Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2497
Peer-review started: July 26, 2021
First decision: October 22, 2021
Revised: October 25, 2021
Accepted: January 29, 2022
Article in press: January 29, 2022
Published online: March 16, 2022
Processing time: 227 Days and 17.2 Hours
Immune checkpoint inhibitors (ICIs) targeting the programmed death (PD)-1 pathway have substantially changed the clinical management of metastatic urothelial carcinoma (mUC); however, the response rate remains low. There are ongoing efforts to identify robust biomarkers that can effectively predict the treatment response to ICIs. Previous studies have suggested that ERBB2/3 mutations are associated with the efficacy of ICIs in gallbladder carcinoma.
We present a 59-year-old man with mUC harboring ERBB2/3 mutations (in-frame insertion of ERBB2 and ERBB3 amplification), negative PD-ligand 1 expression, and low tumor mutation burden. He received anti-PD-1 antibodies and paclitaxel as second-line treatment. After two cycles of treatment, the lung metastases had significantly shrunk, achieving good partial remission. After six cycles of combination therapy, the patient received sindilimab 200 mg once every 3 wk as maintenance monotherapy. At the last follow-up, the patient continued to exhibit a partial response and progression-free survival for as long as 19 mo.
ERBB2/3 mutations may represent a predictive biomarker for selecting a subgroup of mUC patients who will benefit from ICIs.
Core tip: Immune checkpoint inhibitors (ICIs) have substantially changed the clinical management of metastatic urothelial carcinoma (mUC); however, the response rate to monotherapy remains low. Previous studies have suggested that ERBB2/3 mutations are associated with the efficacy of ICIs in gallbladder carcinoma. The present case of mUC harboring ERBB2/3 mutations, negative programmed death (PD)-ligand 1 expression, and low tumor mutation burden showed durable response to anti-PD-1 antibodies combined with paclitaxel as second-line treatment. Further studies are required to investigate this finding.
- Citation: Yan FF, Jiang Q, Ru B, Fei XJ, Ruan J, Zhang XC. Metastatic urothelial carcinoma harboring ERBB2/3 mutations dramatically respond to chemotherapy plus anti-PD-1 antibody: A case report. World J Clin Cases 2022; 10(8): 2497-2503
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2497.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2497
Bladder cancer is considered to be one of the most aggressive neoplasms worldwide[1]. For patients with distant metastases, the 5-year survival rate is as low as approximately 5%[2]. Cisplatin based combination regimens have remained the standard first-line treatment for metastatic urothelial carcinoma (mUC) over the past decade. In the past, following the failure of first-line chemotherapy, paclitaxel, docetaxel, ifosfamide or gemcitabine monotherapy have been the most commonly used drugs, but are associated with low efficacy.
Several immune checkpoint inhibitors (ICIs) have been approved in recent years as first-line treatment for patients who ineligible to cisplatin or as second-line treatment for patients with mUC of the bladder. Despite the success of immune checkpoint blockades as a strategy for activating an antitumor immune response and promoting cancer regression, only a subset of patients experienced a durable clinical benefit. However, low objective response rates (13%-31%) have been observed in mUC[3-5].
The level of programmed death (PD)-1 expression and tumor mutation burden (TMB) are the two most commonly used predictive biomarkers but they are not sufficient[6-9]. Therefore, there is an urgent need to identify biomarkers that can predict patient response or resistance to ICIs. Several clinical trials have attempted to identify robust biomarkers that can effectively predict the treatment response to ICIs in a subgroup analysis, including high levels of microsatellite instability (MSI-H), a mismatch repair deficiency (dMMR)[10], or tumor infiltrating cytotoxic T lymphocytes (TILs)[11,12]. It is suggested that ERBB2/3 mutations are associated with the efficacy of ICIs[13].
Here, we report a case of mUC harboring ERBB2/3 mutations, in which the level of PD-1 expression was negative and TMB was 3.4/Mb, demonstrating a durable response to anti-PD-1 antibodies in combination with chemotherapy as second-line therapy.
A 59-year-old man presented to our department complaining of bloody sputum for 2 wk on March 2020. He was diagnosed with urothelial cancer > 13 years ago.
In May 2006, the patient presented with intermittent hematuria for 6 mo. On June 18, 2006, he received transurethral resection of bladder tumor in a local hospital, and immunohistochemistry revealed invasive UC (grade 3). Due to repeated local recurrence, the patient underwent repeated (10 times) transurethral resection of bladder tumor from June 2006 to July 2017. On July 5, 2017, the patients received laparoscopic total cystectomy and ileal neobladder. Postoperative pathology showed high-grade papillary UC (WHO grade III) with muscularis invasion (rpT2N0M0, stage II). Pathology confirmed that the surgical margin was negative. In July 2018, the patient presented to a local hospital because of intermittent hematuria for 1 mo. Cystoscopy showed urethral neoplasm. Resection biopsy of the neoplasm confirmed high-grade papillary UC (WHO grade III). The TNM stage was rT0N0M1, stage IV. The patient received six cycles of gemcitabine and cisplatin (GP) as first-line chemotherapy from July 7, 2018 to January 19, 2019. In March 2020, the patient presented to our department complaining of bloody sputum for 2 wk.
In May 2006, the patient presented with intermittent hematuria for 6 mo. On June 18, 2006, he received transurethral resection of bladder tumor in local hospital, and the immunohistochemistry results revealed invasive urothelial cancer (grade 3). Due to repeated local recurrence, the patient received repeated (10 times) of transurethral resection of bladder tumor from June 2006 to July 2017. On July 5, 2017, the patients received laparoscopic total cystectomy and ileal neobladder, the postoperative pathology showed high-grade papillary urothelial carcinoma (WHO grade III) with muscularis invasion (rpT2N0M0, stage II). Pathology confirmed that the surgical margin was negative. In July 2018, the patient presented to local hospital for intermittent hematuria for 1 mo. The cystoscope showed neoplasm on urethra. The resection biopsy of the neoplasm confirmed high-grade papillary urothelial carcinoma (WHO grade III). The TNM stage was rT0N0M1, stage IV. The patient received six cycles of GP (gemcitabine and cisplatin) as first-line chemotherapy from July 7, 2018 to January 19, 2019. On March 2020, the patient presented at our department complaining of bloody sputum for half a month.
The patient’s previous medical history was hypertension, without a family history of cancer.
The Eastern Cooperative Oncology Group score was 0 to 1, and the numeric pain intensity scale score was 0. There was an old surgical scar of about 11 cm in the lower abdomen.
Routine blood examination, blood biochemistry and urinalysis were normal. Serum tumor markers including -fetoprotein, carcinoembryonic antigen, cancer antigen (CA)125, CA 19-9, and ferritin were routinely monitored, and all were normal.
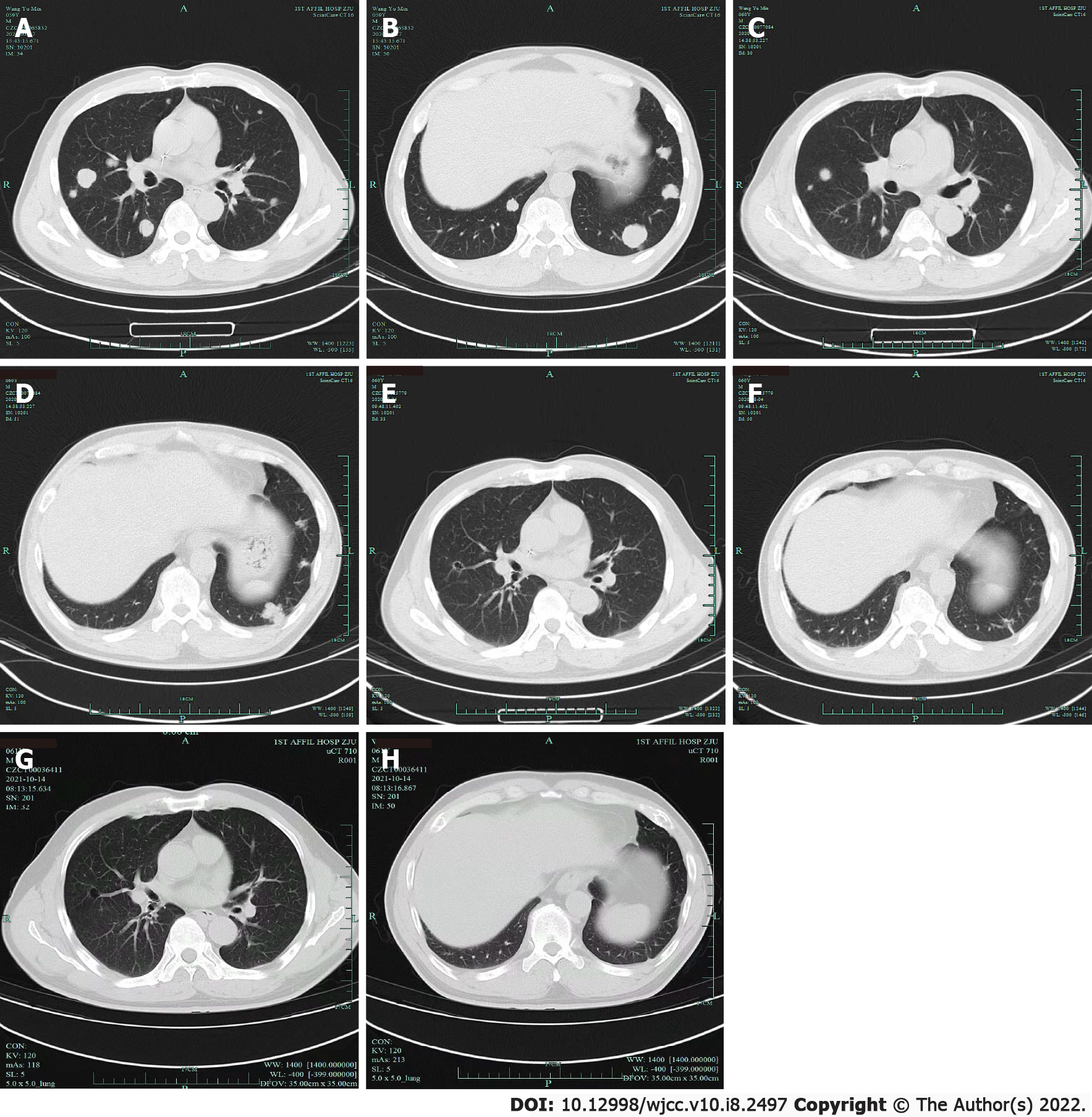
Electrocardiography was normal. Chest computed tomography (CT) showed multiple lung metastases (Figure 1A). Enhanced abdominal CT showed postoperative changes of bladder cancer. Next-generation sequencing (NGS) showed PD-ligand 1 (PD-L1) < 1%, TMB 3.4/Mb, in-frame insertion of ERBB2 [c.2313-2323dup ATACGTGATGGC (p.Y772-A775dup), 21.6%] and ERBB3 amplification (2.5 times).
mUC (cT0N0M1, stage IV).
The patient refused CT-guided percutaneous lung biopsy. Since March 19, 2020, the patient received six cycles of paclitaxel 300 mg plus sindilimab 200 mg once every 3 wk as second-line therapy and subsequently received sindilimab 200 mg once every 3 wk as maintenance treatment.
After two cycles of treatment, chest CT revealed that the lung metastases were markedly reduced in size (Figure 1C and D). After six cycles, chest CT revealed further reduction of the lung metastases (Figure 1E and F). The patient received review irregularly in a local hospital or in our central hospital. At the time of the last follow-up on July 5, 2021, the patient exhibited a durable partial response (Figure 1G and H) and progression-free survival (PFS) was 19 mo. No obvious side effects were observed and the patient was satisfied with the treatment.
ICIs have revolutionized the treatment of a range of solid tumors, including lung cancer, melanoma, esophageal cancer, and colorectal cancer with MSI-H for their durable clinical benefit and lower toxic effects[14,15]. Since 2016, US Food and Drug Administration has approved five ICIs (atezolizumab, nivolumab, pembrolizumab, avelumab and durvalumab) the for the treatment of mUC (Table 1). Although ICIs are effective at treating metastatic urothelial bladder cancer, only a small proportion of patients receive a definite benefit. Currently, no single biomarker can clearly predict treatment response. To better predict the patients who are the mostly likely to benefit from ICIs, several ongoing trials have been conducted to identify effective biomarkers. With the wide application of NGS, an increasing number of new biomarkers are being discovered.
| Anti-PD-L1 antibodies | Approvals of FDA | Clinical trials |
| US FDA approval of anti-PD-L1 antibodies in UC | ||
| Atezolizumab | May 18, 2016: As second-line monotherapy for patients with locally advanced or metastatic urothelial carcinoma (UC) who have disease progression during or following platinum-containing chemotherapy or have disease progression within 12 mo of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy | IMvigor 210 |
| Initial approval April 2017 and modified June 19, 2018 (restricted to PD-L1+): as first-line monotherapy for patients with locally advanced or metastatic UC who: 1) are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells covering ≥ 5% of the tumor area), as determined by an FDA-approved test, or 2) are not eligible for any platinum-containing chemotherapy regardless of PD-L1 status | IMvigor 210, IMvigor130 | |
| Avelumab | May 9, 2017: As second-line monotherapy for patients with locally advanced or metastatic UC whose disease progressed during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy | JAVELIN1b |
| June 30, 2020: As maintenance treatment for patients with locally advanced or metastatic UC that has not progressed with first-line platinum-containing chemotherapy | JAVELIN Bladder 100 | |
| Durvalumab | May 1, 2017: As second-line monotherapy for patients with locally advanced or metastatic UC who have disease progression during or following platinum-containing chemotherapy or who have disease progression within 12 mo of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy | NCT01693562 |
| US FDA approval of anti-PD-1 antibodies in UC | ||
| Anti-PD-1 antibodies | Approvals of FDA | Clinical trials |
| Nivolumab | February 2, 2017: As second-line monotherapy for patients with locally advanced or metastatic UC who have disease progression during or following platinum-containing chemotherapy or have disease progression within 12 months of neoadjuvant or adjuvant treatment with a platinum-containing chemotherapy. | Checkmate 275 |
| Pembrolizumab | May 18, 2017: As second-line monotherapy for patients with locally advanced or metastatic UC who have disease progression during or following platinum-containing chemotherapy or within 12 mo of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy | Keynote-045 |
| May 18, 2017: As first-line monotherapy for patients with locally advanced or metastatic UC who are not eligible for cisplatin-containing chemotherapy. | Keynote-052 | |
The epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases consists of four members: EGFR1/ERBB1/HER1, ERBB2/HER2, ERBB3/HER3, and ERBB4/HER4(16). Signaling through these receptors regulates many key cellular activities, including cell division, migration, adhesion, differentiation and apoptosis[17]. ERBB2/3 mutations (including point mutations and amplification) are observed in many types of solid tumors (e.g., breast cancer, gastric cancer, lung cancer and UC). An ERBB2 in-frame insertion into exon 20 has been associated with tyrosine kinase inhibitor resistance in lung adenocarcinoma[18]. Moreover, ERBB3 overexpression has been associated with resistance to a large number of therapies in some cancers[19,20]. ERBB2/3 mutations are associated with the treatment efficacy of PD-L1 monoclonal antibodies for gallbladder carcinoma[13]. ICI monotherapy after the failure of first-line treatment is another reason for the low response rate associated with ICIs.
Ongoing trials are investigating the regimens of ICIs combined with chemotherapy. The rationale is chemotherapy induces immunogenic cell death resulting in tumor antigens releasing and increasing MHC-I-mediated tumor antigen presentation which may enhance the effects of the immune response within the tumor. Another mechanism is directly modulating the activity and/or quantity of immunosuppressive cellular subsets[21,22]. Several trials have explored the efficacy of ICIs in combination with chemotherapy for mUC. IMvigor-130 is a double blind, three-arm, multicenter, phase 3 trial investigating the use of atezolizumab as monotherapy or combined with platinum-based chemotherapy comparing with chemotherapy alone as first-line treatment for patients with locally advanced or metastatic bladder carcinoma[23]. The addition of atezolizumab to platinum-based chemotherapy as a first-line treatment prolonged PFS in patients with mUC (mPFS 8.2 mo (95%CI: 6.5-8.3) in the atezolizumab plus platinum-based chemotherapy group and 6.3 (6.2-7.0) mo in the placebo plus platinum-based chemotherapy group (stratified hazard ratio: 0.82, 95%CI: 0.70–0.96; one-sided P = 0.007). In addition, the median overall survival was 16.0 (13.9–18.9) mo in the atezolizumab plus platinum-based chemotherapy group and 13.4 (12.0–15.2) mo in the placebo plus platinum-based chemotherapy group (0.83, 0.69–1.0; one-sided P = 0.027). A similar three-arm, multicenter, phase 3 clinical trial (KEYNOTE-036) was established to investigate pembrolizumab as a monotherapy or combined with platinum-based chemotherapy against standard chemotherapy plus a placebo as first-line treatment. A phase 2 study also investigated cisplatin combined with gemcitabine plus ipilimumab compared with chemotherapy alone for patients with mUC. The objective response rate was as high as 69% and the completed response rate was 17%[2].
We first reported metastatic bladder UC harboring an ERBB2 in-frame insertion in an exon 20 mutation and ERBB3 amplification treated with paclitaxel plus sindilimab as second-line treatment. Although PD-L1 expression was negative and the TMB was low, the patient still achieved a durable response, with lung metastases being significantly reduced. At the last follow-up, the PFS was 19 mo. We will continue to focus on the follow-up treatment of this patient. However, we only included one case in this report, further studies and cases are required to confirm the relationship between ERBB2/3 mutations and response to ICIs in mUC.
This case indicates that mUC patients with ERBB2/3 mutations may benefit from ICIs. Further studies and cases are required to explore the ability of ERBB2/3 mutations to predict the efficacy of ICIs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kołat D S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15470] [Article Influence: 2578.3] [Reference Citation Analysis (2)] |
| 2. | Galsky MD, Pal SK, Lin SW, Ogale S, Zivkovic M, Simpson J, Derleth C, Schiff C, Sonpavde G. Real-World Effectiveness of Chemotherapy in Elderly Patients With Metastatic Bladder Cancer in the United States. Bladder Cancer. 2018;4:227-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, Arranz JÁ, Pal S, Ohyama C, Saci A, Qu X, Lambert A, Krishnan S, Azrilevich A, Galsky MD. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 1275] [Article Influence: 159.4] [Reference Citation Analysis (0)] |
| 4. | van der Heijden MS, Loriot Y, Durán I, Ravaud A, Retz M, Vogelzang NJ, Nelson B, Wang J, Shen X, Powles T. Atezolizumab Versus Chemotherapy in Patients with Platinum-treated Locally Advanced or Metastatic Urothelial Carcinoma: A Long-term Overall Survival and Safety Update from the Phase 3 IMvigor211 Clinical Trial. Eur Urol. 2021;80:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 5. | Vuky J, Balar AV, Castellano D, O'Donnell PH, Grivas P, Bellmunt J, Powles T, Bajorin D, Hahn NM, Savage MJ, Fang X, Godwin JL, Frenkl TL, Homet Moreno B, de Wit R, Plimack ER. Long-Term Outcomes in KEYNOTE-052: Phase II Study Investigating First-Line Pembrolizumab in Cisplatin-Ineligible Patients With Locally Advanced or Metastatic Urothelial Cancer. J Clin Oncol. 2020;38:2658-2666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 6. | Bellmunt J, Bajorin DF. Pembrolizumab for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376:2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Nam K, Frenkl TL, Perini RF, de Wit R, Bajorin DF. Randomized phase III KEYNOTE-045 trial of pembrolizumab vs paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019;30:970-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 344] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 8. | Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, Borghaei H, Ramalingam SS, Brahmer J, Reck M, O'Byrne KJ, Geese WJ, Green G, Chang H, Szustakowski J, Bhagavatheeswaran P, Healey D, Fu Y, Nathan F, Paz-Ares L. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med. 2018;378:2093-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1913] [Cited by in RCA: 2373] [Article Influence: 339.0] [Reference Citation Analysis (0)] |
| 9. | Cimadamore A, Gasparrini S, Santoni M, Cheng L, Lopez-Beltran A, Battelli N, Massari F, Giunchi F, Fiorentino M, Scarpelli M, Montironi R. Biomarkers of aggressiveness in genitourinary tumors with emphasis on kidney, bladder, and prostate cancer. Expert Rev Mol Diagn. 2018;18:645-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 4948] [Article Influence: 618.5] [Reference Citation Analysis (0)] |
| 11. | Daud AI, Loo K, Pauli ML, Sanchez-Rodriguez R, Sandoval PM, Taravati K, Tsai K, Nosrati A, Nardo L, Alvarado MD, Algazi AP, Pampaloni MH, Lobach IV, Hwang J, Pierce RH, Gratz IK, Krummel MF, Rosenblum MD. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest. 2016;126:3447-3452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 415] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 12. | Loo K, Tsai KK, Mahuron K, Liu J, Pauli ML, Sandoval PM, Nosrati A, Lee J, Chen L, Hwang J, Levine LS, Krummel MF, Algazi AP, Pampaloni M, Alvarado MD, Rosenblum MD, Daud AI. Partially exhausted tumor-infiltrating lymphocytes predict response to combination immunotherapy. JCI Insight. 2017;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Li M, Liu F, Zhang F, Zhou W, Jiang X, Yang Y, Qu K, Wang Y, Ma Q, Wang T, Bai L, Wang Z, Song X, Zhu Y, Yuan R, Gao Y, Liu Y, Jin Y, Li H, Xiang S, Ye Y, Zhang Y, Jiang L, Hu Y, Hao Y, Lu W, Chen S, Gu J, Zhou J, Gong W, Wang X, Liu X, Liu C, Liu H. Genomic ERBB2/ERBB3 mutations promote PD-L1-mediated immune escape in gallbladder cancer: a whole-exome sequencing analysis. Gut. 2019;68:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (1)] |
| 14. | Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab vs Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5686] [Cited by in RCA: 6739] [Article Influence: 673.9] [Reference Citation Analysis (0)] |
| 15. | Morris VK, Salem ME, Nimeiri H, Iqbal S, Singh P, Ciombor K, Polite B, Deming D, Chan E, Wade JL, Xiao L, Bekaii-Saab T, Vence L, Blando J, Mahvash A, Foo WC, Ohaji C, Pasia M, Bland G, Ohinata A, Rogers J, Mehdizadeh A, Banks K, Lanman R, Wolff RA, Streicher H, Allison J, Sharma P, Eng C. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 295] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 16. | Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4920] [Cited by in RCA: 5111] [Article Influence: 213.0] [Reference Citation Analysis (1)] |
| 17. | Bianco R, Gelardi T, Damiano V, Ciardiello F, Tortora G. Rational bases for the development of EGFR inhibitors for cancer treatment. Int J Biochem Cell Biol. 2007;39:1416-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Mazières J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, Besse B, Blons H, Mansuet-Lupo A, Urban T, Moro-Sibilot D, Dansin E, Chouaid C, Wislez M, Diebold J, Felip E, Rouquette I, Milia JD, Gautschi O. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31:1997-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 517] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 19. | Morrison MM, Williams MM, Vaught DB, Hicks D, Lim J, McKernan C, Aurisicchio L, Ciliberto G, Simion C, Sweeney C, Cook RS. Decreased LRIG1 in fulvestrant-treated luminal breast cancer cells permits ErbB3 upregulation and increased growth. Oncogene. 2016;35:1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Ghasemi R, Rapposelli IG, Capone E, Rossi C, Lattanzio R, Piantelli M, Sala G, Iacobelli S. Dual targeting of ErbB-2/ErbB-3 results in enhanced antitumor activity in preclinical models of pancreatic cancer. Oncogenesis. 2014;3:e117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 620] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 22. | Correale P, Del Vecchio MT, La Placa M, Montagnani F, Di Genova G, Savellini GG, Terrosi C, Mannucci S, Giorgi G, Francini G, Cusi MG. Chemotherapeutic drugs may be used to enhance the killing efficacy of human tumor antigen peptide-specific CTLs. J Immunother. 2008;31:132-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, Garcia-Del-Muro X, De Giorgi U, Mencinger M, Izumi K, Panni S, Gumus M, Özgüroğlu M, Kalebasty AR, Park SH, Alekseev B, Schutz FA, Li JR, Ye D, Vogelzang NJ, Bernhard S, Tayama D, Mariathasan S, Mecke A, Thåström A, Grande E; IMvigor130 Study Group. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1547-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 580] [Article Influence: 116.0] [Reference Citation Analysis (0)] |