Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2577
Peer-review started: September 13, 2021
First decision: November 11, 2021
Revised: November 16, 2021
Accepted: January 29, 2022
Article in press: January 29, 2022
Published online: March 16, 2022
Processing time: 178 Days and 15.1 Hours
Insertion of a left ventricular assist device (LVAD) and heart transplantation (HT) improve the survival of patients with heart failure. In addition, cardiac rehabilitation (CR) further increases the functional capacity. This case report describes a successful case of CR after LVAD insertion and subsequent HT.
In the present case, during the LVAD insertion period, peak oxygen consumption (VO2) increased by 12.16% after CR. HT was performed 7 mo after the LVAD insertion, and the patient participated in phases I and II CR. The peak VO2 increased from 17.24 to 22.29 mL/kg/min. This improvement was more significant than that reported in previous studies on CR after LVAD insertion or HT. The patient’s quality of life also improved. The total average score of the short form-36 questionnaire increased from 29.5 points at admission to 53.3 points 9 mo after HT.
A tailored CR program after LVAD insertion or HT may improve the patients’ quality of life and increase survival.
Core Tip: This case report describes a successful case of cardiac rehabilitation (CR) after left ventricular assist device (LVAD) insertion and subsequent heart transplantation (HT). Increase in the peak oxygen consumption was more significant than that reported in previous studies on CR after LVAD insertion or HT. A tailored exercise program for each phase led to improvement in the patient’s quality of life.
- Citation: Yang TW, Song S, Lee HW, Lee BJ. Cardiac rehabilitation in a heart failure patient after left ventricular assist device insertion and subsequent heart transplantation: A case report. World J Clin Cases 2022; 10(8): 2577-2583
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2577.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2577
Heart transplantation (HT) is the last resort for patients with end-stage heart failure (HF). HT improves the survival of patients with HF[1]; furthermore, a better prognosis can be expected with cardiac rehabilitation (CR) after HT[2]. The limited supply of donor organs remains a major barrier for HT. Mechanical circulatory support, such as a left ventricular assist device (LVAD), is widely used to overcome this limitation. LVAD is used either as a bridge-to-transplant (BTT) or as a destination treatment (DT), especially in patients ineligible for HT due to their poor condition[3]. CR after LVAD insertion improves functional capacity[4]. Herein, we report a successful case of CR after LVAD insertion and subsequent HT.
A 53-year-old man was admitted to the cardiology department because of dyspnea.
Transthoracic echocardiography showed severe mitral regurgitation, mitral chordae rupture, and an ejection fraction of 24%. Despite valve replacement, the patient had a poor prognosis; consequently, HT was planned. The patient urgently required HT, although a donor heart was unavailable; therefore, continuous-flow type LVAD (Heartmate II, Abbott, IL, United States) insertion was performed 1 mo after admission.
The patient was diagnosed with dilated cardiomyopathy with atrial fibrillation.
The patient had chronic kidney disease and had been taking anti-thrombotic medications due to old cerebral infarction. There was no specific family history of related heart disease.
During the physical examination, the patient had a blood pressure of 90/60 mmHg, heart rate of 106 bpm, body temperature of 36.2 °C, respiratory rate of 16 breaths/min, and oxygen saturation of 99% when oxygen was supplied at 2 L/min via a nasal cannula.
The serum pro-brain natriuretic peptide level was elevated to 5250 pg/mL, and the serum creatinine level was also elevated to 1.73 mg/dL.
Chest X-ray showed cardiomegaly with pulmonary edema.
The patient was finally diagnosed with acute aggravation of HF.
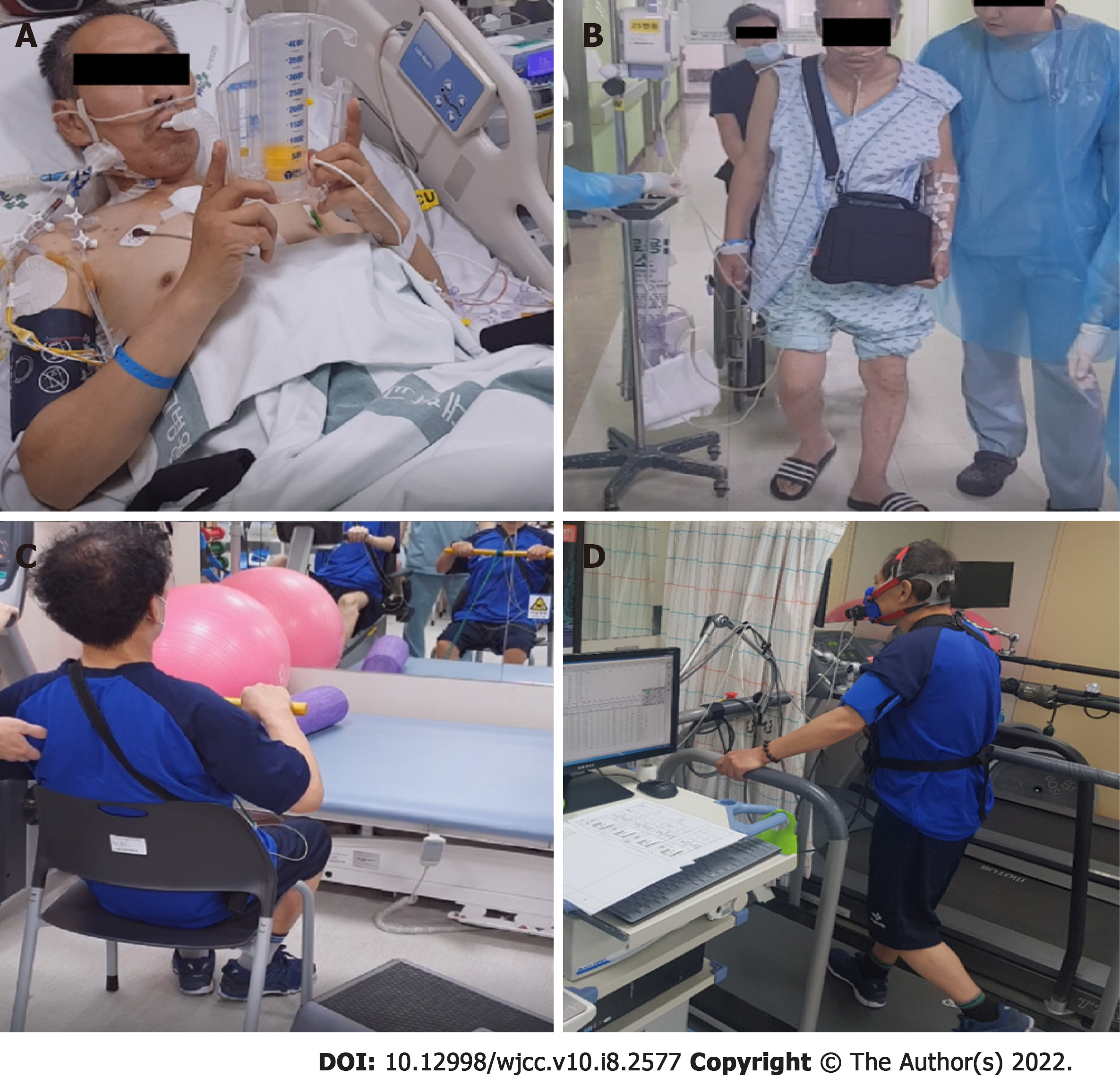
After LVAD insertion, phase I CR, which included chest physiotherapy and aerobic exercise using a portable lower limb ergometer, was conducted in the intensive care unit with a focus on preventing respiratory complications, joint contracture, and muscle atrophy (Figure 1A).
The patient was made to march and to ambulate in the general ward (Figure 1B). The first cardiopulmonary exercise test (CPET) was conducted 3 mo after the LVAD insertion (Figure 1C). The patient's cardiac parameters were as follows: Peak oxygen consumption (VO2), 18.01 mL/min/kg; respiratory exchange ratio, 1.02; minute ventilation (VE)/volume of exhaled CO2 (VCO2) slope, 29.7; resting heart rate (HR), 100 beats per minute (bpm); maximum HR, 140 bpm; and heart rate recovery (HRR), 4 bpm after 1 min. The intensity of exercise was determined by the Karvonen formula using HR.
After discharge, the patient participated in the phase II CR program and peak VO2 increased by 12% of the initial value to 20.20 mL/min/kg. HT was performed 7 mo after the LVAD insertion. During phase I CR, the exercise intensity was determined using a rating of perceived exertion (RPE) scale instead of the HR. Four months after HT, peak VO2 decreased to 17.24 mL/min/kg, and HRR began after 2 min. The phase II CR comprised aerobic and resistance exercises twice a week for 12 wk. After completing the CR program, peak VO2 increased by 30% to 22.29 mL/min/kg (Table 1).
| Post-LVAD1, 81 d (August 8, 2019) | Post-LVAD, 207 d (December 12, 2019) | Post-HT1, 116 d (April 13, 2020) | Post-HT, 162 d (May 29, 2020) | |
| Protocol | Modified Bruce | Modified Bruce | Modified Bruce | Modified Bruce |
| Duration | 7 min 44 s | 12 min 23 s | 9 min 55 s | 14 min 06 s |
| VO2 peak, mL/min/kg (% of the predicted) | 18.01 (47%) | 20.20 (57%) | 17.24 (47%) | 22.29 (62%) |
| VO2 at AT, mL/min/kg | 15.81 | 15.97 | 13.03 | 16.78 |
| METs | 5.2 | 5.7 | 5.0 | 6.4 |
| Resting HR, bpm | 100 | 85 | 86 | 88 |
| Maximum HR, bpm (% of the predicted) | 140 (83%) | 159 (95%) | 107 (64%) | 129 (77%) |
| HRR after 1 min | -4 | -14 | +2 | +5 |
| Resting BP, mmHg | NM | NM | 115/88 | 119/86 |
| Maximum BP, mmHg | NM | NM | 130/67 | 147/88 |
| VE/VCO2 slope | 29.7 | 30.6 | 34.0 | 31.6 |
| RER | 1.02 | 0.98 | 1.01 | 1.11 |
The patient’s quality of life also improved. The total average score of the short form-36 questionnaire increased from 29.5 before the LVAD insertion to 53.3 points 9 mo after HT.
There are several cases of CR after LVAD insertion where LVAD was used as a DT, but not as a BTT[5,6]. Furthermore, few cases have been reported worldwide in which CR was performed after LVAD insertion and subsequent HT. The patient demonstrated a greater improvement in cardiopulmonary parameters than those reported in previous studies[2,4], although 8 mo had passed between admission and HT. The patient underwent an LVAD insertion and HT sequentially, and each phase required different considerations. Major considerations during phase I CR were exercise intensity and sternal precautions; therefore, low-intensity exercise, such as that which elevates HR by 20 bpm or an RPE of 12, was used (Table 2). In addition, resistance band exercises that focused on hip flexors and knee extensors were performed. The intensity of the resistance exercise was set to about 30% of one repetition maximum. Strength can be estimated using increase in band length from that of the initial length and material of the band. Moreover, based on the Holten diagram, resistance exercise was performed at an intensity that could be performed for 4-5 sets with a short interval of 15 repetitions per set. After median sternotomy, trunk and arm activities were restricted postoperatively to ensure adequate healing and the band elongation ratio was set to 75% to limit the range of motion.
| Aerobic exercise | Exercise type | Intensity | Duration of one session | Sessions/d | Days/wk |
| Post LVAD | |||||
| Phase I | Supervised indoor walking | HR < resting HR + 20 or RPE of 12 | 5-10 min, increase up to 20-30 min | 2 | 3 |
| Lower limb ergometer | 10-20 Watt | ||||
| Phase II | Lower limb ergometer, treadmill, box step up | 40%-55% based on the Karvonen formula | Warm-up 10 min, main exercise 20 min, cool-down 10 min | 1 | 2 |
| Post HT | |||||
| Phase I | Supervised indoor walking | RPE of 12 | 5-10 min, increase up to 20-30 min | 2 | 3 |
| Lower limb ergometer | 10-20 Watt | ||||
| Phase II | Lower limb ergometer | RPE of 13 | Warm-up 10 min, main exercise 20 min, cool-down 10 min | 1 | 2 |
| Treadmill | |||||
| Box step up | |||||
| Resistance training | Exercise type | Strength % of 1RM | Repetitions per set | Sets per exercise/d | Days/wk |
| Phase I | Resistance band exercises | 20%-30% | 8-15 | 3-5 | 2-3 |
| Phase II | Overhead press, biceps curl, leg press machine, squat | 40%-60% | 8-15 | 3-5 | 2-3 |
During phase II CR after the LVAD insertion, the exercise intensity was set based on the Karvonen formula after CPET. Usually, aerobic exercise intensity for patients with cardiovascular disease was set to 40–80% of the exercise capacity using the Karvonen formula. The patient was classified as belonging to the high risk group according to the American Association of Cardiovascular and Pulmonary Rehabilitation Risk Stratification Criteria because the rest ejection fraction was < 40% and congestive heart failure occurred. Therefore, the target intensity was set to 40%–55%. The phase II CR was conducted for 3 mo, twice a week. As a continuous-flow type LVAD was inserted in the patient, blood pressure could be measured with a vascular Doppler device. Blood pressure was measured before, during, and after exercise, and the mean arterial pressure was monitored to maintain it between 70 and 90 mmHg. Hypertension would affect the LVAD capacity to pump blood forward; hypotension and LVAD blood flow alterations might be related to under-filling of the left ventricle secondary to high pump speed, RV failure, and arrhythmias[7]. LVAD may increase the risk of cerebrovascular disorders caused by thrombus; therefore, it is vital to monitor the pump speed and flow rate. Care of the driveline is also essential; an inappropriate position could cause pressure injury, and excessive sweating could cause wound infection. Additionally, since excessive sweating and dehydration can reduce venous return and negatively affect LVAD function, regular water intake was recommended before and after exercise. Furthermore, rapid changes of posture from supine to upright positions were avoided. To reduce the risk of adverse events, warming up and cooling down were performed gradually for 10 min each. Breath-holding Valsalva maneuver was avoided, and the patient's vital signs and condition were monitored for 15 min after exercise.
In a previous study on CR after LVAD, peak VO2 increased by approximately 10%[4]. In the present case, peak VO2 improved by 12.16%, despite the prolonged hospitalization. The patient did not experience any LVAD-associated adverse events. The patient had a gout attack and underwent cholecystectomy for acute cholecystitis; therefore, hospital stay was extended to 5 mo. The patient was able to secure sufficient rehabilitation sessions during the hospitalization period and maintain a relatively long phase I CR. Even after the phase II CR was implemented, the medical staff and the patient continued to communicate to encourage the continuation of rehabilitation treatment. In addition, when the patient moved to a place nearby the hospital, access to the rehabilitation center improved further. The participation rates in CR among patients with HF remain low, ranging from 14% to 43% worldwide[8]. Longer and continuous exercise training interventions could improve physical fitness and quality of life. Also, a tailored exercise program for each phase led to improvement in the patient's quality of life. In phase I CR, low-intensity exercise for the purpose of reconditioning was performed in consideration of the patient's overall condition. In phase II CR, exercise capacity was improved by performing moderate- or high-intensity exercise. Mechanisms contributing to the greater fatigability in patients with HF are likely caused by alterations in the skeletal muscle metabolism, resulting in greater glycolytic capacity and reduced oxidative capacity of the muscle and reduced blood perfusion to the muscle[9]. In this case, the time interval from acute aggravation of HF to LVAD insertion was about 1 mo. For this reason, although a decrease in skeletal muscle dysfunction occurred, this change was relatively reversible and could be overcome by continuing rehabilitation.
A denervated autonomic nervous system is a key physiological change after HT. Loss of vagal inhibition to the sinoatrial node causes resting tachycardia with an HR of 100 bpm. The chronotropic response is caused by changes in blood catecholamine concentration owing to the loss of sympathetic innervation. As a result, the HR response to exercise is blunted, with a lower peak HR (20% approximately)[10]. The exercise intensity should be determined based on RPE. Even in the same phase of CR, the target intensity settings were different after LVAD insertion or HT, so that CR was organically intervened at each stage. In this case, the maximum HR decreased from 159 to 107 bpm after HT, and HRR began 2 min after the peak exercise. According to the Fick equation, the peak VO2 also decreased from 20.20 to 17.24 mL/min/kg.
The patient participated in phases I and II CR, and peak VO2 increased from 17.24 to 22.29 mL/min/kg at 5 mo after HT. Compared to a previous study in which VO2 increased by 2.34 mL/min/kg, the patient described herein showed a greater improvement in functional capacity[2]. The maximum HR rose from 64% of the predicted value to 77%, indicating sympathetic reinnervation; however, HRR was still delayed. Since parasympathetic reinnervation is expected to continue for 2 years after HT, further follow-up is necessary[11,12].
The number of end-stage HF patients requiring LVAD or HT is gradually increasing. During CR in these patients, proper exercise prescription and a tailored exercise program for each phase are essential. One-year survival after HT is at least 85%, and median survival exceeds 12 years. Therefore, further research is needed to elucidate the impact of improved functional capacity after CR on the survival rate.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rehabilitation
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Avtaar Singh SS, Ito S S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Bhagra SK, Pettit S, Parameshwar J. Cardiac transplantation: indications, eligibility and current outcomes. Heart. 2019;105:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Hsieh PL, Wu YT, Chao WJ. Effects of exercise training in heart transplant recipients: a meta-analysis. Cardiology. 2011;120:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Tsiouris A, Paone G, Nemeh HW, Borgi J, Williams CT, Lanfear DE, Morgan JA. Short and long term outcomes of 200 patients supported by continuous-flow left ventricular assist devices. World J Cardiol. 2015;7:792-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (2)] |
| 4. | Kerrigan DJ, Williams CT, Ehrman JK, Saval MA, Bronsteen K, Schairer JR, Swaffer M, Brawner CA, Lanfear DE, Selektor Y, Velez M, Tita C, Keteyian SJ. Cardiac rehabilitation improves functional capacity and patient-reported health status in patients with continuous-flow left ventricular assist devices: the Rehab-VAD randomized controlled trial. JACC Heart Fail. 2014;2:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 5. | Bachmann JM, Duncan MS, Shah AS, Greevy RA Jr, Lindenfeld J, Keteyian SJ, Thomas RJ, Whooley MA, Wang TJ, Freiberg MS. Association of Cardiac Rehabilitation With Decreased Hospitalizations and Mortality After Ventricular Assist Device Implantation. JACC Heart Fail. 2018;6:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Park WH, Seo YG, Sung JD. Exercise therapy for an older patient with left ventricular assist device. Ann Rehabil Med. 2014;38:396-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Adamopoulos S, Corrà U, Laoutaris ID, Pistono M, Agostoni PG, Coats AJS, Crespo Leiro MG, Cornelis J, Davos CH, Filippatos G, Lund LH, Jaarsma T, Ruschitzka F, Seferovic PM, Schmid JP, Volterrani M, Piepoli MF. Exercise training in patients with ventricular assist devices: a review of the evidence and practical advice. A position paper from the Committee on Exercise Physiology and Training and the Committee of Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 8. | Chun KH, Kang SM, Cardiac rehabilitation in heart failure. Int J Heart Fail 2021; 3(1): 1-14. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Keller-Ross ML, Larson M, Johnson BD. Skeletal Muscle Fatigability in Heart Failure. Front Physiol. 2019;10:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Frontera WR, DeLisa JA, Gans BM, Walsh NE, Robinson LR. DeLisa’s Physical medicine and rehabilitation: principles and practice. 5th ed. 6: Lippincott Williams & Wilkins, 2013. |
| 11. | Awad M, Czer LS, Hou M, Golshani SS, Goltche M, De Robertis M, Kittleson M, Patel J, Azarbal B, Kransdorf E, Esmailian F, Trento A, Kobashigawa JA. Early Denervation and Later Reinnervation of the Heart Following Cardiac Transplantation: A Review. J Am Heart Assoc. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Imamura T, Kinugawa K, Okada I, Kato N, Fujino T, Inaba T, Maki H, Hatano M, Kinoshita O, Nawata K, Kyo S, Ono M. Parasympathetic reinnervation accompanied by improved post-exercise heart rate recovery and quality of life in heart transplant recipients. Int Heart J. 2015;56:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |