Published online May 16, 2023. doi: 10.12998/wjcc.v11.i14.3176
Peer-review started: February 27, 2023
First decision: March 10, 2023
Revised: March 24, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: May 16, 2023
Processing time: 78 Days and 1.7 Hours
Follicular lymphoma (FL) is a type of B-cell lymphoma that originates at the germinal center and has a low malignancy rate. FL has become the most common inert lymphoma in Europe and America but has a relatively low incidence in Asia.
To explore the clinical features, curative effects, and prognostic factors of FL.
Completed medical records of 49 patients with FL who were admitted to the Ningbo First Hospital from June 2010 to June 2021 were examined. These patients were definitively diagnosed by pathological biopsy or immunohistochemical staining. The diagnostic criteria were based on the 2008 World Health Orga
The age of onset in patients ranged from 24 to 76 years, with a median age of 51 years. Most patients developed the disease at 40–59 years of age, and the male:female ratio was 1.6:1. No significant difference was noted in the curative effect between the non-chemotherapy, combined chemotherapy, and other chemotherapy regimens (P > 0.05). Hemoglobin (Hb) level < 120 g/L, Ki-67 value > 50%, bone marrow involvement, and clinical stages III–IV were associated with a poor prognosis of FL (P < 0.05). However, the influence of other indicators was not statistically significant. Risk grouping was performed using the FLIPI, and the results showed that 24.5%, 40.8%, and 34.7% of patients were in the low-, moderate-, and high-risk groups, respectively. According to the survival analysis results, the survival rate of patients was lower in the high-risk group than in the other low-risk and moderate-risk groups (P < 0.05).
FL mainly occurs in middle-aged and elderly men, primarily affecting lymph nodes and bone marrow. Hb level, Ki-67 value, bone marrow involvement, and clinical staging were used to evaluate prognosis.
Core Tip: Follicular lymphoma (FL) is a B-cell lymphoma originating from the germinal center and has a low malignancy rate. Completed medical records of 49 patients with follicular lymphoma admitted from June 2010 to June 2021 were retrospectively reviewed. FL was more common in middle-aged and elderly men than in women, mainly involving the lymph nodes and bone marrow. However, an optimal treatment strategy remains unclear. Hemoglobin levels, Ki-67 values, bone marrow involvement, and clinical stage can be used to evaluate prognosis.
- Citation: Wu H, Sun HC, Ouyang GF. Clinical features and prognostic factors in 49 patients with follicular lymphoma at a single center: A retrospective analysis. World J Clin Cases 2023; 11(14): 3176-3186
- URL: https://www.wjgnet.com/2307-8960/full/v11/i14/3176.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i14.3176
Follicular lymphoma (FL) is a B-cell lymphoma derived from the germinal center of follicles with the genetic hallmark t (14; 18) (q32; q21). Characterized by a low malignancy rate, FL has become the most common inert lymphoma in Europe and America, accounting for approximately 22% of non-Hodgkin lymphomas (NHL)[1]. In contrast, the incidence of FL in Asia is relatively low[2]. For example, patients with FL in China accounted for approximately 6%–10% of all NHL patients in 2008-2010 period[3]. To provide deep insights into the clinical features, curative effects, and prognostic factors of FL in China and to provide guidance for clinical diagnosis and treatment, this retrospective analysis aimed to examine the clinical data, such as clinical features, curative effects, and prognostic survival factors, of patients with FL who were diagnosed and treated at our hospital in recent years. Through this study, we aim to improve the understanding of this disease among clinicians and assist with diagnosis and treatment in clinical practice.
Completed medical records of 49 patients with FL who were admitted to the Ningbo First Hospital from June 2010 to June 2021 were collected. These patients were definitively diagnosed by pathological biopsy or immunohistochemical staining. The diagnostic criteria were based on the 2008 World Health Organization (WHO) classification of lymphomas[4]. Ann Arbor staging was performed according to the imaging and bone marrow examination results. Risk stratification was performed for all patients based on the International Prognostic Index (IPI), age-adjusted IPI, Follicular Lymphoma International Prognosis Index (FLIPI), and Follicular Lymphoma International Prognosis Index 2 (FLIPI2)[5,6]. Medical follow-up was conducted through hospitalization, outpatient services, or telephone calls from the date of diagnosis to June 2021. After completing all treatments, patients in the remission stage [Complete remission (CR) + Partial remission (PR)] were followed up once every 2–3 mo in the first 2 years and once every 6 mo thereafter. The extent of follow-up was mainly determined according to the treatment of the patient at the onset of FL and related abnormal laboratory examination findings.
General indicators: The clinical data of 49 patients with FL were retrospectively analyzed. These included age, sex, initial site of FL, the presence or absence of B symptoms, number of extralymphatic involvement sites, hepatomegaly, and splenomegaly.
Laboratory indicators: These indicators included hemoglobin (Hb) level, platelet count (PLT), white blood cell (WBC) count, absolute lymphocyte (LYM) count, albumin (ALB) level, globulin (GLB) level, lactate dehydrogenase (LDH) level, hydroxybutyrate dehydrogenase (HBDH) level, β2-microglobulin (β2-MG) level, immune phenotype, pathological grade, and Ki-67 value.
Clinical stage: All patients underwent computed tomography (CT) and bone marrow biopsy, and clinical staging was performed based on the Ann Arbor standards.
Survival and prognostic indicators: Survival time, overall survival (OS), progression-free survival (PFS), and related prognostic factors (such as age, pathological grade, clinical stage, LDH level, Hb level, and Ki-67 value) were analyzed.
The initial therapeutic schemes were classified as follows: Scheme I involved specifically targeted therapy without combined chemotherapy, including rituximab combined with lenalidomide in 10 patients and single-agent lenalidomide or rituximab in 4 patients (total: 14 patients); Scheme II involved rituximab combined with chemotherapy regimen, including rituximab or lenalidomide. Combined cyclophosphamide + doxorubicin + vincristine + prednisone (CHOP) regimen was administered to 30 patients, and rituximab-combined cyclophosphamide + vincristine + prednisone (COP) regimen was administered to 2 patients (total: 32 patients); Scheme III involved other regimens, including rituximab plus bortezomib combined with CHOP regimen in 2 patients and an FC (fluorouracil + cyclophosphamide) regimen in 1 patient, and was not included in the comparison of curative effects. The drug doses in all schemes were calculated based on the standard dose and body surface area.
The short-term curative effect was evaluated according to the evaluation criteria for malignant lymphomas, including CR, PR, stable disease (SD), and progressive disease (PD), while CR + PR constituted the objective remission rate.
The patients were evaluated once every two regular chemotherapy sessions with respect to curative effects and then re-evaluated after every two courses of treatment. Patients were classified based on CR, unconfirmed complete remission (CRu), PR, SD, PD, and relapse (those who reached CR/CRu in the early stages). Patients who met the first three criteria were regarded to have effective samples, whereas those meeting the remaining criteria were regarded to have ineffective samples.
Statistical analyses were performed using SPSS version 25.0. Data with normal distribution are presented as mean ± SD, and data with non-normal distribution are presented as median. Fisher’s exact probability method was used to compare the curative effects of the different schemes. Survival data and prognosis were analyzed using the Kaplan–Meier method, and survival rates were compared using the log-rank test. Survival time, OS, PFS, and related prognostic factors (such as age, pathological grade, clinical stage, regular indicators, and Ki-67 value) were analyzed. P < 0.05 indicated statistically significant difference for all tests.
The clinical data of 49 patients with FL were retrospectively analyzed, and the clinical characteristics, efficacy, prognosis, and survival factors were analyzed to improve the clinicians’ understanding of this disease and provide guidance for clinical diagnosis and treatment.
Follow up results: Loss to follow-up (LTFU) was noted in three patients (LTFU rate: 6.1%), with a median follow-up time of 52 (2–85) mo.
Sex and age: Among the 49 patients, 30 (61.2%) were male and 19 (38.8%) were female patients, with male:female ratio of 1.6:1. The age at disease onset ranged from 24 to 76 years, with a median age of 51 years [17 (34.7%) patients aged > 60 years and 32 (65.3%) patients aged ≤ 60 years]. Overall, approximately 51% (n = 25) developed the disease between 40 and 59 years of age.
Pathological grade and clinical stage: The pathological grade and clinical stage of the 49 patients were determined according to the 2008 WHO classification of lymphomas and the Ann Arbor staging standard. Eventually, 3 (6.1%) patients had Grade I FL, 16 (32.7%) had Grade II FL, and 30 (61.2%) had Grade III FL. In addition, 17 (34.7%) patients had Stage I FL, 32 (65.3%) had Stage II–III FL, and 31 (63.3%) had B symptoms. Among patients with Stage III–IV FL, 19 (59.4%) had B symptoms, 20 (40.9%) had anemia (Hb level < 120 g/L), 6 (18.8%) had PLT count ≤ 80 × 109/L, 24 (75.0%) had LYM count > 1 × 109/L, and 29 (90.6%) had increased LDH level.
Initial site and extra-lymphatic involvement: Overall, 35 patients (71.4%) initially presented with enlarged lymph nodes and 14 (28.6%) initially presented with extralymphatic involvement. Among those initially presenting with extralymphatic involvement, the symptoms included an enlarged abdominal mass, splenomegaly, enlarged leg mass, enlarged tonsils, gastrointestinal tract involvement, and bone marrow involvement. Overall, 19 patients (38.8%) had extralymphatic node tissue or organ involvement, including 10 (20.4%) patients with bone marrow involvement, which was confirmed by clinical imaging such as endoscopy and CT and pathological biopsy results.
Hepatosplenomegaly: Based on physical examination and imaging data of the whole cohort, 2 (4.1%) patients had hepatomegaly and 10 (31.3%) had splenomegaly, including 3 patients with megalosplenia.
Blood routine examination: Overall, 20 (40.6%) patients had anemia (Hb level = 93 ± 4 g/L), 6 (18.8%) had PLT count ≤ 80 × 109/L (median, 65 × 109/L), 11 (22.4%) had leukopenia (median WBC count, 4.0 × 109/L), 7 (14.3%) had leukocytosis (median WBC count, 10.0 × 109/L), and 24 (75.0%) had LYM count > 1 × 109/L (median, 1.97 ± 0.67 × 109/L).
Biochemical indicators: There were 6 (12.4%) patients with decreased ALB level, 5 (10.2%) had increased GLB level, and 35 (71.4%) had increased LDH level (median, 317 IU/L).
Immune phenotypes: Among the 49 patients, B-cell-related common antigen CD20+ was expressed in 44 patients (89.8%), Bcl-2+ in 25 (51.0%) patients, Bcl-6+ in 32 (65.3%) patients, and CD10+ in 35 (71.4%) patients. The Ki-67 value was measured in the pathological specimens of 39 patients, with the results ranging from 5% to 90% (median, 30%). The Ki-67 value was ≤ 50% in 27 (55.1%) patients and > 50% in 22 (44.9%) patients.
Other indicators: Among the 49 patients, β2-MG was detected in 47 patients, including 22 patients (46.8%) with increased β2-MG level (median, 3.33 mg/L).
Curative effects: Among the 49 patients, the effective rate was 50.0% for patients with Scheme I (7/14), 75.0% for patients with Scheme II (24/32), and 100% for patients undergoing Scheme III (3/3). The results of the Fisher's exact test showed no significant differences in curative effects between the three schemes (P > 0.05) (Table 1).
| Scheme | Number of cases | CR | PR | SD | PD | ORR (%) |
| I | 14 | 3 (21.4) | 4 (28.6) | 5 (35.7) | 2 (14.3) | 50.0 |
| II | 32 | 11 (34.4) | 13 (40.6) | 3 (9.4) | 5 (15.6) | 75.0 |
| III | 3 | 1 (33.3) | 2 (66.7) | 0 | 0 | 100.00 |
The influence of different factors on the curative effect: The analysis results of the influence of various factors on the curative effect showed that anemia could affect the curative effect; hence, it was identified as an independent influencing factor. Among the 13 patients with anemia, 6 (46.2%) patients had CR+PR, while among 36 patients without anemia, 26 (72.2%) had CR + PR. Fisher's exact test results showed that the effective treatment rate was higher in patients without anemia than in patients with anemia (P < 0.05). Sex, age, B symptoms, involvement of more than four lymph nodes, pathological grade, clinical stage, bone marrow involvement, hepatomegaly, splenomegaly, increased LYM count, thrombocytopenia, leukopenia, increased LDH level, increased β2-MG level, and other indicators had no significant influence on the curative effect (P > 0.05).
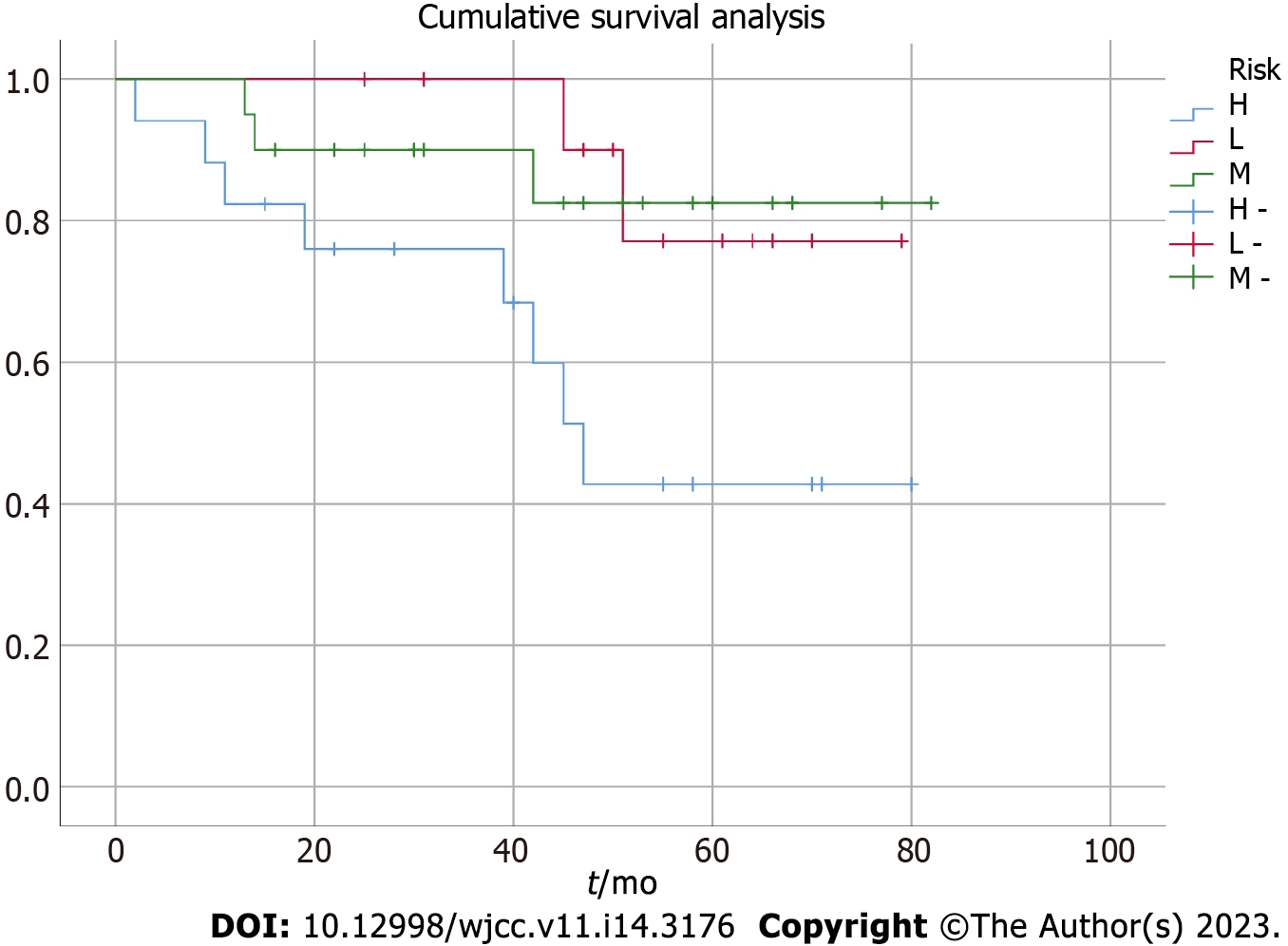
Survival analysis: All patients were followed up via telephone (follow-up time was calculated from the end of the last hospitalization to October 2020), with a median follow-up period of 52 (2–85) mo. The overall median survival was 41 mo in the 49 patients, while the 3-year and 5-year OS was 81.6% and 62.3%, respectively. The 3-year and 5-year PFS was 71.4% and 40.8%, respectively. According to the risk stratification (low-, moderate-, and high-risk groups) and analysis results of the five FLIPI-based adverse prognostic factors (age > 60 years, stages III–IV, involvement of more than four lymph nodes, Hb level < 120 g/L, and increased LDH level) affecting the survival rate, the survival rate was significantly lower (P < 0.05) in the high-risk group than in the other two groups. However, no significant difference was noted in the survival rate between the low- and moderate-risk groups (P > 0.05) (Table 2). The survival and total survival curves for the three risk groups are shown in Figure 1.
| Group | Risk factors | Cases, n (%) | 3-year OS, % (SE) | 3-year PFS, % (SE) | 5-year OS, % (SE) | 5-year PFS, % (SE) |
| Low risk | 0–1 | 12 (24.5) | 100 | 75.0 (0.18) | 83.3 (0.12) | 75.0 (0.18) |
| Medium risk | 2 | 20 (40.8) | 90.0 (0.08) | 75.0 (0.08) | 85.0 (0.11) | 70.0 (0.16) |
| High risk | ≥ 3 | 17 (34.7) | 76.5 (0.10) | 47.1 (0.13) | 47.1 (0.13) | 23.6 (0.20) |
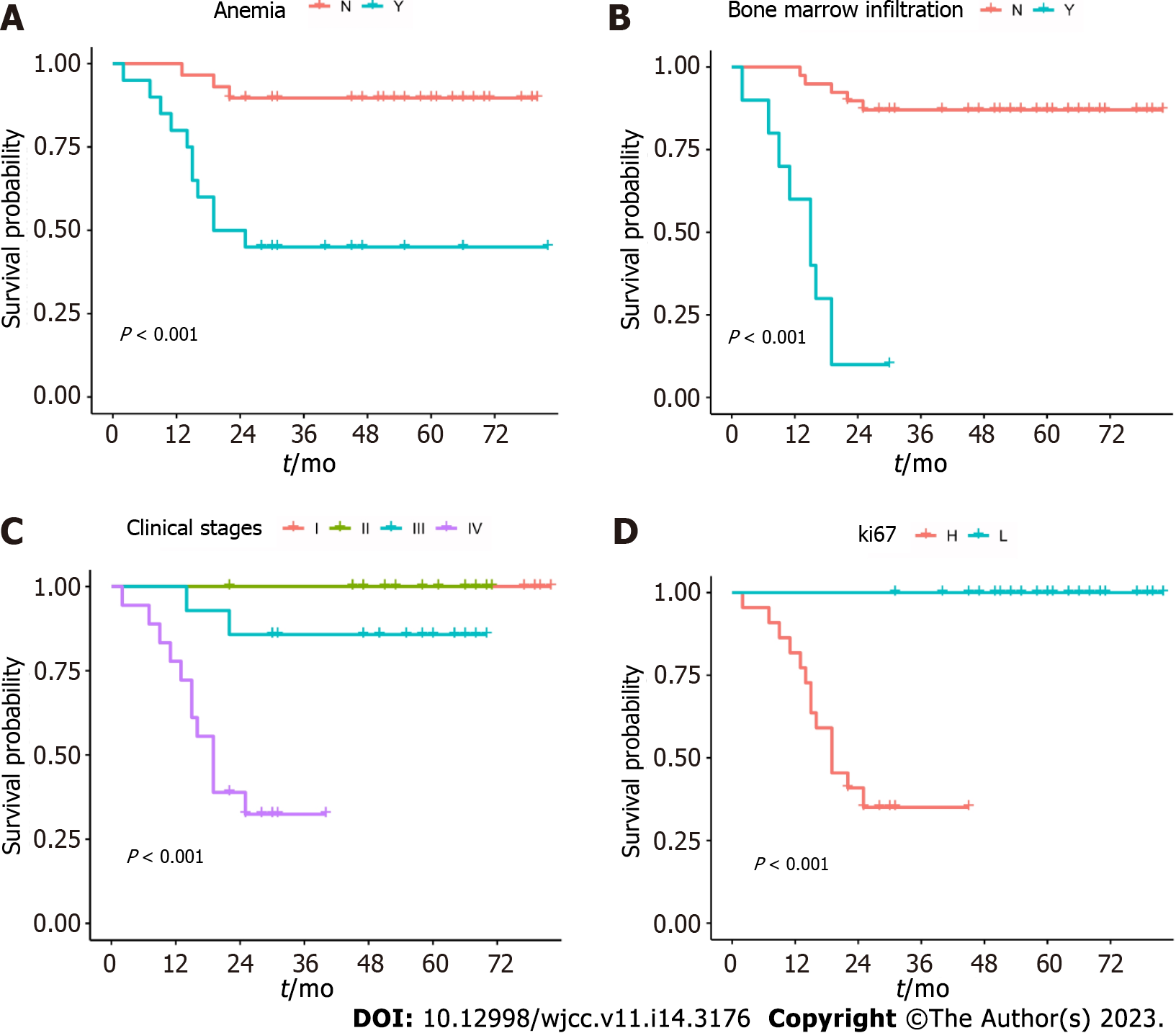
Prognostic analysis: Univariate analysis showed that anemia, a Ki-67 value > 50%, bone marrow involvement, and clinical stages III–IV were the factors inducing a poor prognosis (P < 0.05). No significant difference was noted in the prognosis between sex, age, B symptoms, pathological grade, number of involved lymph nodes, hepatosplenomegaly, decreased ALB level, leukopenia, increased GLB level, increased LDH level, leukocytosis, increased LYM count, increased β2-MG level, immune phenotype expression (CD10+, Bcl-2+, and Bcl-6+), and other factors (P > 0.05) (Table 3). The survival curves of the four adverse factors affecting prognosis are shown in Figure 2.
| Factors | 3-year OS, % (SE) | 5-year OS, % (SE) | Log-rank (P value) | |
| Sex | ||||
| M (30) | 86.7 (0.05) | 63.3 (0.09) | 0.185 | |
| F (19) | 78.9 (0.09) | 63.1 (0.13) | ||
| Age (yr) | ||||
| ≤ 60 (32) | 84.3 (0.05) | 71.9 (0.08) | 0.584 | |
| > 60 (17) | 82.4 (0.08) | 70.6 (0.13) | ||
| The presence or absence of B symptoms | ||||
| Y (31) | 77.4 (0.08) | 58.1 (0.13) | 0.237 | |
| N (18) | 88.9 (0.06) | 72.2 (0.09) | ||
| Stage | ||||
| I-II (17) | 94.1 (0.05) | 94.1 (0.05) | 0.043 | |
| III-IV (32) | 78.1 (0.06) | 65.6 (0.10) | ||
| Pathological grading | ||||
| I (3) | 100.0 | 100.0 | 0.321 | |
| II (16) | 87.5 (0.07) | 68.7 (0.12) | ||
| III (30) | 80.0 (0.05) | 73.3 (0.11) | ||
| BM infiltration | ||||
| Y (10) | 70.0 (0.13) | 30.0 (0.15) | 0.007 | |
| N (39) | 89.7 (0.04) | 79.5 (0.08) | ||
| Involved lymph node area | ||||
| ≤ 4 (36) | 86.1 (0.05) | 72.2 (0.08) | 0.054 | |
| > 4 (13) | 76.9 (0.12) | 53.8 (0.19) | ||
| Hepatomegaly | ||||
| Y (2) | 100.0 | 100.0 | 0.835 | |
| N (47) | 85.1 (0.05) | 74.4 (0.08) | ||
| Splenomegaly | ||||
| Y (10) | 80.0 (0.10) | 60.0 (0.14) | 0.169 | |
| N (39) | 84.6 (0.05) | 76.9 (0.09) | ||
| LDH | ||||
| Normal (14) | 85.7 (0.10) | 71.4 (0.10) | 0.312 | |
| High (35) | 82.9 (0.05) | 68.6 (0.09) | ||
| Hb | ||||
| < 120 g/L (20) | 75.0 (0.09) | 45.0 (0.12) | 0.011 | |
| ≥ 120 g/L (29) | 89.7 (0.04) | 75.9 (0.09) | ||
| WBC | ||||
| ≤ 4 × 109/L (11) | 100 | 72.7 (0.18) | 0.481 | |
| > 4 × 109/L (38) | 84.2 (0.05) | 65.8 (0.08) | ||
| WBC | ||||
| ≤ 10 × 109/L (42) | 88.1 (0.05) | 66.7 (0.08) | 0.813 | |
| > 10 × 109/L (7) | 71.4 (0.21) | 57.1 (0.21) | ||
| LYM | ||||
| ≤ 1 × 109/L (25) | 80.0 (0.09) | 68.0 (0.15) | 0.494 | |
| > 1 × 109/L (24) | 87.5 (0.05) | 66.7 (0.09) | ||
| PLT | ||||
| ≤ 80 × 109/L (9) | 100 | 66.7 (0.19) | 0.998 | |
| > 80 × 109/L (40) | 82.5 (0.06) | 70.0 (0.08) | ||
| ALB | ||||
| ≤ 35 g/L (6) | 83.3 (0.15) | 66.7 (0.19) | 0.941 | |
| > 35 g/L (43) | 87.4 (0.05) | 67.2 (0.08) | ||
| GLB | ||||
| ≤ 34 g/L (44) | 87.9 (0.05) | 66.5 (0.08) | 0.758 | |
| > 34 g/L (5) | 80.0 (0.18) | 80.0 (0.18) | ||
| Ki-67 | ||||
| ≤ 50% (27) | 96.2 (0.04) | 91.3 (0.06) | 0.004 | |
| > 50% (22) | 83.3 (0.15) | 31.2 (0.25) | ||
| Bcl-6+ | 32 | 83.9 (0.07) | 74.6 (0.09) | 0.926 |
| Bcl-6- | 17 | 100 | 68.6 (0.19) | |
| CD10+ | 35 | 85.7 (0.07) | 64.6 (0.11) | 0.293 |
| CD10- | 14 | 90.9 (0.09) | 90.9 (0.09) | |
| Bcl-2+ | 25 | 91.4 (0.06) | 76.4 (0.09) | 0.493 |
| Bcl-2- | 24 | 80 (0.10) | 66.7 (0.15) | |
FL is an inert NHL originating from the germinal center of follicles in the lymph nodes. Although individuals of any age can develop this disease, it is more common in adults, particularly men[1]. In this study, the peak age at FL onset ranged from 40 to 59 years, with a median age of 51 years, and the male:female ratio was 1.6:1. Initial lymph node enlargement was the most common clinical feature. However, nearly half of the patients with FL had extralymphatic and organ involvement. The most commonly affected sites include the liver, spleen, bone marrow, and gastrointestinal tract[2-4]. In this study, 31.3% of the patients had splenomegaly, 20.4% had bone marrow involvement, 4.1% had hepatomegaly, and 4.1% had gastrointestinal tract involvement, similar to findings of other reports China and abroad, with the exception of low hepatomegaly incidence[5].
The immune phenotype of FL is vital for its diagnosis and treatment. Bcl-2, Bcl-6, and CD10 expression has been detected in most patients[6,7]. In terms of the immune phenotype detection results of 40 patients, 51.0% of patients expressed Bcl-2+, 65.3% expressed Bcl-6+, and 71.4% expressed CD10+, which is lower than the data reported abroad[8,9], which may be related to the small size of this sample.
The FLIPI is an important prognostic tool for patients with FL before treatment. As revealed in an international multicenter study involving 4,167 FL patients, age (≤ 60 years or > 60 years), Ann Arbor stage (Stage I–II or Stage III–IV), Hb level (< 120 g/L or > 120 g/L), LDH level (normal or increased), and number of lymph node area involved (≤ 4 or > 4) could affect the prognosis of patients[4]. According to the findings of the present study, clinical stage, bone marrow involvement, Ki67 value, and decreased Hb level can affect the prognosis of patients; however, age, LDH level, and number of involved lymph node areas were not associated with the prognosis of patients, which may be associated with the small sample size of the study. Analysis of the three different risk groups based on the FLIPI score demonstrated that the 5-year OS and PFS of patients were significantly lower in the high-risk group than in the low-risk and moderate-risk groups, which is consistent with the results reported abroad[10,11].
As reported in some articles abroad, the positive expression of molecular biological markers such as Bcl-2 and Bcl-6 could affect the curative effect and prognosis of patients with FL[7,12]. However, the positive expression of Bcl-2 and Bcl-6 was not significantly correlated with the prognosis of patients in this study[13]. The Southwest Oncology Group has selected Ki-67 value as an early indicator to predict the clinical progression of tumors and an important parameter for identifying NHL prognosis[14]. In this study, a Ki-67 value of 50% was selected as the cutoff point, and the analysis showed that the 5-year OS of patients > 50% was significantly lower than OS of patients ≤ 50%, which is in line with the results of a previous study[15]. Therefore, Ki-67 Levels must be measured during pathological examinations.
Currently, there is no consensus on the therapeutic schemes for FL. With continuous exploration of the pathogenesis, clinical features, and prognosis of FL, rituximab has been confirmed as an important drug for treating FL, as it can improve the total remission rate and prolong the remission duration in patients[16,17]. In this study, 32 newly treated patients with FL received rituximab therapy in conjunction with chemotherapy, and the overall response rate was 75%, which was slightly higher than that (67%) reported elsewhere[18].
Because of the long course of the disease, high relapse rate after remission, and strong tendency to transform into diffuse large B-cell lymphoma, it is difficult to employ conventional therapies to improve the quality of life and disease-free survival rate of patients with FL. The key molecules, proteins, and pathways expressed in the development of FL are expected to become new treatment targets, including CD20 antibody, PI3K inhibitor, BTK pathway inhibitor, immunomodulator, and anti-PD1. Among these, obinutuzumab has received much attention, with many studies demonstrating that obinutuzumab can significantly prolong PFS and OS in patients with FL and can even become a candidate to replace rituximab because it has been launched in the market and is widely used. These drugs provide additional therapeutic options for patients with FL, further improving the remission rate and prolonging the survival time of patients, all of which can be attributed to the progress of individualized treatment based on further risk stratification of FL.
FL mainly occurs in men aged 50–69 years who specifically present with lymph node involvement. Currently, most FL patients are treated with chemotherapy, and there is a lack of a standard therapeutic scheme. Rituximab combined with chemotherapy can improve the remission rate and prolong the survival time of patients with FL, and therapy integrating fludarabine is expected to become the first-line therapeutic scheme. Advanced clinical stage, high Ki-67 value, anemia, and bone marrow involvement affect the prognosis of patients with FL.
Follicular lymphoma (FL) is a B-cell lymphoma that originates at the germinal center and has a low malignancy rate.
To gain a deeper understanding of the clinical characteristics, efficacy, and prognostic factors of FL in China and to provide guidance for clinical diagnosis and treatment, we retrospectively analyzed the clinical data of 49 patients with FL who were diagnosed and treated at our hospital in recent years.
This study aimed to explore the clinical features, curative effects, and prognostic factors of FL.
The medical records of 49 patients with FL admitted to the Ningbo First Hospital from June 2010 to June 2021 were retrospectively reviewed to compare the curative effects of different therapeutic schemes and analyze the related prognostic factors.
The age at onset in the 49 patients ranged from 24 to 76 years, with a median age of 51 years. The male:female ratio was 1.6:1. No significant difference was noted in the curative effect between the non-chemotherapy, combined chemotherapy, and other chemotherapy regimen groups. Hemoglobin (Hb) level < 120 g/L, Ki-67 value >50%, bone marrow involvement, and clinical stages III–IV were associated with a poor prognosis. However, the influence of the other indicators on prognosis was not statistically significant. Risk grouping was performed using the Follicular Lymphoma International Prognosis Index. The results showed that 24.5%, 40.8%, and 34.7% of patients were in the low-, moderate-, and high-risk groups, respectively. According to the survival analysis results, the survival rate of patients was lower in the high-risk group than in the other two groups.
FL mainly occurs in middle-aged or older men who mainly present with lymph node and bone marrow involvement. Hb level, Ki-67 value, bone marrow involvement, and clinical stage can be used for prognostic estimation.
To analyze the clinical characteristics, efficacy, prognosis, and survival factors of 49 patients in order to improve clinicians' understanding of the disease and provide guidance for clinical diagnosis and treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Klapper W, Germany; Shimokawa T, Japan S-Editor: Wang JL L-Editor: A P-Editor: Zhao S
| 1. | Zelenetz AD, Abramson JS, Advani RH, Andreadis CB, Bartlett N, Bellam N, Byrd JC, Czuczman MS, Fayad LE, Glenn MJ, Gockerman JP, Gordon LI, Harris NL, Hoppe RT, Horwitz SM, Kelsey CR, Kim YH, LaCasce AS, Nademanee A, Porcu P, Press O, Pro B, Reddy N, Sokol L, Swinnen LJ, Tsien C, Vose JM, Wierda WG, Yahalom J, Zafar N. Non-Hodgkin's lymphomas. J Natl Compr Canc Netw. 2011;9:484-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Gross SA, Zhu X, Bao L, Ryder J, Le A, Chen Y, Wang XQ, Irons RD. A prospective study of 728 cases of non-Hodgkin lymphoma from a single laboratory in Shanghai, China. Int J Hematol. 2008;88:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Yang QP, Zhang WY, Yu JB, Zhao S, Xu H, Wang WY, Bi CF, Zuo Z, Wang XQ, Huang J, Dai L, Liu WP. Subtype distribution of lymphomas in Southwest China: analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol. 2011;6:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019-5032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1444] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 5. | Li Y, Zhang Y, Wang W, Wei C, Zhao D, Zhang W. Follicular Lymphoma in China: Systematic Evaluation of Follicular Lymphoma Prognostic Models. Cancer Manag Res. 2022;14:1385-1393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U, Pro B, Pileri S, Pulsoni A, Soubeyran P, Cortelazzo S, Martinelli G, Martelli M, Rigacci L, Arcaini L, Di Raimondo F, Merli F, Sabattini E, McLaughlin P, Solal-Céligny P. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27:4555-4562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 543] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 7. | Khanlari M, Chapman JR. Follicular lymphoma: updates for pathologists. J Pathol Transl Med. 2022;56:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Sakurai M, Mori T, Kato K, Kanaya M, Mizuno S, Shiratori S, Wakayama T, Uchida N, Kobayashi H, Kubo K, Amano I, Ohta T, Miyazaki Y, Kanda J, Fukuda T, Atsuta Y, Kondo E; Adult Lymphoma Working Group of the Japan Society for Hematopoietic Cell Transplantation (JSHCT). Outcome of allogeneic hematopoietic stem cell transplantation for follicular lymphoma relapsing after autologous transplantation: analysis of the Japan Society for Hematopoietic Cell Transplantation. Bone Marrow Transplant. 2021;56:1462-1466. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Zinzani PL, Flinn IW, Yuen SLS, Topp MS, Rusconi C, Fleury I, Le Dû K, Arthur C, Pro B, Gritti G, Crump M, Petrich A, Samineni D, Sinha A, Punnoose EA, Szafer-Glusman E, Spielewoy N, Mobasher M, Humphrey K, Kornacker M, Hiddemann W. Venetoclax-rituximab with or without bendamustine vs bendamustine-rituximab in relapsed/refractory follicular lymphoma. Blood. 2020;136:2628-2637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 10. | Gordon MJ, Smith MR, Nastoupil LJ. Follicular lymphoma: The long and winding road leading to your cure? Blood Rev. 2023;57:100992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 11. | Batlevi CL, Sha F, Alperovich A, Ni A, Smith K, Ying Z, Soumerai JD, Caron PC, Falchi L, Hamilton A, Hamlin PA, Horwitz SM, Joffe E, Kumar A, Matasar MJ, Moskowitz AJ, Moskowitz CH, Noy A, Owens C, Palomba LM, Straus D, von Keudell G, Zelenetz AD, Seshan VE, Younes A. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer J. 2020;10:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 12. | Dreyling M, Ghielmini M, Rule S, Salles G, Vitolo U, Ladetto M; ESMO Guidelines Committee. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v83-v90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 13. | Maurer MJ, Bachy E, Ghesquières H, Ansell SM, Nowakowski GS, Thompson CA, Inwards DJ, Allmer C, Chassagne-Clément C, Nicolas-Virelizier E, Sebban C, Lebras L, Sarkozy C, Macon WR, Feldman AL, Syrbu SI, Traverse-Glehan A, Coiffier B, Slager SL, Weiner GJ, Witzig TE, Habermann TM, Salles G, Cerhan JR, Link BK. Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am J Hematol. 2016;91:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 14. | Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131:68-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 312] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 15. | Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, Phillips E, Sangha R, Schlag R, Seymour JF, Townsend W, Trněný M, Wenger M, Fingerle-Rowson G, Rufibach K, Moore T, Herold M, Hiddemann W. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med. 2017;377:1331-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 553] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 16. | Pastore A, Jurinovic V, Kridel R, Hoster E, Staiger AM, Szczepanowski M, Pott C, Kopp N, Murakami M, Horn H, Leich E, Moccia AA, Mottok A, Sunkavalli A, Van Hummelen P, Ducar M, Ennishi D, Shulha HP, Hother C, Connors JM, Sehn LH, Dreyling M, Neuberg D, Möller P, Feller AC, Hansmann ML, Stein H, Rosenwald A, Ott G, Klapper W, Unterhalt M, Hiddemann W, Gascoyne RD, Weinstock DM, Weigert O. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015;16:1111-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 474] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 17. | Casulo C, Byrtek M, Dawson KL, Zhou X, Farber CM, Flowers CR, Hainsworth JD, Maurer MJ, Cerhan JR, Link BK, Zelenetz AD, Friedberg JW. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J Clin Oncol. 2015;33:2516-2522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 636] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 18. | Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, Millenson MM, Cohen AD, Schuster SJ, Lebovic D, Dhodapkar M, Avigan D, Chapuy B, Ligon AH, Freeman GJ, Rodig SJ, Cattry D, Zhu L, Grosso JF, Bradley Garelik MB, Shipp MA, Borrello I, Timmerman J. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol. 2016;34:2698-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 817] [Article Influence: 90.8] [Reference Citation Analysis (0)] |