8]. Earlier reports have also confirmed that patients with SPG4 gene mutation-related diseases were mostly male, suggesting that in some cases sex is a stronger contributing factor to the time of onset of disease symptoms than age[9]. This may be associated with the higher levels of estrogen and progesterone in female patients[10,11]. Due to the genetic and clinical heterogeneity of HSP, its phenotype is complex, and the diagnosis is difficult, which may be attributed to the effects of genetic factors, environmental modifiers, penetrance, and sex[8,12,13].
In the present study, a novel mutation, c.1053dupA, p. (Gln352Thrfs*15), was detected in the SPG4 gene in the family with HSP. This variant is a frameshift mutation that results in a Gln 352-to-Thr substitution and a new reading frame and is terminated at codon 15 downstream of the amino acid at position 352, causing early translation termination compared with the wild-type protein. This may lead to truncation of the coding protein synthesis, thereby losing its normal function. This novel variant was predicted to be deleterious by Mutation Taster analysis (http://www.mutationtaster.org/MT69/MutationTaster69.cgi?bases_inserted=A&end_insdel=1054&start_insdel=1053&transcript_stable_id_text=ENST00000315285&sequence_type=CDS&gene=spast). The DNA sequence variants were named following the guidelines of the Human Genome Variation Society (https://varnomen.hgvs.org/recommendations/general/).
In 2009 and 2010, two mutations of the SPG4 gene were reported in two families, including c.1055A > C, p. (Gln352Pro) and c.1054C > T, p. (Gln352X), respectively[14,15]. Both are mutations caused by a single-base substitution, one being a missense mutation and the other being a nonsense mutation. These two previously detected mutations and our newly identified mutation are located within the conserved adenosine triphosphatases associated with diverse cellular activities cassette, resulting in changes in activity of the spastin protein and loss of function. The findings of the present study suggest that mutations in this region are not uncommon. To date, at least 80 genes and several variants have been found to be associated with HSP, of which those of SPG4 cause the most common type of HSP[9,16,17]. Most of the mutations of the SPG4 gene are missense (33%), frameshift (24%), splice-site (16%), nonsense variants, and deletions (12%)[4].
The SPG4 gene encodes a microtubule (MT)-severing protein, namely spastin, which is an MT-severing enzyme containing an MTbinding domain and adenosine triphosphatases associated with diverse cellular activity domains with adequate severing activity, playing an important role in axon development, synaptic formation, and spinal cord maturation[18-20]. A number of factors lead to mutations in the SPG4 gene and the onset of related disease symptoms, of which the main factor is a decrease in the level of the functional spastin protein, resulting in insufficient MT cutting[18-21]. Another study found that aggregation of the mutant spastin protein caused toxicity, while it could not explain the underlying mechanisms and possible consequences[21]. Qiang et al[22] demonstrated that the function acquisition mechanism of SPG4 gene is more meaningful than its function loss mechanism.
Only symptomatic treatment of HSP is currently available. In the present study, the proband was treated with methylcobalamin and baclofen, which only relieved his low back pain, while the other symptoms were not improved significantly. Therefore, it is critically important to find an effective treatment for this disease. A recent study proposed a new direction of targeted therapeutic application[23]. The results of this investigation showed that the mutation of the SPG4 gene was not only associated with haploinsufficiency causing decreased spastin function but also could be one of the important pathogenic factors of spastin function dysregulation. The phosphorylation of S268 mediated by HIPK2 may contribute to the stability of the spastin protein and rescue HSP neurite defects.
In conclusion, a newly pathogenic mutation was proposed, expanding the existing knowledge of the spectrum of mutations of the SPG4 gene. The findings may provide a reliable basis for further research on the genetic etiology and pathogenesis of HSP. The diagnosis and typing of HSP through genetic analysis can control and treat the disease as well as avoid the transmission of pathogenic genes in the family.
The authors thank all participants for their active contribution to this study. The authors also thank reviewers for their comments and suggestions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Orlacchio A, Italy; Yahya FS, Iraq S-Editor: Liu XF L-Editor: Filipodia P-Editor: Cai YX
| 1. | Murala S, Nagarajan E, Bollu PC. Hereditary spastic paraplegia. Neurol Sci. 2021;42:883-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Shribman S, Reid E, Crosby AH, Houlden H, Warner TT. Hereditary spastic paraplegia: from diagnosis to emerging therapeutic approaches. Lancet Neurol. 2019;18:1136-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 3. | Walusinski O. A historical approach to hereditary spastic paraplegia. Rev Neurol (Paris). 2020;176:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Lallemant-Dudek P, Durr A. Clinical and genetic update of hereditary spastic paraparesis. Rev Neurol (Paris). 2021;177:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Meyyazhagan A, Orlacchio A. Hereditary Spastic Paraplegia: An Update. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 6. | Schüle R, Holland-Letz T, Klimpe S, Kassubek J, Klopstock T, Mall V, Otto S, Winner B, Schöls L. The Spastic Paraplegia Rating Scale (SPRS): a reliable and valid measure of disease severity. Neurology. 2006;67:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Harding AE. Classification of the hereditary ataxias and paraplegias. Lancet. 1983;1:1151-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 649] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Parodi L, Fenu S, Barbier M, Banneau G, Duyckaerts C, Tezenas du Montcel S, Monin ML, Ait Said S, Guegan J, Tallaksen CME, Sablonniere B, Brice A, Stevanin G, Depienne C, Durr A; SPATAX network. Spastic paraplegia due to SPAST mutations is modified by the underlying mutation and sex. Brain. 2018;141:3331-3342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Erfanian Omidvar M, Torkamandi S, Rezaei S, Alipoor B, Omrani MD, Darvish H, Ghaedi H. Genotype-phenotype associations in hereditary spastic paraplegia: a systematic review and meta-analysis on 13,570 patients. J Neurol. 2021;268:2065-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 10. | Orlacchio A, Kawarai T, Gaudiello F, Totaro A, Schillaci O, Stefani A, Floris R, St George-Hyslop PH, Sorbi S, Bernardi G. Clinical and genetic study of a large SPG4 Italian family. Mov Disord. 2005;20:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Hu R, Sun H, Zhang Q, Chen J, Wu N, Meng H, Cui G, Hu S, Li F, Lin J, Wan Q, Feng H. G-protein coupled estrogen receptor 1 mediated estrogenic neuroprotection against spinal cord injury. Crit Care Med. 2012;40:3230-3237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Boutry M, Morais S, Stevanin G. Update on the Genetics of Spastic Paraplegias. Curr Neurol Neurosci Rep. 2019;19:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Rodrigues R, Silva R, Branco M, Brandão E, Alonso I, Ruano L, Loureiro JL. Determinants of age at onset in a Portuguese cohort of autosomal dominant spastic paraplegia. J Neurol Sci. 2020;410:116646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Loureiro JL, Miller-Fleming L, Thieleke-Matos C, Magalhães P, Cruz VT, Coutinho P, Sequeiros J, Silveira I. Novel SPG3A and SPG4 mutations in dominant spastic paraplegia families. Acta Neurol Scand. 2009;119:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Alvarez V, Sánchez-Ferrero E, Beetz C, Díaz M, Alonso B, Corao AI, Gámez J, Esteban J, Gonzalo JF, Pascual-Pascual SI, López de Munain A, Moris G, Ribacoba R, Márquez C, Rosell J, Marín R, García-Barcina MJ, Del Castillo E, Benito C, Coto E; Group for the Study of the Genetics of Spastic Paraplegia. Mutational spectrum of the SPG4 (SPAST) and SPG3A (ATL1) genes in Spanish patients with hereditary spastic paraplegia. BMC Neurol. 2010;10:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Yang JO, Yoon JY, Sung DH, Yun S, Lee JJ, Jun SY, Halder D, Jeon SJ, Woo EJ, Seok JM, Cho JW, Jang JH, Choi JK, Kim BJ, Kim NS. The emerging genetic diversity of hereditary spastic paraplegia in Korean patients. Genomics. 2021;113:4136-4148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | McDermott C, White K, Bushby K, Shaw P. Hereditary spastic paraparesis: a review of new developments. J Neurol Neurosurg Psychiatry. 2000;69:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Liu Q, Zhang G, Ji Z, Lin H. Molecular and cellular mechanisms of spastin in neural development and disease (Review). Int J Mol Med. 2021;48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Sandate CR, Szyk A, Zehr EA, Lander GC, Roll-Mecak A. Author Correction: An allosteric network in spastin couples multiple activities required for microtubule severing. Nat Struct Mol Biol. 2020;27:400. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Kelliher MT, Saunders HA, Wildonger J. Microtubule control of functional architecture in neurons. Curr Opin Neurobiol. 2019;57:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 21. | Rehbach K, Kesavan J, Hauser S, Ritzenhofen S, Jungverdorben J, Schüle R, Schöls L, Peitz M, Brüstle O. Multiparametric rapid screening of neuronal process pathology for drug target identification in HSP patient-specific neurons. Sci Rep. 2019;9:9615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Qiang L, Piermarini E, Baas PW. New hypothesis for the etiology of SPAST-based hereditary spastic paraplegia. Cytoskeleton (Hoboken). 2019;76:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Sardina F, Pisciottani A, Ferrara M, Valente D, Casella M, Crescenzi M, Peschiaroli A, Casali C, Soddu S, Grierson AJ, Rinaldo C. Spastin recovery in hereditary spastic paraplegia by preventing neddylation-dependent degradation. Life Sci Alliance. 2020;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |