Published online May 6, 2023. doi: 10.12998/wjcc.v11.i13.3062
Peer-review started: November 28, 2022
First decision: December 19, 2022
Revised: February 6, 2023
Accepted: March 31, 2023
Article in press: March 31, 2023
Published online: May 6, 2023
Processing time: 148 Days and 2.2 Hours
Urothelial carcinoma (UC) is a common malignancy of the urinary system that can occur anywhere from the renal pelvis to the proximal urethra. Most UCs are in the bladder and have multifocal growth. Upper urinary tract UC (UTUC), which occurs in the renal pelvis or ureter, accounts for only 5% to 10% of UCs.
In March 2015, a 70-year-old male who initially presented to a local hospital with a complaint of painless hematuria was diagnosed with UTUC of the right renal pelvis. The doctors administered radical nephroureterectomy and bladder cuff excision. Although the doctors recommended intravesical chemotherapy and regular follow-up, he rejected this advice. In December 2016, the patient presented at our hospital with dysuria. We identified UC in the residual bladder and administered radical cystectomy and left cutaneous ureterostomy. In November 2021, he presented again with urethral bleeding. We detected urethral UC as the cause of urethral orifice bleeding and administered radical urethrectomy. Since then, he has visited regularly for 6-mo follow-ups, and was in stable condition as of December 2022.
UTUC is prone to seeding and recurrence. Adjuvant instillation therapy and intense surveillance are crucial for these patients.
Core Tip: Urothelial carcinoma (UC) is a common malignancy in the urinary system, and typically grows from multiple foci. UC is most common in the bladder, and upper urinary tract UC (UTUC) is rare. We describe a male who initially presented at a local hospital in 2015 at the age of 70 years with a complaint of painless hematuria. The doctors diagnosed UTUC of the right renal pelvis. After radical nephroureterectomy and bladder cuff excision, the doctors recommended intravesical chemotherapy and regular follow-up, but he rejected this advice. He presented at our hospital again with dysuria in 2016. We identified UC in the residual bladder and performed radical cystectomy and left cutaneous ureterostomy. Unfortunately, he presented again with urethral orifice bleeding in 2021, and we identified urethral UC as the cause. We thus administered radical urethrectomy. Since this last surgery, he has received regular 6-mo follow-ups and has remained in a stable condition. Treatment for upper UTUC should include adjuvant instillation as immunotherapy and intense surveillance.
- Citation: Zhang JQ, Duan Y, Wang K, Zhang XL, Jiang KH. Metachronous urothelial carcinoma in the renal pelvis, bladder, and urethra: A case report. World J Clin Cases 2023; 11(13): 3062-3069
- URL: https://www.wjgnet.com/2307-8960/full/v11/i13/3062.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i13.3062
Urothelial carcinoma (UC) is a common urological malignancy. Bladder tumors account for 90% to 95% of UCs, and upper urinary tract UCs (UTUCs) account for only 5 to 10%[1,2]. Urethral cancer is a rare malignancy of the urinary system (< 1% of all malignancies), and the predominant histological type is UC[3]. Recurrence of UTUC in the bladder occurs in 22% to 47% of these patients, and recurrence in the contralateral upper urinary tract occurs in only 2% to 6%[4,5]. A metachronous UC is a primary UC in which a second primary cancer is diagnosed more than 6 mo after the first primary cancer. There are no previous reports of metachronous UC in the upper urinary tract, bladder, and urethra. Herein, we report such a case to improve recognition and management of this disease.
A 70-year-old male was admitted to our department with a complaint of bloody urethral discharge during the previous month.
The patient reported bloody urethral discharge for one month. He had no flank pain or urethral pain.
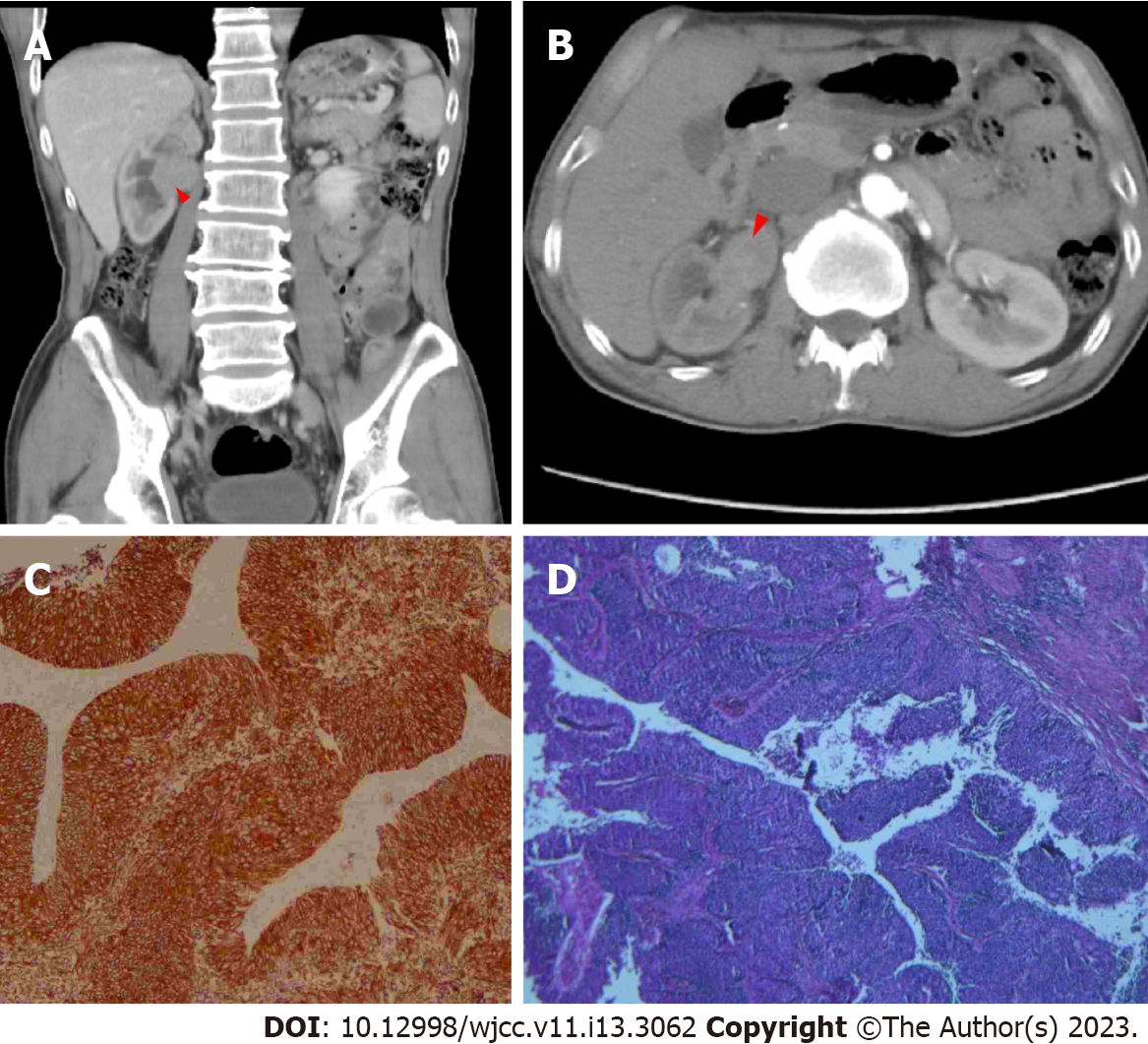
In March 2015, this patient was admitted to a local hospital for painless hematuria. Percussion of the kidneys indicated no enlargement and no renal mass. A cystoscopy indicated hematuria from the right ureteral orifice, but no mass in the bladder or urethra. Computed tomography (CT) of the abdomen showed a tumor with a size of 3.6 cm × 2.9 cm in the right renal pelvis (Figure 1). The urine cytology results were negative.
The doctors performed radical nephroureterectomy and bladder cuff resection. Postoperative pathology showed that the tumor was a high-grade UC that invaded the subepithelial connective tissue (Figure 1). There was no evidence of local or distant metastases. These findings led to a diagnosis of upper UTUC of the right renal pelvis with a clinical stage of T1N0M0. We recommended Bacillus Calmette-Guérin (BCG) intravesical chemotherapy, but he declined and was lost to follow-up.
In December 2016, the patient came to our department because of dysuria for three months. Percussion of the kidneys and inspection of the lymph nodes indicated no enlargement. The red blood cell count was 4.10 × 1012/L and the hemoglobin concentration was 131.0 g/L. Blood biochemistry showed elevated levels of cancer antigen 19-9: 48.32 U/mL and non-small cell lung cancer antigen: 9.51 U/mL.
Ultrasonography showed multiple masses in the bladder, and a chest X-ray showed a hyperdense nodule with a diameter of 4 mm in the inferior lobe of the right lung (data not shown). Enhanced CT of the urinary tract showed multiple masses in the bladder, the largest of which was about 6.5 cm × 5.0 cm (Figure 2), but there was no evidence of enlarged lymph nodes in the abdominal cavity, the retroperitoneal space, or the pelvic cavity. A cystoscopy confirmed multiple tumors in the bladder, and a biopsy showed no umbrella cells, but evidence of pathological mitotic figures and tissue consisting of low-grade UC. We therefore, performed radical cystectomy and left cutaneous ureterostomy. The postoperative pathology results showed the tumor was a low-grade UC that invaded the superficial muscle of bladder wall (Figure 2), but there were no positive margins or involved lymph nodes. Immunohistochemistry showed positive staining for CK7, CK20, CK (L), CK5/6, P40, P53, PHH3, and Ki-67, but no staining for CK (H). There were no local or distant metastases. We therefore diagnosed the patient as having bladder UC with a clinical stage of T2N0M0.
The patient denied any history of injury to the penis, scrotum, or perineum before development of bloody urethral discharge.
He reported smoking 10 cigarettes daily for over 40 years, and consuming white wine for more than 30 years. He quit smoking and consuming white wine in 2017. There were no similar cases in his family.
Percussion of the kidneys indicated no enlargement and no renal mass. We observed a 15 cm surgical scar at the right waist, and another 7 cm surgical scar at the lower right abdomen. We also observed that a stoma bag on the left side of the abdomen contained light yellow urine. All the vital signs were stable and there were no other abnormal findings.
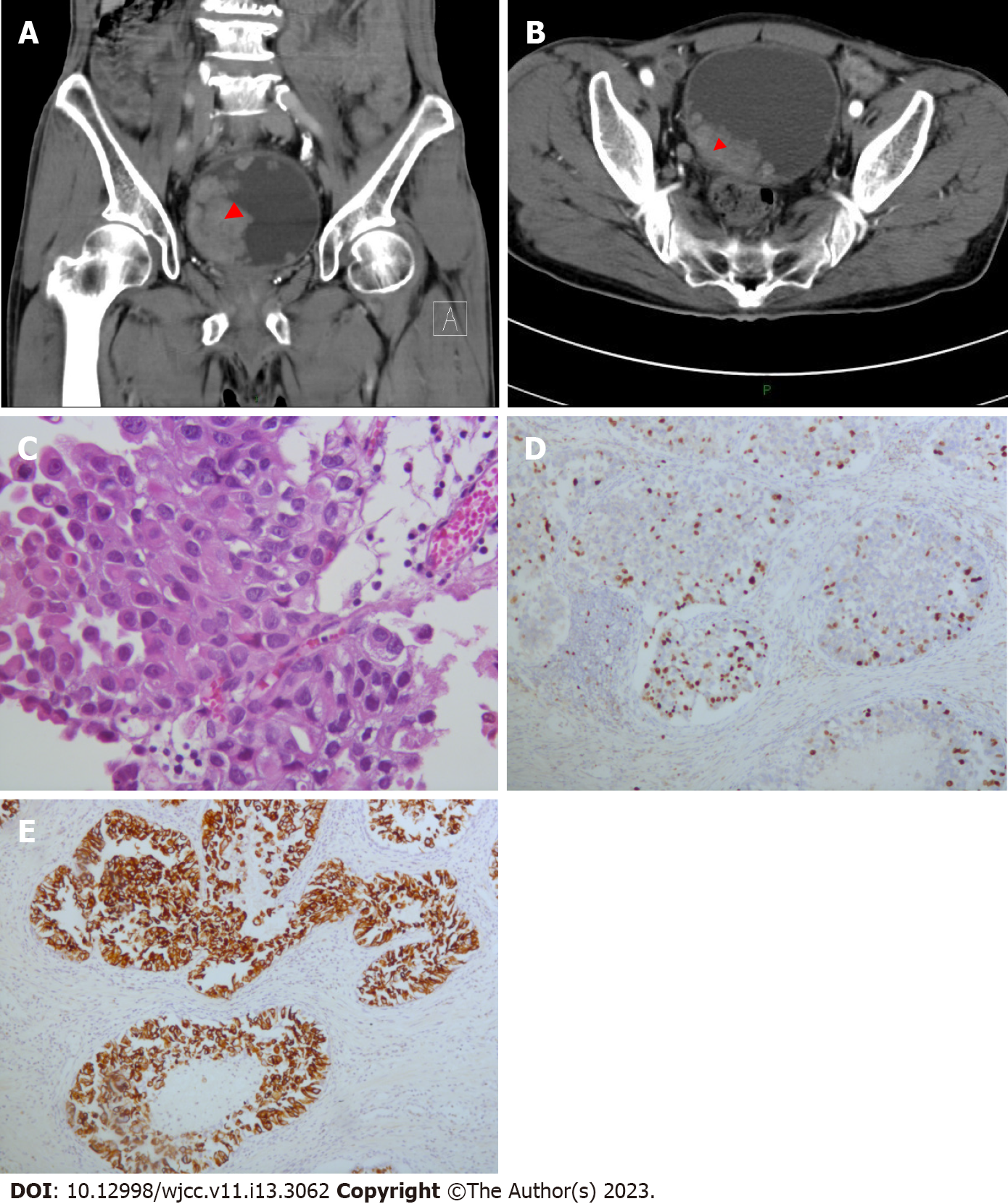
Urethroscopy showed a cauliflower-shaped neoplasm with a size of 2.0 cm × 1.5 cm × 0.5 cm in the anterior urethra, and a biopsy revealed the tissue consisted of low-grade UC. The routine blood test showed high levels of white blood cells (12.07 × 109/L) and neutrophils (89.0%), but low levels of red blood cells (4.02 × 1012/L), hemoglobin (131.0 g/L), and lymphocytes (4.1%). The blood biochemistry showed low levels of estimated glomerular filtration rate (61 mL/min/1.73 m2) and total protein (61.8 g/L), but a high level of uric acid (438 μmol/L). The coagulation test and the prostate specific antigen test showed no abnormal changes. The results of all other examinations were normal.
Except for the absence of the right kidney, there were no abnormal findings in the color Doppler ultrasonography.
After admission, the patient was diagnosed with urethral UC.
We decided radical urethrectomy was the most suitable treatment. Postoperative pathology showed that the urethral tumor consisted of 80% low-grade papillary UC and 20% high-grade papillary UC, and that the tumor invaded the lamina propria. There was no evidence of local or distant metastasis or lymph node involvement. We therefore diagnosed the patient with urethral UC with a clinical stage of T1N0M0[6].
Since this surgery, the patient has visited every 6 mo for follow-up, and was in stable condition as of December 2022. Figure 3 summarizes the treatment timeline.
The urinary tract is divided into two parts: The upper region includes the kidneys and ureters and the lower region includes the bladder and urethra. UC is a common malignant tumor of the urinary system. The bladder is the most common site of UC, UTUC is rare[4,5], and urethral UC is even more rare (< 1% of all malignancies)[3]. A metachronous UC, in which a second primary cancer is diagnosed more than 6 mo after the first primary cancer, occurs in a small number of these patients.
The main clinical manifestations of UTUC are hematuria, dysuria, and flank pain. Hematuria is the most common sign, and is painless when it is the sole manifestation. Flank pain is a sign of obstruction and/or hydronephrosis. When a blood clot or tumor clot passes through the tubules, it can trigger renal-colic-like pain. However, when the obstruction is incomplete and hydronephrosis progresses, the resulting flank pain can be dull and chronic. Nevertheless, a patient can also be asymptomatic and diagnosed incidentally from imaging tests. When UC worsens, the patient may develop a flank or abdominal mass, bone pain, anorexia, and weight loss. In these cases, detailed evaluations of metastasis are necessary and a poor prognosis is likely[7,8]. Previous research reported that after surgical treatment, recurrence in the bladder occurred in 22% to 47% of cases, and recurrence in the contralateral upper tract occurred in 2% to 6% of cases[4,5].
Several medical techniques are essential for the diagnosis of UTUC. Ureteroscopy is commonly employed for detection and to determine treatment strategy. A biopsy specimen can be used to determine disease grade. However, the use of diagnostic ureteroscopy is associated with a higher risk of bladder recurrence after radical nephroureterectomy[9,10]. Cystoscopy should be considered because UTUCs are often in the bladder. Cytology can be helpful in diagnosis and determination of treatment, but its sensitivity is low and it has limited ability to detect the origin of tumor cells. CT is commonly utilized because of its high accuracy, ease of use, and wide availability[11,12].
There is a general consensus regarding treatments to be used for UTUC. Radical nephroureterectomy combined with bladder cuff excision is the gold standard treatment for a tumor of the renal pelvis or proximal ureter that is large, high-grade, and suspected of being invasive, provided there is a normal contralateral kidney.
Previous analyses of the pathological characteristics of UTUCs reported that papillary lesions were associated with better outcomes, and sessile lesions with poor outcomes[13,14]. Tumor invasion of muscle is also associated with poor outcome[15]. There is a high rate of ipsilateral recurrence in patients with upper urinary tract tumors, probably because of the multifocality of this tumor and downstream seeding[16-18].
UC of urethra tends to be malignant and to follow bladder cancer. Chen et al[19] found that the majority of patients with UC in the urethra (26 of 35 cases, 74%) had high-grade tumors, and more than three-quarters of patients (23 of 30, 77%) had a previous history of either high-grade papillary UC (n = 22) or UC in situ (n = 1) of the bladder[19]. It was reported that approximately 2% to 5% of patients with superficial bladder cancer and 40% to 60% of those with muscle-invasive bladder cancer developed urethral cancer[3]. Another study reported that 4% to 8% of male patients developed recurrent UC in the remnant urethra after cystectomy[20]. Erckert et al[21] found that the overall incidence of urethral cancer among 2052 events of primary and recurrent bladder tumors was 6.1%. Therefore, prophylactic urethrectomy should be recommended for patients with bladder cancer to prevent subsequent involvement of urethra, although the current guidelines have no relevant recommendations. In regard to urethral UC following bladder cancer, the monoclonality of multifocal cancers in the urinary tract indicate the possible seeding or implantation of bladder cancer cells to the retained urethra after cystectomy[20].
Our patient had metachronous primary UC in the right renal pelvis (March 2015), bladder (December 2016), and urethra (November 2021). The short interval between the first second episodes may be explained by the patient’s rejection of the recommended BCG intravesical chemotherapy and the loss to follow-up. Because UTUC is prone to multifocality and downstream seeding, adjuvant immunotherapy is necessary. Adjuvant intracavitary instillation of BCG is likely to improve the outcome of patients receiving kidney-sparing surgery. Nevertheless, Foerster et al[22] found no difference of recurrence rates between patients who received adjuvant instillations of BCG and untreated patients. On the other hand, some evidence showed that adjuvant instillation of mitomycin within 72 h of surgery reduced the recurrence rate within the first year[23]. Also, recent evidence suggests that early single adjuvant intracavitary instillation of mitomycin C in patients with low-grade UTUC might reduce the risk of local recurrence[1,24]. Therefore, the re-appearance of cancer in our patient could be attributable to the lack of adjuvant instillation of BCG and mitomycin.
Follow-up is also important to prevent worsening of the patient’s condition. A retrospective study of 275 patients with UTUC reported the prevalence in the bladder was 46% and in the urethra was 2%; the prevalence of contralateral recurrence was 1%, distant metastasis was 7.5%, and local metastasis was 6%. These researchers concluded that UTUC was a unique disease with synchronous and metachronous recurrence that requires long-term surveillance[25].
The interval between our patient’s second and third episodes was 5 years, and the third episode involved the urethra, a rare location of UC. Although there are reports of similar cases, these previous patients only had one or two episodes[26,27]. In one rare case, UC spread from the prostatic urethra to the brain[28]. There is a possibility that cutaneous diversion could make it easier to seed the remnant urethra, because if the urethra was used for voiding then viable cancer cells may be shed by urine flow[20].
Analysis of oncogenes can help elucidate the mechanisms of disease recurrence. However, this information was unavailable in our patient.
Although UTUC is relatively rare, the possibility of multifocality and downstream seeding indicates the need for intense surveillance and the use of adjuvant instillation of mitomycin to prevent metastasis and recurrence. After radical operation, UC may recur in adjacent downstream tissues, thus emphasizing the significance of long-term patient follow-up.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morozov S, Russia; Mao YM, China S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, Gontero P, Liedberg F, Masson-Lecomte A, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF, Seisen T, Soukup V, Sylvester RJ. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022;81:75-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 860] [Article Influence: 215.0] [Reference Citation Analysis (0)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11916] [Article Influence: 2979.0] [Reference Citation Analysis (4)] |
| 3. | Janisch F, Abufaraj M, Fajkovic H, Kimura S, Iwata T, Nyirady P, Rink M, Shariat SF. Current Disease Management of Primary Urethral Carcinoma. Eur Urol Focus. 2019;5:722-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Xylinas E, Rink M, Margulis V, Karakiewicz P, Novara G, Shariat SF; Upper Tract Urothelial Carcinoma Collaboration (UTUCC). Multifocal carcinoma in situ of the upper tract is associated with high risk of bladder cancer recurrence. Eur Urol. 2012;61:1069-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Li WM, Shen JT, Li CC, Ke HL, Wei YC, Wu WJ, Chou YH, Huang CH. Oncologic outcomes following three different approaches to the distal ureter and bladder cuff in nephroureterectomy for primary upper urinary tract urothelial carcinoma. Eur Urol. 2010;57:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Grivas PD, Davenport M, Montie JE, Kunju LP, Feng F, Weizer AZ. Urethral cancer. Hematol Oncol Clin North Am. 2012;26:1291-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Raman JD, Shariat SF, Karakiewicz PI, Lotan Y, Sagalowsky AI, Roscigno M, Montorsi F, Bolenz C, Weizer AZ, Wheat JC, Ng CK, Scherr DS, Remzi M, Waldert M, Wood CG, Margulis V; Upper-Tract Urothelial Carcinoma Collaborative Group. Does preoperative symptom classification impact prognosis in patients with clinically localized upper-tract urothelial carcinoma managed by radical nephroureterectomy? Urol Oncol. 2011;29:716-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Ito Y, Kikuchi E, Tanaka N, Miyajima A, Mikami S, Jinzaki M, Oya M. Preoperative hydronephrosis grade independently predicts worse pathological outcomes in patients undergoing nephroureterectomy for upper tract urothelial carcinoma. J Urol. 2011;185:1621-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Marchioni M, Primiceri G, Cindolo L, Hampton LJ, Grob MB, Guruli G, Schips L, Shariat SF, Autorino R. Impact of diagnostic ureteroscopy on intravesical recurrence in patients undergoing radical nephroureterectomy for upper tract urothelial cancer: a systematic review and meta-analysis. BJU Int. 2017;120:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Guo RQ, Hong P, Xiong GY, Zhang L, Fang D, Li XS, Zhang K, Zhou LQ. Impact of ureteroscopy before radical nephroureterectomy for upper tract urothelial carcinomas on oncological outcomes: a meta-analysis. BJU Int. 2018;121:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Ito A, Shintaku I, Satoh M, Ioritani N, Aizawa M, Tochigi T, Kawamura S, Aoki H, Numata I, Takeda A, Namiki S, Namima T, Ikeda Y, Kambe K, Kyan A, Ueno S, Orikasa K, Katoh S, Adachi H, Tokuyama S, Ishidoya S, Yamaguchi T, Arai Y. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP Monotherapy Study Group Trial. J Clin Oncol. 2013;31:1422-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Cowan NC, Turney BW, Taylor NJ, McCarthy CL, Crew JP. Multidetector computed tomography urography for diagnosing upper urinary tract urothelial tumour. BJU Int. 2007;99:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Fritsche HM, Novara G, Burger M, Gupta A, Matsumoto K, Kassouf W, Sircar K, Zattoni F, Walton T, Tritschler S, Baba S, Bastian PJ, Martínez-Salamanca JI, Seitz C, Otto W, Wieland WF, Karakiewicz PI, Ficarra V, Hartmann A, Shariat SF. Macroscopic sessile tumor architecture is a pathologic feature of biologically aggressive upper tract urothelial carcinoma. Urol Oncol. 2012;30:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Remzi M, Haitel A, Margulis V, Karakiewicz P, Montorsi F, Kikuchi E, Zigeuner R, Weizer A, Bolenz C, Bensalah K, Suardi N, Raman JD, Lotan Y, Waldert M, Ng CK, Fernández M, Koppie TM, Ströbel P, Kabbani W, Murai M, Langner C, Roscigno M, Wheat J, Guo CC, Wood CG, Shariat SF. Tumour architecture is an independent predictor of outcomes after nephroureterectomy: a multi-institutional analysis of 1363 patients. BJU Int. 2009;103:307-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Abouassaly R, Alibhai SM, Shah N, Timilshina N, Fleshner N, Finelli A. Troubling outcomes from population-level analysis of surgery for upper tract urothelial carcinoma. Urology. 2010;76:895-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Harris AL, Neal DE. Bladder cancer--field versus clonal origin. N Engl J Med. 1992;326:759-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Heney NM, Nocks BN, Daly JJ, Blitzer PH, Parkhurst EC. Prognostic factors in carcinoma of the ureter. J Urol. 1981;125:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Johansson S, Angervall L, Bengtsson U, Wahlqvist L. A clinicopathologic and prognostic study of epithelial tumors of the renal pelvis. Cancer. 1976;37:1376-1383. [PubMed] [DOI] [Full Text] |
| 19. | Chen F, Joshi S, Carthon BC, Osunkoya AO. A Contemporary Clinicopathologic Analysis of Primary Urothelial Carcinoma of the Urethra Without Concurrent Renal Pelvic, Ureteral, or Bladder Carcinoma. Int J Surg Pathol. 2022;30:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Kakizoe T, Tobisu K. Transitional cell carcinoma of the urethra in men and women associated with bladder cancer. Jpn J Clin Oncol. 1998;28:357-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Erckert M, Stenzl A, Falk M, Bartsch G. Incidence of urethral tumor involvement in 910 men with bladder cancer. World J Urol. 1996;14:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Foerster B, D'Andrea D, Abufaraj M, Broenimann S, Karakiewicz PI, Rouprêt M, Gontero P, Lerner SP, Shariat SF, Soria F. Endocavitary treatment for upper tract urothelial carcinoma: A meta-analysis of the current literature. Urol Oncol. 2019;37:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Cassell A 3rd, Manobah B, Willie S. Diagnostic and Therapeutic Challenges of Rare Urogenital Cancers: Urothelial Carcinoma of the Renal Pelvis, Ureters and Urethra. World J Oncol. 2021;12:20-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Gallioli A, Boissier R, Territo A, Vila Reyes H, Sanguedolce F, Gaya JM, Regis F, Subiela JD, Palou J, Breda A. Adjuvant Single-Dose Upper Urinary Tract Instillation of Mitomycin C After Therapeutic Ureteroscopy for Upper Tract Urothelial Carcinoma: A Single-Centre Prospective Non-Randomized Trial. J Endourol. 2020;34:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Elawdy MM, Osman Y, Taha DE, El-Halwagy S, El-Hamid MA, Abouelkheir RT. Long-term outcomes of upper tract urothelial carcinoma: A retrospective evaluation of single-center experience in 275 patients. Turk J Urol. 2019;45:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Kisa E, Semiz HS, Küçük Ü, İlbey YÖ. Metastatic primary urothelial carcinoma of the prostatic urethra: A case report. Urologia. 2019;86:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Moez R, Boulma R, Hassen K. Primary urothelial carcinoma of the male anterior urethra; A case report. Ann Med Surg (Lond). 2022;76:103561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 28. | Morita K, Oda M, Koyanagi M, Saiki M. Metastatic brain tumor from urothelial carcinoma of the prostatic urethra. Surg Neurol Int. 2016;7:S488-S491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |