Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.13138
Peer-review started: November 3, 2022
First decision: November 14, 2022
Revised: November 16, 2022
Accepted: November 18, 2022
Article in press: November 18, 2022
Published online: December 16, 2022
Processing time: 40 Days and 16.5 Hours
Rocuronium, a nondepolarizing muscle relaxant, is usually administered during general anesthesia to facilitate endotracheal intubation and keep patients immobile during the surgery. Sugammadex, the selective reversal agent of rocuronium, fully reverses the neuromuscular blockade (NMB) at the end of surgery. Most reports show that sugammadex rapidly achieves a ratio of train-of-four (TOF), a quantitative method of neuromuscular monitoring, of 0.9 which ensures adequate recovery for safe extubation. However, very rare patients with neuromuscular diseases may respond poorly to sugammadex.
A 69-year-old female presented with abdominal fullness and nausea, and was diagnosed with gastroparesis. She underwent gastric peroral endoscopic my
In our case, both prolonged rocuronium-induced NMB and poor response to sugammadex were noted. To optimize the dose of rocuronium, perioperative TOF combined with other neur
Core Tip: Sugammadex reverses rocuronium-induced neuromuscular blocking effect, providing full and rapid recovery from general anesthesia. However, in rare cases, patients with neuromuscular diseases may response poorly to sugammadex. In our patient, suspected neuromuscular disease, renal insufficiency, and a history of delayed recovery from general anesthesia may indicate a delayed recovery course. An optimal dose of rocuronium adjusted by train-of-four neuromuscular monitoring throughout the surgery helps reduce the risk of prolonged recovery from general anesthesia.
- Citation: Wang HC, Lu CW, Lin TY, Chang YY. Unexpected delayed reversal of rocuronium-induced neuromuscular blockade by sugammadex: A case report and review of literature. World J Clin Cases 2022; 10(35): 13138-13145
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/13138.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.13138
General anesthesia consisting of unconsciousness, immobility, analgesia and amnesia facilitates surgical treatment[1]. Steroidal neuromuscular blocking agents, such as rocuronium, are usually administered during general anesthesia to facilitate endotracheal intubation and to keep the patients immobile during the surgery[2,3]. Previous clinical study showed the duration of neuromuscular blocking effect induced by rocuronium 0.6 mg/kg (2 × effective dose 95) is around 23-75 min in adult patients, but varies indivi
The first selective binding reversal agent for steroidal neuromuscular blocking agents, sugammadex (Bridon®), encapsules rocuronium and reverses the neuromuscular blocking effect[13-15]. Many clinical studies have shown full and rapid recovery of muscle strength by sugammadex, even in circumstances of moderate to profound muscular blockade[12,14-20]. A dose of 2 mg/kg sugammadex is recommended when TOF count > 2[21]. Restoration of neuromuscular function using the recommended dose of sugammadex is demonstrated in 95% of patients within 5 min[21]. Here we report a case of prolonged rocuronium-induced neuromuscular blocking effect and delayed reversal by sugammadex. The underlying mechanism and the role of TOF are discussed.
A 69-year-old female (140 cm/55 kg) complained of poor appetite, nausea and vomiting.
The patient had persistent abdominal fullness with intermittent nausea for several years. She visited our hospital for further evaluation because of a recent exacerbation of gastrointestinal symptoms. The gastric emptying scan showed a significantly prolonged oatmeal-based gastric emptying time, particularly in the first hour. The percentage of gastric retention after one hour (93%), two hours (50.25%), three hours (25.10%) or four hours (14.40%) was higher than the normal limit. Due to gastroparesis, the patient was admitted in our hospital for gastric peroral endoscopic myotomy.
The patient had history of hypertension, type 2 diabetes mellitus (DM) and α-thalassemia. She regularly took antihypertensive drugs and oral hypoglycemic agents.
The patient was neither a drinker nor a smoker. No family history of myasthenia gravis, neuromuscular disease or autoimmune diseases was recorded. Her surgical history included laparoscopic cholecystectomy, lumbar spinal surgery and functional endoscopy sinus surgery.
Physical examination revealed dry eye and dry mouth, which may be DM-related, and pale conjunctiva that may be a sign of anemia. Hypoactive bowel sound and mild epigastric tenderness were also noted. Raynaud's phenomena or arthritis-related symptoms were not observed. Notably, non-specific involuntary movement of mouth and twitching of the tongue for years were reported by the patient herself. Since these hyperkinetic movements did not cause any daily functional impairment, she did not seek any medical consultation. Slow movement while changing position and poor hand grip muscle strength were found at the bedside.
Before the surgery, routine examinations including levels of electrolytes (potassium 4.1 mmol/L and sodium 140 mmol/L), coagulation function and liver function (alanine transaminase 9 U/L) were all unremarkable. The complete blood count test showed anemia (Hgb 7.8 g/dL). Furthermore, preoperative laboratory findings displayed an increased level of creatinine (1.52 mg/dL) and blood urea nitrogen (26 mg/dL). A low estimated glomerular filtration rate, 34 mL/min/1.73 m2, was noted during this admission.
Preoperative chest X-ray revealed normal heart size with no significant lung lesion. Electrocardiography revealed a normal sinus rhythm. Abdominal sonography showed bilateral renal cysts and parenchymal renal diseases. Panendoscopy revealed a gastric ulcer scar in the deformed antrum. No obvious obstruction or gastroesophageal reflux was observed in the subsequent barium study.
DM-related refractory gastroparesis.
As the endoscope traveled down the esophagus, increased resistance was noted at the pyloric ring. A mucosal incision was made at 5 cm proximal to the pyloric ring, followed by a submucosal tunneling. One 1.5 cm long myotomy was performed at the pylorus. Finally, the mucosal entry point was closed by endoclips.
On arrival at the operating room, hypertension (190/97 mmHg) with oxygen saturation of 96% on room air was noted. After preoxygenation, rapid sequence induction of general anesthesia was conducted with propofol (100 mg), fentanyl (75 μg) and rocuronium (40 mg). Anesthesia was maintained using sevoflurane (1-1.3 minimum alveolar concentration) through a 7.0# endotracheal tube. During the entire surgery, the Bispectral Index value was kept between 40-60. No supplemental rocuronium was administered intraoperatively.
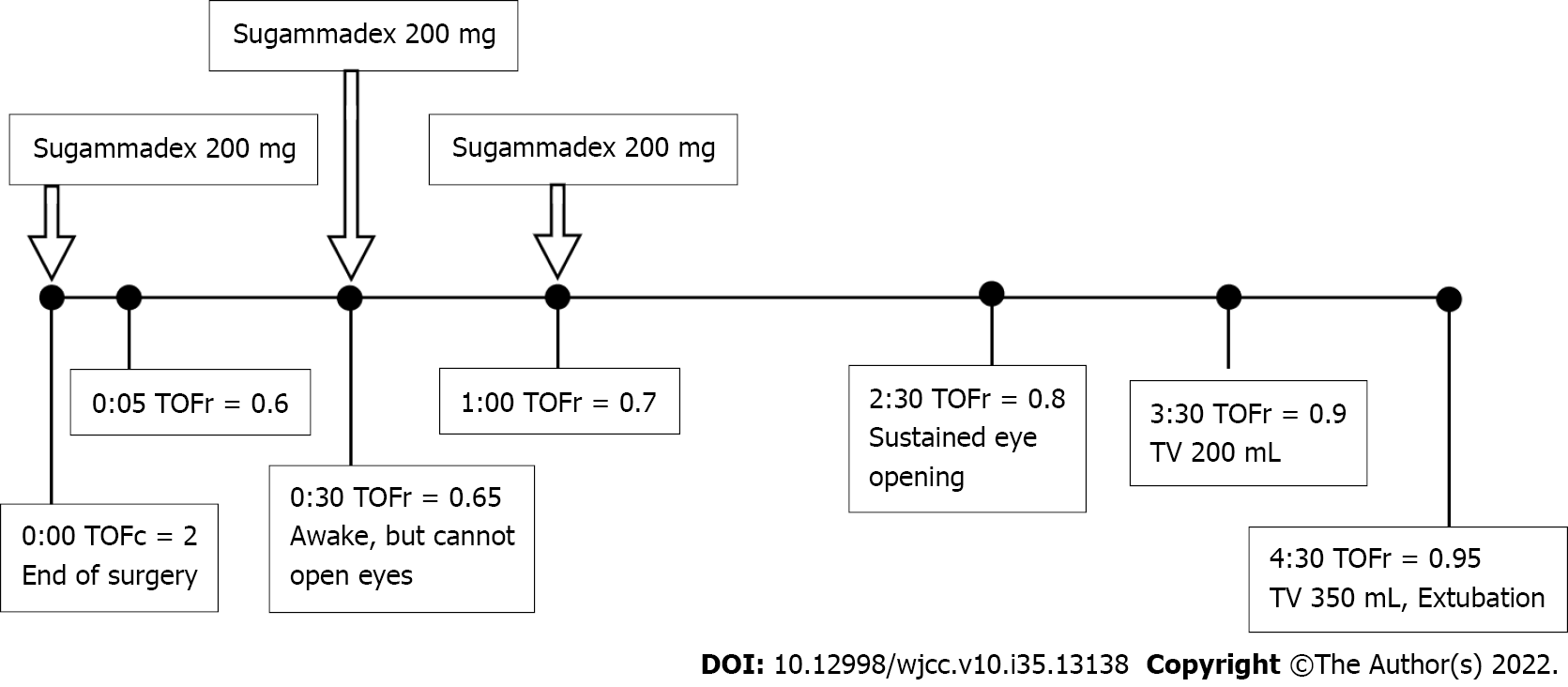
Surgery was completed smoothly within 1.5 h. Before administration of the reversal agent, we turned off sevofluorane and applied TOF to this patient. Her body temperature was 36.5 oC. The TOF count was 2, therefore, the first dose of sugammadex 200 mg (3.6 mg/kg) was administered. After 30 min, the TOF count increased to 4 with a TOF ratio of 0.65. Though the patient was fully awake and able to follow our instructions by slight nodding, spontaneous breathing with a low tidal volume (30-50 mL) was noted. She tried to lift her eyelids but failed. During the following 2 h, her TOF ratio slowly increased to 0.8 after the second and third doses of sugammadex 200 mg. The patient was able to open her eyes and grip her hands. About 3.5 h after the end of surgery, the TOF ratio was 0.9, and her tidal volume during spontaneous breathing increased to 200 mL. The arterial blood gas analysis data were within normal ranges. About 4.5 h after the end of the surgery, her tidal volume reached 350 mL (TOF ratio = 0.95), followed by smooth removal of the endotracheal tube. The course of recovery from general anesthesia in this patient is shown in Figure 1. The patient was then sent to the post-anesthetic care unit for further care. She was transferred to the ward in 1 h without any discomfort.
The patient was discharged uneventfully on postoperative day three. She was followed for 3 mo after the surgery, without recurrent symptoms.
According to previous clinical studies, a variety of predisposing factors prolong the neuromuscular blocking effect of rocuronium, including drug interaction or pathological states. First, rocuronium may have an enhanced effect when interacting with anti-arrhythmic agents, aminoglycosides or local anesthetic agents[22]. During the perioperative period, we did not use these drugs in our patient. Second, low calcium or high magnesium levels lead to delay recovery from NMB[23]. In our case, a normal calcium level (1.25 mmol/L) and mildly low magnesium level (0.43 mmol/L) were found at the end of surgery. Third, some autoimmune diseases such as myasthenia gravis may be linked to prolonged postoperative recovery. In our case, undiagnosed myasthenia gravis was ruled out based on the normal level of acetylcholine receptor antibody (< 0.2 nmol/L). In addition, the levels of Anti-Ro antibody (7 U/mL) or Anti-La antibody (9 U/mL) were within the normal ranges, which excluded Sjogren's syndrome. We also excluded other possible mechanism underlying the observed prolonged effect of rocuronium, including hypothermia, human errors, intravenous infusion problems and inaccurate TOF placement.
In addition to the aforementioned factors, the duration of rocuronium-induced neuromuscular blocking effect varies individually. After reviewing our patient’s medical record, delayed extubation was also found after functional endoscopy sinus surgery in 2010, when cisatracurium (10 mg) and neostigmine (3.5 mg) were used. This document led us to speculate that our case was an outlier who was extremely sensitive to nondepolarizing neuromuscular blocking agents. Furthermore, in our case, renal insufficiency may lead to the long duration of rocuronium. Although a previous study has proven the safe and effective use of rocuronium in patients of renal insufficiency[24], poor clearance leads to a longer duration of rocuronium in patients with renal insufficiency than in patients with normal renal function[25]. Moreover, females are more sensitive to rocuronium than males[26,27]. Taken together, in our case, there were multiple predisposing factors for the long duration of rocuronium-induced neuromuscular blocking effect.
Sugammadex reverses the neuromuscular blocking effect by directly binding to rocuronium[13]. This novel agent encapsulates rocuronium and promotes its dissociation from the acetylcholine receptor[13]. One molecule of sugammadex binds to one molecule of rocuronium, and antagonizes its effect[28]. Therefore, in our case, 600 mg sugammadex (2.75 × 10-4 moles) was sufficient to antagonize the effect of 40 mg rocuronium (6.55 × 10-5 moles). Theoretically, the effect of sugammadex is poor if the binding affinity between sugammadex and rocuronium is low. To date, very few published reports have discussed about the mechanism underlying the possible poor effect of sugammadex. Only one in vitro study revealed that dexamethasone may mitigate the reversal of rocuronium-induced NMB by sugammadex in a dose-dependent manner[29]. In our case, only 0.09 mg/kg dexamethasone was used, which should not significantly impede the action of sugammadex. Furthermore, sugammadex can be used safely and effectively in patients with renal insufficiency[30,31], although the recovery of TOF ratio to 0.9 may be prolonged in patients with end-stage renal disease[32].
Based on our literature review, there are sporadic previously published case reports about delayed reversal of neuromuscular blocking effect by sugammadex, as summarized in Table 1. Notably, except for patients with high magnesium levels, nearly all cases refractory to the rapid effect of sugammadex are patients with neuromuscular or movement disorders, including myasthenia gravis[33], Charcot-Marie-Tooth disease[34] or Parkinson’s disease[35]. A previous study showed that although most researchers have reported the successful use of sugammadex in patients with neuromuscular diseases, unpredictability in response or individual differences still remains an issue[36]. The possible mechanism was reported to be the redistribution of unbound neuromuscular blocking agent molecules from the peripheral compartments to the central compartments[37]. Interestingly, our patient reported tremor of her tongue with involuntary movement of her mouth for years. The associated signs included poor hand grip strength. Since tongue tremor is a rare initial presentation of essential tremor[38], our patient may be a case of undiagnosed essential tremor or other movement disorders. The correlation between muscle weakness and essential tremor had also been demonstrated[39]. In addition, essential tremor or other movement disorders are known to involve gastrointestinal symptoms such as gastroparesis[40] that was our patient’s diagnosis during this admission. Taken together, we speculated that our case may have undiagnosed neuromuscular diseases, which may partially explain her poor response to sugammadex. Further research and evaluation are required to confirm our hypotheses.
| Ref. | Patient data | Past history | Surgery (duration) | Total dose of muscle relaxants; total dose of sugammadex | Time to extubation from first dose of sugammadex | Possible causes of delayed recovery after sugammadex |
| Ortiz-Gómez et al[28], 2014 | 60 yr/Male (115 kg/175 cm) | HTN | Sigmoid colon resection (360 min) | SCC 100 mg + rocuronium 83 mg; 1120 mg | 208 min | An extreme outlier with resistance to sugammadex |
| Altıparmak et al[35], 2019 | 58 yr/Female (75 kg) | HTN, CVD, PD | Hysterectomy (90 min) | Rocuronium 45 mg; 800 mg | 30 min | PD |
| Brown et al[44], 2019 | 58 yr/Male (69 kg/170 cm) | Glaucoma | Robot-assisted laparoscopic prostatectomy (not mentioned) | Vecuronium 9 mg; 400 mg | 27 min | High magnesium |
| Fernandes et al[33], 2019 | 27 yr/Female (110 kg/172 cm) | Myasthenia gravis | Laparoscopic cholecystectomy (not mentioned) | Rocuronium 20 mg; 800 mg | 45 min | Myasthenia gravis |
| Moriwaki and Kayashima[23], 2019 | 37 yr/Female (42 kg/144 cm) | HTN, GDM; preeclampsia | C/S (95 min) | Rocuronium 40 mg; 200 mg | > 15 min | High magnesium, pregnancy |
| Hiramatsu et al[34], 2022 | 63 yr/Male (68 kg/164 cm) | RA, DM, Af, restrictive lung disease | Hip arthroplasty (273 min) | Rocuronium 50 mg; 1200 mg | 18 h | CMTD type 1A |
| Kiss et al[37], 2013 | 25 yr/Male (BMI: 32) | Myasthenia gravis | Thymectomy (120 min) | Rocuronium 50 mg; 12 mg/kg | Not mentioned | Myasthenia gravis |
To date, there have been few studies on the use of TOF in patients of neuromuscular diseases. Some researchers argued about the accuracy of TOF ratio in cases of myasthenia gravis, and recommended the concomitant use of other neuromuscular monitoring techniques such as tetanic stimulation[41]. Others emphasized the placement of neuromuscular monitoring at multiple sites in patients of neuromuscular disorders[42]. We suggest the employment of TOF throughout the surgery to avoid consumption of rocuronium more than the patient’s requirement, since preanesthetic TOF helps predict the requirement of muscle relaxants in patients of myasthenia gravis[43].
Our patient’s subclinical symptoms may have been related to an undiagnosed neuromuscular disease. Poor renal function and a history of delayed recovery from general anesthesia also hint at the possible prolonged effect of rocuronium. Our case may be an outlier who was extremely sensitive to nondepolarizing neuromuscular blocking agents or has poor response to sugammadex. The role of perioperative TOF in optimizing the dose of neuromuscular blocking agents cannot be overemphasized.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao C, China; Velnar T, Slovenia S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Forman SA, Chin VA. General anesthetics and molecular mechanisms of unconsciousness. Int Anesthesiol Clin. 2008;46:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | England AJ, Margarson MP, Feldman SA. Tracheal intubation conditions after one minute: rocuronium and vecuronium, alone and in combination. Anaesthesia. 1997;52:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Fuchs-Buder T, Schlaich N, Ziegenfuss T. [Rocuronium for anesthesia induction in elective procedures. Time course of muscular blockade and intubation after administration of 2-compartment ED95 (0.6 mg/kg) and dose reduction (0.4 mg/kg)]. Anaesthesist. 1999;48:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Magorian T, Flannery KB, Miller RD. Comparison of rocuronium, succinylcholine, and vecuronium for rapid-sequence induction of anesthesia in adult patients. Anesthesiology. 1993;79:913-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 262] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Fuchs-Buder T, Schreiber JU, Meistelman C. Monitoring neuromuscular block: an update. Anaesthesia. 2009;64 Suppl 1:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Kim KS, Cheong MA, Lee HJ, Lee JM. Tactile assessment for the reversibility of rocuronium-induced neuromuscular blockade during propofol or sevoflurane anesthesia. Anesth Analg. 2004;99:1080-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Difficult Airway Society Extubation Guidelines Group; Popat M, Mitchell V, Dravid R, Patel A, Swampillai C, Higgs A. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia. 2012;67:318-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 320] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 8. | Duţu M, Ivaşcu R, Tudorache O, Morlova D, Stanca A, Negoiţă S, Corneci D. Neuromuscular monitoring: an update. Rom J Anaesth Intensive Care. 2018;25:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Marymont JH, Vender JS, Gray J, Landry E, Gupta DK. Intraoperative acceleromyography monitoring reduces symptoms of muscle weakness and improves quality of recovery in the early postoperative period. Anesthesiology. 2011;115:946-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Alenezi FK, Alnababtah K, Alqahtani MM, Olayan L, Alharbi M. The association between residual neuromuscular blockade (RNMB) and critical respiratory events: a prospective cohort study. Perioper Med (Lond). 2021;10:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Shear T, Vender JS, Gray J, Landry E. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesth Analg. 2013;117:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Plaud B, Baillard C, Bourgain JL, Bouroche G, Desplanque L, Devys JM, Fletcher D, Fuchs-Buder T, Lebuffe G, Meistelman C, Motamed C, Raft J, Servin F, Sirieix D, Slim K, Velly L, Verdonk F, Debaene B. Guidelines on muscle relaxants and reversal in anaesthesia. Anaesth Crit Care Pain Med. 2020;39:125-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 13. | Gijsenbergh F, Ramael S, Houwing N, van Iersel T. First human exposure of Org 25969, a novel agent to reverse the action of rocuronium bromide. Anesthesiology. 2005;103:695-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Groudine SB, Soto R, Lien C, Drover D, Roberts K. A randomized, dose-finding, phase II study of the selective relaxant binding drug, Sugammadex, capable of safely reversing profound rocuronium-induced neuromuscular block. Anesth Analg. 2007;104:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Suy K, Morias K, Cammu G, Hans P, van Duijnhoven WG, Heeringa M, Demeyer I. Effective reversal of moderate rocuronium- or vecuronium-induced neuromuscular block with sugammadex, a selective relaxant binding agent. Anesthesiology. 2007;106:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | de Boer HD, Driessen JJ, Marcus MA, Kerkkamp H, Heeringa M, Klimek M. Reversal of rocuronium-induced (1.2 mg/kg) profound neuromuscular block by sugammadex: a multicenter, dose-finding and safety study. Anesthesiology. 2007;107:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Sacan O, White PF, Tufanogullari B, Klein K. Sugammadex reversal of rocuronium-induced neuromuscular blockade: a comparison with neostigmine-glycopyrrolate and edrophonium-atropine. Anesth Analg. 2007;104:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Sparr HJ, Vermeyen KM, Beaufort AM, Rietbergen H, Proost JH, Saldien V, Velik-Salchner C, Wierda JM. Early reversal of profound rocuronium-induced neuromuscular blockade by sugammadex in a randomized multicenter study: efficacy, safety, and pharmacokinetics. Anesthesiology. 2007;106:935-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Schaller SJ, Fink H, Ulm K, Blobner M. Sugammadex and neostigmine dose-finding study for reversal of shallow residual neuromuscular block. Anesthesiology. 2010;113:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Pongrácz A, Szatmári S, Nemes R, Fülesdi B, Tassonyi E. Reversal of neuromuscular blockade with sugammadex at the reappearance of four twitches to train-of-four stimulation. Anesthesiology. 2013;119:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Cammu G. Sugammadex: Appropriate Use in the Context of Budgetary Constraints. Curr Anesthesiol Rep. 2018;8:178-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Kim YB, Sung TY, Yang HS. Factors that affect the onset of action of non-depolarizing neuromuscular blocking agents. Korean J Anesthesiol. 2017;70:500-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Moriwaki K, Kayashima K. Prolonged neuromuscular blockade and insufficient reversal after sugammadex administration in cesarean section under general anesthesia: a case report. JA Clin Rep. 2019;5:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Della Rocca G, Pompei L, Coccia C, Costa MG, Cecchini V, Vilardi V, Pietropaoli P. Atracurium, cisatracurium, vecuronium and rocuronium in patients with renal failure. Minerva Anestesiol. 2003;69:605-611, 612, 5. [PubMed] |
| 25. | Craig RG, Hunter JM. Neuromuscular blocking drugs and their antagonists in patients with organ disease. Anaesthesia. 2009;64 Suppl 1:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Adamus M, Gabrhelik T, Marek O. Influence of gender on the course of neuromuscular block following a single bolus dose of cisatracurium or rocuronium. Eur J Anaesthesiol. 2008;25:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Xue FS, Tong SY, Liao X, Liu JH, An G, Luo LK. Dose-response and time course of effect of rocuronium in male and female anesthetized patients. Anesth Analg. 1997;85:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Ortiz-Gómez JR, Palacio-Abizanda FJ, Fornet-Ruiz I. Failure of sugammadex to reverse rocuronium-induced neuromuscular blockade: a case report. Eur J Anaesthesiol. 2014;31:708-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Rezonja K, Sostaric M, Vidmar G, Mars T. Dexamethasone produces dose-dependent inhibition of sugammadex reversal in in vitro innervated primary human muscle cells. Anesth Analg. 2014;118:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Panhuizen IF, Gold SJ, Buerkle C, Snoeck MM, Harper NJ, Kaspers MJ, van den Heuvel MW, Hollmann MW. Efficacy, safety and pharmacokinetics of sugammadex 4 mg kg-1 for reversal of deep neuromuscular blockade in patients with severe renal impairment. Br J Anaesth. 2015;114:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Min KC, Lasseter KC, Marbury TC, Wrishko RE, Hanley WD, Wolford DG, Udo de Haes J, Reitmann C, Gutstein DE. Pharmacokinetics of sugammadex in subjects with moderate and severe renal impairment . Int J Clin Pharmacol Ther. 2017;55:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Kim YS, Lim BG, Won YJ, Oh SK, Oh JS, Cho SA. Efficacy and Safety of Sugammadex for the Reversal of Rocuronium-Induced Neuromuscular Blockade in Patients with End-Stage Renal Disease: A Systematic Review and Meta-Analysis. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Fernandes HDS, Ximenes JLS, Nunes DI, Ashmawi HA, Vieira JE. Failure of reversion of neuromuscular block with sugammadex in patient with myasthenia gravis: case report and brief review of literature. BMC Anesthesiol. 2019;19:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Hiramatsu S, Moriwaki K, Nakao M, Tsutsumi YM. Rocuronium-induced respiratory paralysis refractory to sugammadex in Charcot-Marie-Tooth disease. Can J Anaesth. 2022;69:364-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Altıparmak B, Korkmaz Toker M, Uysal A, Pınarbaşı A, Gümüş Demirbilek S. Delayed recovery from neuromuscular block after sugammadex in a patient with Parkinson's disease. Br J Anaesth. 2019;123:e459-e460. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Gurunathan U, Kunju SM, Stanton LML. Use of sugammadex in patients with neuromuscular disorders: a systematic review of case reports. BMC Anesthesiol. 2019;19:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Kiss G, Lacour A, d'Hollander A. Fade of train-of-four ratio despite administration of more than 12 mg kg(-1) sugammadex in a myasthenia gravis patient receiving rocuronium. Br J Anaesth. 2013;110:854-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Kusanale A, Wilson A, Brennan P. Tongue tremor: a rare initial presentation of essential tremor. Br J Oral Maxillofac Surg. 2011;49:e82-e83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Özer G, Kirmaci ZİK, Adigüzel H, Ergun N. Correlation of proximal and distal muscle strength with upper limb functional ability in patients with essential tremor. Acta Neurol Belg. 2020;120:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Pradeep S, Mehanna R. Gastrointestinal disorders in hyperkinetic movement disorders and ataxia. Parkinsonism Relat Disord. 2021;90:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Itoh H, Shibata K, Nitta S. Neuromuscular monitoring in myasthenic syndrome. Anaesthesia. 2001;56:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Matsui S, Tanaka S, Kiyosawa K, Tanaka T, Kawamata M. [Anesthetic Management of a Patient with Facioscapulohumeral Muscular Dystrophy: Importance of Monitoring Neuromuscular Function at Multiple Sites]. Masui. 2015;64:1273-1276. [PubMed] |
| 43. | Mann R, Blobner M, Jelen-Esselborn S, Busley R, Werner C. Preanesthetic train-of-four fade predicts the atracurium requirement of myasthenia gravis patients. Anesthesiology. 2000;93:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Brown AF, Cobert J, Dierkes J, Kuhn CM, Grant SA. Delayed Neuromuscular Blockade Reversal With Sugammadex After Vecuronium, Desflurane, and Magnesium Administration: A Case Report. A A Pract. 2019;13:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |